Summary
Melanoma-associated chondroitin sulfate proteoglycan (MCSP; also called HMW-MAA, CSPG4, NG2, MSK16, MCSPG, MEL-CSPG, or gp240) is a well characterized melanoma cell-surface antigen. In this study, a new bispecific T-cell engaging (BiTE) antibody that binds to MCSP and human CD3 (MCSP-BiTE) was tested for its cytotoxic activity against human melanoma cell lines. When unstimulated peripheral mononuclear blood cells (PBMC) derived from healthy donors were co-cultured with melanoma cells at effector: target (E:T) ratios of 1:1, 1:5 or 1:10, and treated with MCSP-BiTE antibody at doses of 10, 100 or 1,000 ng/mL, all MCSP-expressing melanoma cell lines (n=23) were lysed in a dose- and E:T ratio-dependent fashion, whereas there was no cytotoxic activity against MCSP-negative melanoma cell lines (n=2). To investigate whether T cells from melanoma patients could act as effector cells, we co-cultured unstimulated PBMC with allogeneic melanoma cells from 13 patients (4 stage I/II, 3 stage III, and 6 stage IV) or with autologous melanoma cells from 2 patients (stage IV). Although cytotoxic activity varied, all 15 PBMC samples mediated significant redirected lysis by the BiTE antibody. When PBMC or CD8+ T cells were pre-stimulated by anti-CD3 antibody OKT3 and IL-2, the MCSP-BiTE concentrations needed for melanoma cell lysis decreased up to 1000-fold. Because MCSP is expressed on most human melanomas, immunotherapy with MCSP/CD3-bispecific antibodies merits clinical investigation.
Keywords: Melanoma, MCSP, BiTE antibody, PBMC, T cell
Introduction
Bispecific T-cell engaging antibodies, abbreviated as BiTE antibodies, are designed to redirect cytotoxic T cells to malignant cells.1-3 BiTE antibodies are based on a 55-kDa, single-chain bispecific antibody construct. One arm of the antibody binds to a specific cancer antigen and the other arm binds to a CD3 subunit of T-cell receptors, the most potent trigger proteins for T-cell activation. In this manner, the activity of BiTE antibodies does not require the generation of specific T-cell clones or antigen presentation by dendritic cells. This mode of action may prevent cancer cells from escaping immune attack by downregulation of MHC class I molecule expression or by selection for defects in peptide antigen generation and surface transport. Any pre-existing T-cell clone can be engaged by a BiTE antibody to recognize a defined surface antigen on tumor cells.2 BiTE antibodies activate T cells only when bound to target cells expressing the proper surface antigen.4 They engage not only cytotoxic CD8+ T cells but also CD4+ T cells, and induce an immunological synapse as needed for delivery of perforin and granzymes.5 The activated T cells also release robust and highly significant amount of inflammatory cytokines interferon gamma, tumor necrosis factor alpha, interleukin (IL)-6, IL-4, IL-10 and IL-2.2, 4 In addition, BiTE antibodies allow repeated target cell elimination by cytotoxic T cells (CTLs),6 which may be required for treatment of tumors with low levels of tumor-infiltrating lymphocytes.
Two BiTE antibodies are currently being tested in clinical trials. Blinatumomab targets CD19 and has shown impressive tumor regression in patients with relapsed or refractory non-Hodgkin's lymphoma,3 and acute lymphoblastic leukemia.7 MT110 is a BiTE antibody that targets epithelial cell adhesion molecule (EpCAM, CD326), a cancer stem cell antigen. A phase I dose-escalation trial of MT110 for treatment of lung and gastrointestinal cancer is underway.2, 8, 9
We generated humanized BiTE antibody targeting melanoma-associated chondroitin sulfate proteoglycan (MCSP), a well characterized antigen expressed on the surface of human melanoma cells and their progenitor cells, and CD3 of the T-cell receptor.1, 10-14 MCSP is also expressed on cells of melanocytic lineage as well as basal cells of the epidermis, and is believed to be a marker of epidermal and hair follicle progenitor cells.13 MCSP expression has been found in normal human tissues such as chondrocytes, smooth muscle cells, the neuromuscular junction of human postnatal skeletal muscle, and microglial and mesangial cells of renal glomeruli.13 Likewise, MCSP is frequently expressed on neurons and glial cells of the developing and adult brain, where it is referred to as NG2 in both rodents and humans.15 However no adverse events or toxicities have thus far been reported that would indicate non-recognition of MCSP on normal tissues by MCSP-directed therapies.13 Therefore, MCSP may represent a clinically attractive target for immunotherapies based on MCSP-specific antibodies or genetically engineered MCSP-specific T cells.10, 13, 16
We here determined its cytotoxic efficacy against a large panel of human melanoma-derived cell lines co-cultured with T cells from either healthy donors or melanoma patients. Likewise, we investigated the effect of T-cell pre-stimulation by anti-CD3 monoclonal antibody OKT3 in combination with IL-2; we hypothesized that this would increase the efficacy of redirected lysis by the BiTE antibody.
Materials and Methods
BiTE antibodies
Human MCSP-BiTE antibody and MEC14 BiTE (control BiTE) were obtained from Micromet (Munich, Germany). As recently reported,14 standard DNA cloning and monoclonal antibody cloning technologies were used to construct both antibodies.17, 18 The final MCSP-BiTE product was selected from a panel of MCSP-BiTE antibodies that had different epitope specificities. Each MCSP-BiTE candidate showed stability as a single-chain antibody in conjunction with an anti-CD3 single-chain antibody. The final candidate had the strongest and most specific binding to the most membrane-proximal domain of MCSP, based on antitumor activity in cytotoxic assays.14 The control antibody contains the same CD3 binding arm but does not recognize melanoma cells or any antigens that bind to the cell membrane. Antibody preparations containing primarily the monomeric form (>97%) of each BiTE antibody were used in this study.
Cell lines
Melanoma cell lines established from biopsied/resected metastatic lesions and stored in the John Wayne Cancer Institute's (JWCI) specimen bank were used in this study. Thirty-five melanoma cell lines were selected based on the availability of single-cell suspensions and minced frozen tissues for planned future xenograft animal studies.19, 20 All melanoma cells were cultured in RPMI 1640 (Mediatech Inc, Manassas, VA), 10% FBS (Omega Scientific, Tarzana, CA), 25 mM HEPES (Mediatech Inc), and 2 mM L-glutamine (Lonza, Walkersville, MD). All cell lines were grown in a humidified atmosphere of 5% CO2.
Peripheral mononuclear blood cells (PBMC) collection and preparation
After informed consent, blood specimens were obtained from 10 healthy donors and 13 melanoma patients under a protocol approved by the Institutional Review Board. Patients presented to the JWCI clinic from August 2009 to January 2010 with melanoma or a history of melanoma. Patients who had received immunotherapy, chemotherapy or biochemotherapy within 4 weeks prior to the blood draw were excluded. Specimens were collected in heparinized syringes, and PBMC were isolated from blood using a Lymphoprep tube according to the manufacturer's directions (Nycomed Pharma, Oslo, Norway). The mononuclear cell layer was extracted, washed and resuspended in RPMI 1640 with 10% FBS.
In addition to the 13 PBMC specimens that were prospectively collected from melanoma patients, we obtained 2 cryopreserved PBMC specimens from JWCI's specimen bank. These specimens, both from patients with clinically evident stage IV melanoma, were selected because autologous cell line had been screened for MCSP expression in our study and those were MCSP-strongly positive cell lines.
Generation of stimulated PBMC and CD8+ T cells
A cell culture dish was coated with 1 μg/mL of OKT3 antibody (Centocor Ortho Biotech Inc, Horsham, PA), incubated for 1 hour at 37°C, and washed once with PBS. PBMC (3-5×107) in RPMI 1640 supplemented with 10% FBS were added to the cell culture dish with 20 U/mL of IL-2 (Novartis, Basel, Switzerland), and the cells were thus stimulated for 2 days. On the third day, the cells were collected and washed once with RPMI 1640 supplemented with 10% FBS, and an additional 20 U/mL of IL-2 was added for one day. On the fourth day, stimulated PBMC were collected, washed once and resuspended in RPMI 1640 supplemented with 10% FBS. Stimulated CD8+ T cells were isolated from the stimulated PBMC using a negative isolation kit for human CD8 T cells (Dynal Biotech, Oslo, Norway), according to the manufacturer's instructions.
Flow cytometric analysis (FACS)
To detect MCSP expression in melanoma cell lines, or to detect CD3, CD4 and CD8 expression in PBMC from melanoma patients, melanoma cell lines or PBMC were incubated on ice in 25 μL of cold buffer containing 1 mg/mL human IgG (Sigma-Aldrich, Saint Louis, MO) for 15 minutes, to block nonspecific binding due to Fc-receptor binding. Cells were stained with fluorochrome-conjugated monoclonal antibodies or isotype-matched controls for 30 minutes at 4°C. After staining, the cells were washed twice with PBS plus 0.5% fetal calf serum (FCS) by centrifuging at 250g for 5 min and discarding the supernatant. The cells were then resuspended in staining buffer prior to FACS.
Chemical reagents and antibodies
MCSP expression was assessed by fluorescein-labeled anti-NG2/MCSP monoclonal antibody (mouse IgG1, clone LMH-2) and a fluorescein-labeled isotype-matched control mouse antibody, both of which were obtained from R&D Systems (Minneapolis, MN). Staining with 7-amino actinomycin D (7-AAD; BD Biosciences, San Jose, CA) was used to detect cell death; staining with annexin V (BD Biosciences, San Jose, CA) was used to detect early stages of apoptosis. Fluorescein isothiocyanate (FITC)-labeled anti-CD4 monoclonal antibody (mAb) (mouse IgG1k, Clone L200), phycoerythrin (PE)-labeled CD8 mAb (rat IgG1k, clone HIT8a), and PE-Cy5-labeled anti-CD3 mAb (mouse IgG2ak, clone HIT3a) were obtained from BD Bioscience (San Diego, CA). FITC-, PE-labeled mouse IgG1, and PE-Cy5-labeled mouse IgG2ak (BD Bioscience, San Diego, CA) were used as isotype-matched controls.
FACS-based cytotoxicity assay
Melanoma cells were labeled with the fluorescent membrane dye PKH67 in order to distinguish them from effector cells upon FACS analysis. The PKH67 kit (Sigma-Aldrich, Saint Louis, MO) was applied in accordance with the manufacturer's instructions. In brief, melanoma cell lines were washed twice in PBS and resuspended in 1 mL of Diluent C per 1 × 107 cells. Another 1 mL Diluent C containing 4 μL PKH67 dye per 1 × 107 cells was added. Cells were labeled for 5 minutes before the reaction was stopped by adding an equal volume of FCS. After three washing steps in cell culture medium, labeled target cells were counted and resuspended in RPMI 1640 with 10% FCS.
Each well of 24-well culture plates (Corning Incorporated, Corning, NY) was seeded with 1 mL of dye-labeled melanoma cells (1∼10×104 cells/mL) adjusted for concentration of PBMC or CD8+ T cells; 2-3 hours later, after melanoma cells had attached to the plates, 500 μL of PBMC suspension and 5 μL of MCSP-BiTE antibody in RPMI 1640 with 10% FCS were added. Reactions were performed using 0, 0.1, 1, 10, 100 or 1000 ng/mL MCSP-BiTE with effector:target (E:T) ratios of 1:1, 1:3, 1:5 or 1:10. A control BiTE antibody was tested at a dose of 100 ng/mL with an E:T ratio 1:3. Two different sets of FACS-based cytotoxicity assays were done. To observe MCSP-BiTE antibody effect with stimulated PBMC or CD8+ T cells, the reactions were stopped and 7-AAD and annexin V were added at 18 hours or 6 days after adding MCSP-BiTE to the wells. To observe MCSP-BiTE antibody effect with unstimulated PBMC, propidium iodide (PI) was added 6-7 days after adding MCSP-BiTE to the wells. Samples were analyzed using a FACSCalibur (BD Biosciences, San Jose, CA). All cytotoxicity assays were performed in triplicate. The total number of melanoma cells corresponded to the number of PKH67-stained cells that had a structurally intact cell membrane. The subgroup of melanoma cells that also stained with PI or 7AAD represented dead cells; cells that stained with annexin V represented cells in early stages of apoptosis.
Statistical analysis
The median MCSP: isotype ratio was used to determine the relative intensity of MCSP according to the following criteria: <1.0, negative; 1.0-1.4, moderately positive; and >1.4, strongly positive. Correlation between the relative intensity of MCSP and MCSP-BiTE cytotoxicity, and correlation between the percentage of CD3+ T cells in PBMC and MCSP-BiTE cytotoxicity were examined using Pearson correlation coefficients. Because MCSP-BiTE treatment results in complete target cell lysis, an indicator of the anti-tumor effect of MCSP-BiTE (live melanoma cell number) at different E:T ratios and antibody dosages was calculated according to the following formula: the total number of melanoma cells (all PKH67-positive cells) multiplied by the percentage of live cells (both 7-AAD and annexin V negative cells or PI-negative cells) in each culture well. The MCSP-BiTE cytotoxicity was calculated by the formula: [1 – live melanoma cell number (sample) / live melanoma cell number (control)] × 100. The results of control BiTE or MCSP-BiTE 0 ng/mL served as the control. The results were assessed by ANOVA, general linear model, and linear regression model. All the analysis was done using SAS 9.2 (Cary, NC) and P value less than 0.05 was considered statistically significant.
Results
MCSP expression in 35 human melanoma cell lines
Because MCSP is reportedly expressed by more than 90% of all human melanoma lesions and more than 80% of cells within those lesions,13 melanoma cell lines negative for MCSP are expected to be rare. We first screened JWCI's panel of 35 melanoma cell lines to determine the level of MCSP surface expression. MCSP-negative and MCSP-positive cell lines were used to determine the antigen specificity of redirected lysis by MCSP-BiTE. The 35 melanoma cell lines were established from 34 patients whose metastatic lesions were biopsied or resected from the skin (n=17), subcutaneous tissue (n=5), lymph nodes (n=5), brain (n=2), liver (n=1), lung (n=1), kidney (n=1), colon (n=1), pancreas (n=1) and muscle (n=1). Single-stained FACS analysis demonstrated that 33 lines (94%) expressed MCSP at moderate levels (5 lines from lymph nodes, skin, lung, and subcutaneous metastasis) or high levels (28 lines); only two cell lines (6%) were MCSP negative. The negative cell lines were established from a stage III subcutaneous tissue metastasis and a stage IV skin metastasis.
Redirected lysis by MCSP-BiTE of melanoma cell lines with PBMC from healthy donors
To determine if MCSP expression by melanoma cells is necessary for a cytotoxic effect of MCSP-BiTE, we co-cultured 23 MCSP-positive and the 2 MCSP-negative human melanoma lines with PBMC from healthy donors, with or without MCSP-BiTE. Anti-tumor activity was measured for various MCSP-BiTE doses and E:T ratios by FACS-based cytotoxicity assay after 6-7 days of co-culture. This time period was adapted from another investigator's study using a different BiTE antibody.18 Though new expression of early T-cell activation markers CD69 and granzyme B by CD4+ or CD8+ T cells could be detected after 48-72 hours,21 significant lysis of melanoma cell lines did not occur during this time period. However, after 6-7 days, all 23 MCSP-positive cell lines showed lysis. The expression level of MCSP had a nonsignificant positive correlation with the cytotoxicity of MCSP-BiTE (for instance 0.35 at E:T ratio 1:5 at BiTE 1000ng/mL). This in part may be due to the small sample size and incidental to an allogeneic reaction between melanoma cell line and donor PBMC. When melanoma cells were co-cultured with 10, 100 and 1000 ng/mL MCSP-BiTE, average percent cytolysis of the 23 MCSP-positive cell lines was 42.80 ± 10.14 (SE)%, 55.03 ± 8.82%, and 76.48 ± 6.60%, respectively, at an E:T ratio of 1:10; 15.55 ± 7.25%, 38.98 ± 10.17%, and 49.10 ± 8.64%, respectively, at an E:T ratio of 1:5; and 12.70 ± 6.58%, 25.79 ±7.93%, 33.10 ±8.04%, respectively, at an E:T ratio of 1:1 (Figure 1A). MCSP-BiTE had no cytotoxic effect on the two MCSP-negative cell lines (Figure 1B). Some lysis of melanoma cell lines by an allogeneic T-cell reaction was observed without MCSP-BiTE treatment at all E:T ratios (Figure 1C), but all dose of MCSP-BiTE significantly increased the percent cytolysis. Increasing the E:T ratio (p< 0.0001) or the dose of MCSP-BiTE (p< 0.0001) also significantly increased the lysis of MCSP-positive cells (Figures 1A and C).
Figure 1.
Cytotoxicity of MCSP-BiTE antibody treatment in human melanoma cell lines. Twenty-three MCSP-positive (1A) or 2 MCSP-negative melanoma cell lines (1B) were co-cultured with effector cells (PBMC obtained from healthy donors) at E:T ratios of 1:10, 1:5, or 1:1. Cells were then treated with MCSP-BiTE antibody in concentrations of 0, 10, 100 or 1,000 ng/mL. Percent change in cytotoxicity from baseline (MCSP-BiTE 0 ng/mL) is shown as the mean and standard error (SE) of triplicate determinations. C: Means and SE of 23 MCSP-positive melanoma cell lines. Number of live melanoma cells after co-culture with or without effector cells at E:T ratios of 1:10, 1:5 and 1:1, and treatment with 0 or 100 ng/mL of MCSP-BiTE. The anti-tumor effect of MCSP-BiTE was calculated according to the following formula: live melanoma cell number = total melanoma cell counts (PKH67 positive cells) × percentage of live melanoma cell (PI negative cells).
MCSP-BiTE-redirected lysis of melanoma cell lines co-cultured with PBMC from melanoma patients
We next tested whether PBMC isolated from melanoma patients would similarly function with MCSP-BiTE in lysing MCSP-positive melanoma cells. One representative melanoma cell line (M27-HI) was selected from our previous screening as target cell line based on its high level of MCSP expression. Because CD3+ T cells is essential for MCSP-BiTE antibody-mediated melanoma lysis, PBMC from 13 melanoma patients were analyzed by triple-stain FACS analysis for proportions of CD3+, CD4+ and CD8+ T cells. CD3+ T cells comprised 29.01 ± 11.51% (mean ± SD) of PBMC, as compared with approximately 70-80% reported for PBMC from normal donors.22 The CD4+ and CD8+ T cell subsets represented 65.39 ± 14.16% and 29.75 ± 14.66 % of CD3+ cells, respectively (Table 1). To equilibrate CD3+ cell number to that of normal healthy donors, we used a 3:1 ratio of patients' PBMC.
Table 1. CD3+-positive cells in PBMC from 13 patients with melanoma.
| # | Age | Sex | Stage | Status | Primary site | Most recent tumor | CD3+ in PBMC (%) | CD4+ in CD3 (%) | CD8+ in CD3 (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | F | IA | AWD | Extremity | Leg | 38.28 | 77.37 | 20.87 |
| 2 | 64 | M | IA | AWD | Head and Neck | Face | 37.1 | 55.47 | 34.5 |
| 3 | 50 | M | IB | AWD | Head and Neck | Ear | 35.28 | 74.41 | 20.81 |
| 4 | 47 | M | IIB | AWD | Trunk | Back | 35.38 | 67.72 | 30.73 |
| 5 | 45 | M | III | AWD | Head and Neck | oral cavity | 47.97 | 53.55 | 35.96 |
| 6 | 54 | M | IIIB | AWD | Head and Neck | Head | 40.7 | 82.41 | 10.96 |
| 7 | 77 | M | IIIB | NED | Head and Neck | Lymph node | 19.94 | 37.96 | 59.58 |
| 8 | 63 | M | IV | AWD | Head and Neck | Head | 18.61 | 51.74 | 43.33 |
| 9 | 61 | F | IV | AWD | Head and Neck | Ear | 22.68 | 82.63 | 14.99 |
| 10 | 56 | F | IV | AWD | not available | Lung/chest wall | 11.11 | 58.15 | 38.33 |
| 11 | 70 | M | IV | NED | Trunk | Back | 15.58 | 83.83 | 5.91 |
| 12 | 32 | F | IV | NED | Head and Neck | Liver | 19.39 | 61.17 | 37.26 |
| 13 | 44 | M | IV | NED | Head and Neck | Lung/chest wall | 35.17 | 63.72 | 33.52 |
AWD: alive with disease
NED: no evidence of disease
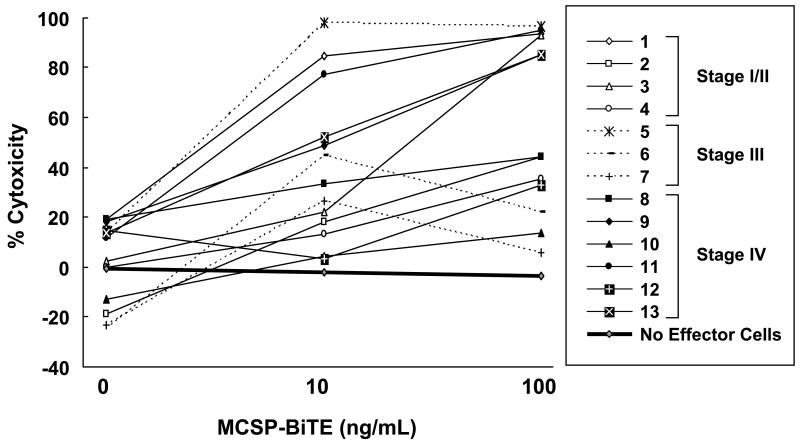
We separately co-cultured PBMC from 13 patients (4 with stage I/II, 3 with stage III, and 6 with stage IV) with M27-HI cells using a FACS-based cytotoxicity assay. Anti-tumor cell activity was measured for MCSP-BiTE doses of 0, 10, and 100 ng/mL, and for a control BiTE dose of 100 ng/mL. As shown in Figure 2, the cytotoxicity widely varied but was significantly higher with MCSP-BiTE doses of 10 ng/mL and 100 ng/mL (p<0.008). There was no significant difference in cytotoxicity between co-cultures treated with control BiTE or 0 ng/ml MCSP-BiTE. No cytotoxicity was observed in the absence of PBMC. Our data suggest that MCSP-BiTE can redirect lysis of melanoma cells by effector T cells from patients with early or advanced melanoma, and that efficacy depends on antibody dose.
Figure 2.
Anti-tumor effects of MCSP-BiTE antibody with melanoma patients' PBMC against the MCSP-positive melanoma cell line (M27-HI). PBMC from 13 melanoma patients were cocultured with M27-HI at an E:T ratio 3:1; cells were treated with MCSP-BiTE antibody (0, 10 or 100 ng/mL) or control BiTE (100 ng/mL). The results of control BiTE that binds CD3 but not MCSP were used as baseline data. The numbers 1-13 correspond to the patient numbers in column 1 of Table 1.
Unlike PBMC from healthy donors, PBMC from some melanoma patients did not readily respond to MCSP-BiTE treatment. Because cytotoxic activity of MCSP-BiTE was dependent on E:T ratio in our previous study, we speculated that the cytotoxic activity of MCSP-BiTE with PBMC from melanoma patients might depend on the percentage of CD3+ T cells (Table 1). Though it was not statistically significant, the percentage of CD3+ T cells in a PBMC specimen was positively correlated with the cytotoxic activity of MCSP-BiTE (0.43 at 10ng and 0.37 at 100ng MCSP-BiTE treatment), supporting a role of the E:T ratio.
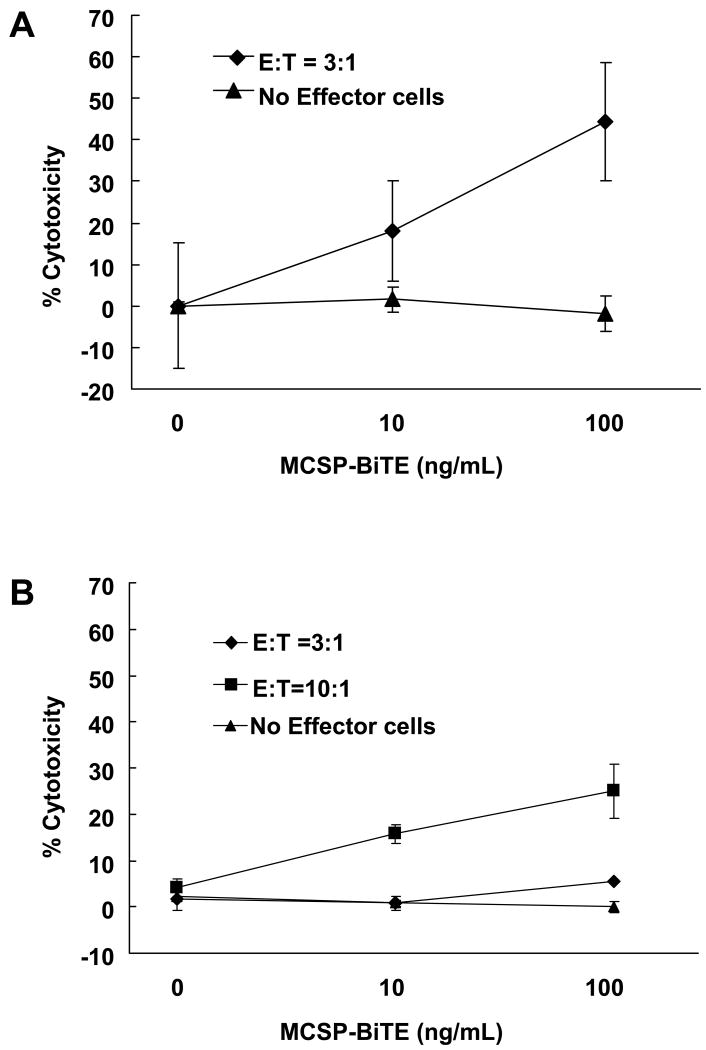
MCSP-BiTE effect in melanoma cell lines co-cultured with autologous PBMC
To provide a more clinically relevant assessment of MCSP-BiTE anti-tumor activity, and avoid possible allogeneic effects from the assay, we co-cultured two PBMC specimens with autologous melanoma cell lines (M34-HI or M35-HI) derived from stage IV metastases. We used FACS-based cytotoxicity assay to measure cytotoxicity for each MCSP-BiTE concentration (0, 10, and 100 ng/mL) and the control BiTE (100 ng/mL). In cell line M34-HI, there was a significant dose-dependent anti-tumor effect (p<0.05) at an E:T ratio 3:1; cytotoxicity was 18.19 ± 6.81% and 41.11 ± 14.00% at MCSP-BiTE concentrations of 10 and 100 ng/mL, respectively (Figure 3A). With the M35-HI cell line, significant dose-dependent cytotoxicity was observed at E:T ratios of 10:1 (p<0.02) and 3:1 (p=0.04) when compared to no effector cells. At an E:T ratio of 10:1, the cytotoxicity was 15.17 ± 1.74% and 24.99 ± 5.79%, and at an E:T ratio of 3:1, the cytotoxicity was 0.92 ± 0.30% and 5.48 ± 0.27% upon treatment with 10 and 100 ng/mL of MCSP-BiTE (Figure 3B). These cytotoxic effects were not observed without effector cells in both cell lines (Figure 3A and B). At an E:T ratio of 3:1, MCSP-BiTE cytotoxicity for M35-HI cell lines was weaker than that of autologous PBMC from M34-HI or majority of allogeneic PBMC from melanoma patients; however, cytotoxicity markedly increased at an E:T ratio of 10:1. As was observed for PBMC from allogeneic melanoma patients, MCSP-BiTE can also effectively redirect autologous effector T cells to cause lysis of melanoma cells in a dose-dependent manner, which was apparently affected by the E:T ratio.
Figure 3.
Anti-tumor effects of MCSP-BiTE antibody using MCSP-positive autologous melanoma cells and PBMC. Cells were treated with MCSP-BiTE antibody (0, 10 or 100 ng/mL) or control BiTE (100 ng/mL). Means from triplicate determination and SE are shown. A: Autologous PBMC for M34-HI melanoma cell line was cocultured with M34-HI melanoma cell line at an E:T ratio 3:1. B: Autologous PBMC for M35-HI melanoma cell line was cocultured with M35-HI cell line at an E:T ratio 3:1 and 10:1.
Pre-stimulation of PBMC and use of CD8+ T cells enhances the cytotoxic effect of MCSP-BiTE
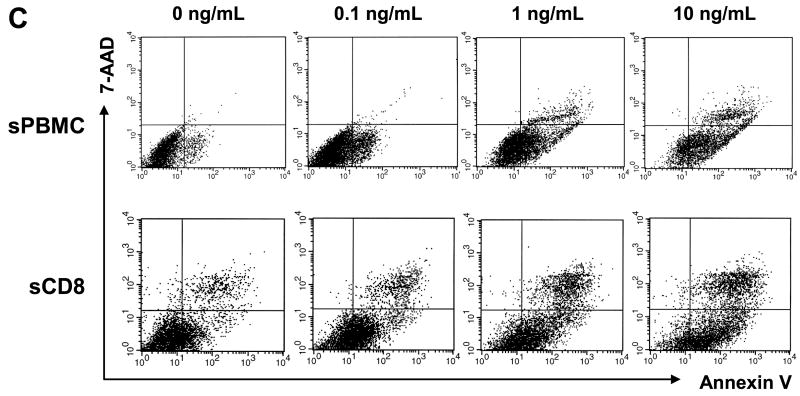
We next examined whether pre-stimulation of T cells with OKT3 and IL-2 can promote the anti-tumor cell effect of MCSP-BiTE. A strongly MCSP-positive cell line (M11-HI) was incubated with stimulated PBMC or CD8+ T cells from 4 healthy donors at MCSP-BiTE concentrations of 0, 0.1, 1 or 10 ng/mL. FACS-based cytotoxicity assay was performed at 18 hours and on day 6, at an E:T ratio of 1:1. Annexin V and 7AAD were used instead of propidium iodide to detect early apoptosis and death of melanoma cells. Data from the four PBMC specimens were averaged for analysis.
As shown in Figure 4A, MCSP-BiTE antibody (0.1, 1 or 10 ng/mL) caused significant dose-dependent cytotoxicity of melanoma cells co-cultured with stimulated PBMC or CD8+ T cells for 18 hours (p<0.01) or 6 days (p<0.01). Cytotoxicity was slightly weaker with PBMC versus CD8+ T cells at both time points. The number of live cells was lower at 6 days than at 18 hours, even in co-cultures not treated with MCSP-BiTE, however it might be because effector T cells and target melanoma cells were allogeneic (Figure 4B). As shown in Figure 4C, FACS-based cytotoxicity assay using annexin V and 7AAD identified early apoptosis subpopulations (annexin V positive but 7AAD negative) at 18 hours of co-culture. Early apoptosis and cell death increased with increasing concentrations of MCSP-BiTE when melanoma cells were co-cultured with stimulated PBMC (p< 0.01) or CD8+ T cells (P< 0.03) for 18 hours. Early apoptosis of melanoma cells was no longer observed at 6 days because MCSP-BiTE had caused almost complete lysis of melanoma cells.
Figure 4.
Use of OKT-3 and IL-2 to stimulate PBMC or CD8+ T cells from healthy donors increased the efficacy of MCSP-BiTE redirected lysis in MCSP-positive melanoma cell (M11-HI). A: Percent change of cytotoxicity in M11-HI cell line co-cultured with stimulated PBMC (sPBMC) or CD8+ T cells (sCD8) from healthy donors at E:T ratio 1:1. Value of MCSP-BiTE 0 ng/mL was used as a baseline in stimulated PBMC and CD8+ T cells, respectively. B: Number of live melanoma cells after FACS-based cytotoxicity assay. The anti-tumor effect of MCSP-BiTE was calculated according to the following formula: live melanoma cell number = total melanoma cell number (PKH67 positive) × percentage of live melanoma cell (7-AAD negative and annexin V negative). All data from 4 sets of PBMC or CD8+ T cells co-cultured with M11-HI was averaged. Each bar shows the mean number of live melanoma cells and SE. C: Melanoma cells were pre-stained with PKH-67 and co-cultured with stimulated PBMC or CD8+ T cells for 18 hours in presence of MCSP-BiTE 0, 0.1, 1 and 10 ng/mL. 7-AAD and annexin V were used to detect dead melanoma cells and early apoptotic cells, respectively. sPBMC: stimulated PBMC, sCD8: stimulated CD8+ T cell.
Compared to unstimulated PBMC, stimulated PBMC or CD8+ T cells showed a stronger and faster anti-tumor cell effect in response to MCSP-BiTE, even if melanoma cells were treated at lower doses of 0.1, 1 or 10 ng/mL.
Discussion
A variety of T cell-based therapies have been developed for advanced melanoma. Adoptive T cell therapy,23 T cell gene therapy24 and other approaches occasionally have produced partial or complete responses or disease stabilization. Although these results indicate that T cell therapies are potentially useful, it has been necessary to culture T cells in vitro and expand them prior to reinfusion for immunotherapy.25
In this study, we demonstrated that MCSP antigen expression on the melanoma cell surface is a promising target for BiTE-based immunotherapy. MCSP expression varied among melanoma cell lines, but overall 94% of melanoma cell lines established from various metastatic sites were positive for MCSP expression. MCSP-BiTE antibody treatment mediated lysis of MCSP-positive melanoma cells co-cultured with PBMC or CD8+ T cells from healthy donors or with allogeneic or autologous PBMC from melanoma patients. MCSP-BiTE antibody can redirect and activate unstimulated PBMC from healthy donors for lysis of MCSP-expressing melanoma cells. Lysis has been observed even at the very low E:T ratio of 1:10. Because PBMC are a mixture of T cells, B cells, NK cells and monocytes, all E:T ratio based on PBMC is typically lower than the physiological ratio of CD3+ T cells to melanoma cells.
We here also showed that PBMC from patients with clinically evident distant metastatic melanoma could act as effector cells for BiTE antibody-induced lysis of target melanoma cells, although the average CD3+ T-cell percentage of these PBMC was only 29.82 ± 11.94%, which is lower than the 70-80% reported for PBMC from healthy donors.22 This may indicate that the T cell proportion in the peripheral blood of melanoma patients might drop with tumor burden. We also expected that the cytotoxic activity of MCSP-BiTE might depend on the percentage of CD8+ T cells among CD3+ T cells in PBMC from melanoma patients. However, the potency of MCSP-BiTE was not significantly different when PBMC had a CD8+ T-cell percentage above (7 samples) versus below (6 samples) the median of 33.52%. Dose-dependent cytotoxic effects of MCSP-BiTE were highly significant (p<0.0001) in both groups. These preliminary data suggest that even though the percentage of CD8+ T cells among CD3+ T cells tends to be lower in PBMC from melanoma patients, this difference did not compromise engagement of T cells by MCSP-BiTE antibody. This might be because MCSP-BiTE theoretically could engage every non-naïve T cell via CD3-binding site for redirected melanoma cell lysis.
We also speculated that the cytotoxic activity of MCSP-BiTE might depend on the MHC haplotypes of tumor cells and PBMC from melanoma patients. However, dose-dependent cytotoxic effects of MCSP-BiTE were highly significant (p=0.002) with the HLA-A*02 haplotype (6 samples) which was matched with M27-HI cell line and in other unmatched MHC haplotypes (4 samples). MCSP-BiTE specific cytotoxicity was also observed in co-cultures of autologous melanoma cell lines and PBMC. Both sets of matched melanoma cells/PBMC were from patients with stage IV melanoma, which suggests that MCSP-BiTE might be effective even in the setting of advanced disease. Therefore, the effect did not depend on matching of HLA between T cells and melanoma target cells.
CD3 is not only a common surface marker for all T cells but an extremely potent trigger for T cell activation. BiTE molecules are designed to activate T cells only when multiple BiTE molecules are bound at a certain density on the surface of a target cell. The single-armed binding of BiTE to a T cell in the absence of target cannot activate T cells even at very high concentrations.1 This is important to prevent global T cell activation by BiTE molecules. In this study, our control BiTE had the same CD3-binding arm as MCSP-BiTE but the other arm did not recognize any antigen. Co-culture of this antibody with PBMC did not cause lysis of melanoma cells. The recognition of tumor antigens requires CD3, the T cell receptor, and the co-stimulatory molecule CD28 on the T cell, as well as the peptide antigen and MHC class I and activation molecules on the tumor cell. T cell activation by BiTE only requires CD3 and target antigen, and thus presents a simpler manner of T cell activation. BiTE antibodies can activate any CD3+ T cell, and all CD3+ T cells can participate in the anti-tumor cell effect if they express cytotoxic proteins. CD3+ T cells represent about 70-80% of PBMC, whereas CD8+ T cells (the major CTL) represent about 13-48% of PBMC.22 We observed the activation of both CD4 and CD8 T cells against tumor cells in the presence of MCSP-BiTE,21 and the number of CD4 and CD8 T cells in the MCSP-BiTE co-cultures increased with time. Consistent with our finding, Osada et al18 showed that the number of T cells in a co-culture of CEA/CD3 BiTE (MEDI-565) and target cells was greater than the number of T cells placed into the cultures and was also greater than the number of T cells in the control BiTE cultures after 5 days, suggesting proliferation of the effector T cells. In this study, PBMC showed a slightly weaker effect than CD8+ T cells but antibody dose-dependent cytotoxicity was still significant, even though PBMC are a mixture of effector, naïve and regulatory T cells, B cells and monocytes. When PBMC or CD8+ T cells were stimulated by OKT3 and IL-2, significant melanoma cell lysis and death as well as early apoptosis at 18 hours was MCSP-BiTE dose-dependent. This demonstrates that it may not be necessary to isolate effector cells from PBMC to treat melanoma patients, as is done with adoptive T cell transfer.
The anti-tumor cell effect of MCSP-BiTE requires both the expression of tumor antigen (MCSP) and the presence of CD3+ T cells in and around the lesion. Metastatic melanoma lesions tend to have a lower intratumoral and peritumoral density of CD3+ T cells than primary lesions,26 which is a problem of T-cell-based-therapies. However, BiTE antibodies produced proliferation and activation of effector T cells and caused serial killing of tumor cells by those activated T cells.6, 18 In vitro proliferation and activation of circulating PBMC took 6-7 days without in vitro OKT3 and IL-2 stimulation but only 18 hours with stimulation. Therefore, in vivo treatment might involve concurrent injection of OKT3, IL-2 and MCSP-BiTE, or continuous intravenous/intralesional infusion of MCSP-BiTE alone as has been observed in non-Hodgkin's lymphoma.3 In addition, lymph node metastases may be the most responsive lesions because lymph nodes have many CD3+ T cells and therefore high E:T ratios.
In conclusion, we have demonstrated that MCSP-BiTE antibody has specific cytotoxic activity against MCSP-positive melanoma cell lines in vitro. Because MCSP-specific BiTE antibodies can broadly activate the antitumor activity of T cells, this immunotherapeutic approach merits clinical investigation in patients with MCSP-positive melanoma.
Acknowledgments
Supported by grant P01 CA12582 from the National Cancer Institute, Melanoma Research Alliance (Washington, DC), Dr. Miriam & Sheldon G. Adelson Medical Research Foundation (Boston, MA), Lincy Foundation (Beverly Hills, CA), Amyx Foundation, Inc. (Boise, ID), Alan and Brenda Borstein (Los Angeles, CA), Mr. and Mrs. Louis Johnson (Stanfield, AZ), Heather and Jim Murren (Las Vegas, NV), and by funding from the Wayne and Gladys Valley Foundation (Oakland, CA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
References
- 1.Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther. 2009;11:22–30. [PubMed] [Google Scholar]
- 2.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 3.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 4.Brischwein K, Parr L, Pflanz S, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 5.Haas C, Krinner E, Brischwein K, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 7.Nagorsen D, Baeuerle PA. Immunomodulatory therapy of cancer with T cell-engaging BiTE antibody blinatumomab. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.03.010. EPub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Trerotola M, Guerra E, Alberti S. Letter to the editor: efficacy and safety of anti-Trop antibodies, R. Cubas, M. Li, C. Chen and Q. Yao, Biochim Biophys Acta 1796 (2009) 309-1. Biochim Biophys Acta. 2010;1805:119–120. doi: 10.1016/j.bbcan.2009.12.002. author reply 121-112. [DOI] [PubMed] [Google Scholar]
- 9.Brischwein K, Schlereth B, Guller B, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt P, Kopecky C, Hombach A, et al. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci U S A. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natali PG, Bigotti A, Cavaliere R, et al. Phenotyping of lesions of melanocyte origin with monoclonal antibodies to melanoma-associated antigens and to HLA antigens. J Natl Cancer Inst. 1984;73:13–24. [PubMed] [Google Scholar]
- 12.Kageshita T, Nakamura T, Yamada M, et al. Differential expression of melanoma associated antigens in acral lentiginous melanoma and in nodular melanoma lesions. Cancer Res. 1991;51:1726–1732. [PubMed] [Google Scholar]
- 13.Campoli MR, Chang CC, Kageshita T, et al. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–296. doi: 10.1615/critrevimmunol.v24.i4.40. [DOI] [PubMed] [Google Scholar]
- 14.Bluemel C, Hausmann S, Fluhr P, et al. Epitope distance to the target cell membrane and antigen size determine the potency of T cell-mediated lysis by BiTE antibodies specific for a large melanoma surface antigen. Cancer Immunol Immunother. 2010;59:1197–1209. doi: 10.1007/s00262-010-0844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karram K, Chatterjee N, Trotter J. NG2-expressing cells in the nervous system: role of the proteoglycan in migration and glial-neuron interaction. J Anat. 2005;207:735–744. doi: 10.1111/j.1469-7580.2005.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns WR, Zhao Y, Frankel TL, et al. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 2010;70:3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutterbuese R, Raum T, Kischel R, et al. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother. 2009;32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 18.Osada T, Hsu D, Hammond S, et al. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br J Cancer. 2010;102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torisu-Itakura H, Baeuerle PA, Morton DL. T cell-engaging MCSP-specific BiTE antibody showed anti-tumor activity of at very low effector to target ratios. Proc Japanese Dermatological Association. 2009;108th Abs 10042. [Google Scholar]
- 20.Torisu-Itakura H, Schoellhammer HF, Huynh Y, et al. Anti-tumor activity of a T cell-engaging MCSP-specific BiTE antibody at very low effector to target ratios: A new approach to treat metastatic melanoma. Proc Am Assoc Cancer Res. 2009;50:786. [Google Scholar]
- 21.Schoellhammer HF, Torisu-Itakura H, Sim M, et al. Single-chain bispecific antibody specific for CD3 and melanoma-associated chondroitin sulfate proteoglycan: In vitro and in vivo antimelanoma activity. Proc Am Assoc Cancer Res. 2010;51:1365. [Google Scholar]
- 22.McMichael AJ, Beverly P, Gilks W. Leucocyte Typing III: White Cell Differentiation Antigens. New York: Oxford University Press; 1987. [Google Scholar]
- 23.Berry LJ, Moeller M, Darcy PK. Adoptive immunotherapy for cancer: the next generation of gene-engineered immune cells. Tissue Antigens. 2009;74:277–289. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 24.Uckert W, Schumacher TN. TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol Immunother. 2009;58:809–822. doi: 10.1007/s00262-008-0649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattinoni L, Powell DJ, Jr, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiss J, Timar J, Somlai B, et al. Association of microvessel density with infiltrating cells in human cutaneous malignant melanoma. Pathol Oncol Res. 2007;13:21–31. doi: 10.1007/BF02893437. [DOI] [PubMed] [Google Scholar]