Abstract
Among several pharmacological compounds, Phlebotomine saliva contains substances with anti-inflammatory properties. Herein, we demonstrated the therapeutic activity of salivary gland extract (SGE) of Phlebotomus papatasi in an experimental model of arthritis (collagen-induced arthritis [CIA]) and identified the constituents responsible for such activity. Daily administration of SGE, initiated at disease onset, attenuated the severity of CIA, reducing the joint lesion and pro-inflammatory cytokines release. In vitro incubation of dendritic cells (DC) with SGE limited specific CD4+Th17 cell response. We identified adenosine (ADO) and adenosine monophosphate (5′AMP) as the major salivary molecules responsible for anti-inflammatory activities. Pharmacologic inhibition of ADO A2A receptor or enzymatic catabolism of salivary nucleosides reversed the SGE-induced immunosuppressive effect. Importantly, CD73 (ecto-5′nucleotidase enzyme) is expressed on DC surface during stage of activation, suggesting that ADO is also generated by 5′AMP metabolism. Moreover, both nucleosides mimicked SGE-induced anti-inflammatory activity upon DC function in vitro and attenuated establishment of CIA in vivo. We reveal that ADO and 5′AMP are present in pharmacological amounts in P. papatasi saliva and act preferentially on DC function, consequently reducing Th17 subset activation and suppressing the autoimmune response. Thus, it is plausible that these constituents might be promising therapeutic molecules to target immune inflammatory diseases.
INTRODUCTION
During their evolutionary process, several species of blood-feeding arthropods developed a number of sophisticated and redundant mechanisms to overcome the hemostatic and inflammatory/immune systems of their vertebrate hosts (1). Vasodilators, anticoagulants, inhibitors of platelet aggregation, anti-inflammatory and immunomodulatory molecules are present in the salivary glands and are essential to a successful blood meal (2, 3). Furthermore, these active molecules may contribute in the transmission, as well as establishment, of arthropod-borne diseases (i.e., leishmaniasis by phlebotomines, malaria by anophelines, and Lyme disease by ixodid ticks), through modulation of the host immune response (4, 5). Indeed, arthropod saliva has been shown to inhibit several functions of the immune system including activation of the alternative complement pathway, phagocytosis of pathogens, production of inflammatory cytokines by macrophages and dendritic cells (DCs), and activity of NK cells, as well as T and B cell proliferation (6–11).
In phlebotomines, it has been demonstrated that their saliva is able to selectively inhibit several DC and macrophage functions including antigen presentation, nitric oxide and hydrogen peroxide production, and IFN-γ-induced iNOS gene expression, thus inhibiting intracellular killing by Leishmania major (9, 12). Furthermore, salivary proteins from certain sand fly species favor development of a Th2-type immune response, either in vitro or in vivo, characterized by production of high levels of IL-4 (13, 14). Importantly, sand fly saliva induces release of immunomodulatory mediators such as IL-10 and prostaglandin E2 (PGE2) and inhibits production of protective type 1 cytokines such IL-12, IFN-γ, and TNF-α, all of which enhance survival of the Leishmania parasite. (15–18).
We recently demonstrated that systemic pretreatment of mice with salivary gland extract (SGE) from the Old World species Phlebotomus papatasi and Phlebotomus duboscqi, inhibited neutrophil migration during OVA-induced immune peritonitis (19). By exploring the specific mechanism of saliva action, we found that Phlebotomine saliva acts preferentially on APCs, inhibiting DC’s ability to present antigens to T cells. These anti-inflammatory effects seem to depend on a sequential production of PGE2 and IL-10 by DCs, which act in an autocrine manner (19).
DCs are potent APCs specialized in the initiation of the immune response by direct activation and differentiation of naïve T lymphocytes to specific subtypes (20). Inflamed synovia from arthritic patients contains high numbers of both DC subsets, myeloid and plasmacytoid, which strongly suggests a role for these APCs in disease perpetuation (21–23). During the antigen presentation process, depending on stimuli (i.e., pathogens or autoantigens), DCs that emigrate to inflamed joints produce pro-inflammatory mediators such as interleukins IL-1β, IL-6, IL-12p70, IL-15, IL-18, IL-23p19, and TNF-α that support expansion and differentiation of Th1 and/or Th17 cells, which play a pathologic role in arthritis (24–27). Given the ability of DCs to interact strongly with T cells, inducing and activating the lymphocyte CD4+Th17 subset, it is plausible to suggest that pharmacologic strategies aimed at blocking DC function may deserve attention as a potential therapeutic target of autoimmune diseases.
Taking into account this evidence, we examined here the potential therapeutic effect of P. papatasi SGE on collagen-induced arthritis (CIA). We also identify the constituents of P. papatasi saliva which are responsible for the immunomodulatory activityobserved.
MATHERIALS AND METHODS
Mice
Male DBA/1J mice weighing 18–22 g were housed at the animal facility of the Department of Pharmacology or Immunology, School of Medicine of Ribeirão Preto, University of São Paulo (Brazil), in temperature-controlled rooms (22–25°C) and received water and food ad libitum. All experiments were conducted in accordance with National Institutes of Health (NIH) guidelines on the welfare of experimental animals and with the approval of the Ethics Committee from the School of Medicine of Ribeirão Preto.
Saliva
Salivary glands were prepared from 7- to 10-d-old laboratory-bred females of P. papatasi from the Laboratory of Malaria and Vector Research at the NIH (USA) as previously described (28). Briefly, 50 pairs of salivary glands were dissected under sterile conditions in endotoxin-free PBS, placed in 50 μl of sterile PBS buffer and kept at −70°C until needed. Immediately before use, the glands were disrupted by sonication using a Sonifier 450 homogenizer (Branson). Endotoxin levels were evaluated using the QCL-1000(r) Chromogenic LAL Endpoint Assay kit (Lonza, Switzerland), resulting in negligible levels of endotoxin in the salivary gland supernatant.
Induction of CIA and assessment of arthritis
CIA was elicited in mice as previously described (29). Briefly, male DBA/1J mice (10 wk) received 200 μg diluted in acetic acid of bovine type II collagen (Sigma) emulsified in Freund’s complete adjuvant (Sigma) by intradermal (i.d.) injection at the base of tail on d 0. Mice were boosted i.d. with collagen (200 μg in diluted in acetic acid) emulsified in Freund’s incomplete adjuvant (Sigma) on d 21. Mice were monitored daily for signs of arthritis as described (29). Scores were assigned based on erythema, swelling, or loss of function present in each paw on scale of 0–3, giving a maximum score of 12 per mouse. When mice reached a score of 1 for clinical arthritis, they were treated with P. papatasi SGE (1 gland/animal) or 5′AMP+ADO (~ 20 μM each) by i.v. route daily for 2 wk. To evaluate the salivary nucleosides effectiveness, some animals were either treated when disease was scored 6 or the treatment was interrupted after 1 week. Control mice received the same volume of PBS. Alternatively, in some groups, SGE were previously incubated ± with ADA (4.3 U), an enzyme that catabolizes ADO. Scoring was conducted in a blinded fashion. For histologic assessment, mice were euthanized 35 d post challenge, and the hind limbs were removed and demineralized thoroughly in 10% EDTA for 1–2 wk. The decalcified tissues were trimmed, dehydrated in graded ethanol, and embedded in paraffin. Serial sections (5 μm) were cut and mounted on glass slides precoated with 0.1% poly-L-lysine (Sigma). Histologic assessment was carried out following routine hematoxylin and eosin staining (H&E). Ankle and joint sectionswere prepared and stained with H&E to study the inflammatory cell influx or using safranin-O to determine proteoglycan depletion and cartilage destruction. Quantification of cellular infiltrate was performed by ImageJ software (NIH), in 40 fields (400× magnification) for each animal/group. To measure cytokine concentrations in the inflammatory site, articular tissues were harvested and titered in 1 mL of PBS containing protease inhibitor cocktail Complete (Roche) by tissue-trimmer. Articular homogenates were centrifuged and their supernatants collected and stored at −70°C for determination of IFN-γ, IL-10, IL-17, and TNF-α by ELISA (BD Biosciences) and PGE2 by a radioimmunoassay kit, according to the manufacturer’s instructions (DuPont NEN® Research Products).
DC generation
BM cells were isolated from 6- to 8-wk-old DBA/1 naïve mice and cultured with murine GM-CSF (20 μg/ml) (Peprotech). On d 3, half of the supernatant was gently removed and replaced with the same volume of supplemented medium. On d 6, non-adherent cells were collected and submitted to positive selection using anti-CD11c magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec) to eliminate residual macrophage and granulocyte contamination. Flow cytometric evaluation of purified DCs shows that 90% of cells express CD11c interm or high (marker of DC).
SGE fractionation and HPLC procedures
P. papatasi SGE (60 pairs) were diluted in 60 μl of PBS and submitted to filtration in a Microcon YM-3 (Millipore) (cut-off < 3 KDa), separating SGE into two fractions: filtrate (< 3 kDa) and retentate (> 3 kDa). Molecular sieving HPLC of the filtrate fraction was performed using a Superdex 75 column (3.2 × 300 mm) (Amersham Biosciences) and eluted at 0.05 ml/min with 10 mM HEPES buffer (pH 7.2), containing 0.15 M NaCl. The solvent was delivered using a CM-4100 pump (Thermo Separation Products). The eluent was monitored for UV absorbance at 220 nm (SM-4100 UV spectrophotometer; ThermoSeparation Products) with fractions being collected at 1-min intervals using a FC203-B fraction collector (Gilson Inc.). The column was calibrated using BSA, chicken OVA, carbonic anhydrase, myoglobin, cytochrome c, angiotensin 1, tyrosine, and tryptophan. Reverse phase of filtrate was performed on a C18 column (0.5 × 150mm; Thermo Separation Products) perfused at 25 μl/min using a PerkinElmer ABI 1400 pump. A gradient of 60-min duration from 5–90% acetonitrile in water, containing 0.1% trifluoroacetic acid, was imposed after sample injection. Aliquots of filtrate or these fractions were tested for cytokine-inhibitory activity after evaporation of the solvent and resuspension in RPMI-1640 media. Alternatively, filtrate, ADO (100 μM), or 5′AMP (100 μM) were incubated ± with ADA (4.3 U) and then added to BMDC cultures for 3 h.
Effect of P. papatasi SGE, fractions, and nucleosides in LPS-induced BMDC maturation
BMDCs (1 × 106/ml) in RPMI-1640 supplemented with 10% FBS was incubated with medium, total extract from P. papatasi salivary gland (16 μg/ml), or salivary fractions (retentate or filtrate; 16 μg/ml each) for 12 h. In some experiments, indicated concentrations of ADO, 5′AMP (3–300 μM), ADO synthetic analogous P101 (100–1000 μM), or medium was added to the BMDC culture. For the time-response studies, BMDCs were pretreated with ADO or 5′AMP (100 μM) for −3.0; −0.5; 0.0; +0.5 and +3.0 h of LPS stimulation. In all experiments, LPS (50 ng/ml) or medium was added to the culture and incubated at 37°C in 5% CO2 for 24 h in a total volume of 200 μL per condition. The supernatant was collected to measure TNF-α, IL12p40, and IL-10 production by ELISA assay and PGE2 by radioimmunoassay. The cells were harvested and their surface expression characterized by flow cytometry using antibodies against MHC class-II, CD80, CD86, CD40, CD39, and CD73 conjugated to FITC, PE, or PerCP, as well as control isotypes.
In vivo depletion of IL-10 cytokine
The IgG1 2A5 anti-murine IL-10 was purified from ascite harvested of pristine (Sigma Chemical Co., St Louis, MO)-primed nude backcrossed-BALB/c mice injected with the 2A5 hybridoma cells, as describes elsewhere (30). The groups of vehicle- or nucleosides-treated arthritic mice received at the onset of the disease, 250μg of 2A5MAb or normal rat IgG controls by i.p. route antibody ever each 2 days, weekly during 2 weeks thereafter. Scores were assessed as above described. Draining lymph nodes were harvested and DC maturation was evaluated through surface expression of CD11c and MHC class-II characterized by flow cytometry using specific antibodies.
T cell proliferation
To assess the influence of P. papatasi SGE treatment on antigen presentation, spleens and popliteal and inguinal LN cells harvested from arthritic mice were removed and washed twice with PBS. Tissues were minced, and the cells were filtered through a cell strainer, centrifuged at 500 g at 4°C for 10 min, and resuspended in RPMI-1640 medium at 2.5 × 106 cells/ml. For intracellular staining, non-adherent cells were cultured ± stimulation with collagen-pulsed BMDCs pretreated with PBS or SGE (8 glands/106 BMDC) for a final ratio of 10 total cells:1 BMDC for 96 h. Brefeldin A was added for the last 4 h of stimulation. For surface staining, cells were incubated with anti-CD4 for 30 min at 4°C, washed, and then fixed with BD Cytofix (BD Biosciences). Cells were permeabilized using PBS containing 1% FCS, 0.01% sodium azide, and 0.05% saponin and stained with anti-mouse IFN-γ and anti-mouse IL-17 (all antibodies from BD Biosciences), acquired on FACS Canto II (BD Biosciences), and analyzed using FlowJo software (Tree Star).
To purify CD4+ T cells, total cells were incubated with microbeads coated with antibodies against L3T4 (Miltenyi Biotec) and isolated using magnetic separation. The procedures were performed in accordance with manufacturer’s instructions. To perform in vitro coculture assay, BMDCs were preincubated with SGE, ADO, or medium for 3 h. Isolated CD4+ T cells were added to wells to a cell ratio of 10:1. In some wells, A2AR antagonist was added 2h before SGE or ADO preincubation. In all the experiments, C-II (5μg/mL), plate-bound α-CD3 (5 μg/mL), or medium was added to the culture and incubated for 96 h in a total volume of 200 μL per condition. Supernatants were harvested for determination of IL-17 production and cell proliferation measured by overnight [3H]thymidine incorporation.
Quantitative RT-PCR
Total RNA of BMDCs pre-incubated with ADO (100 μM) was extract 24 h after stimulation with LPS (50 ng/ml) using the Illustra RNAspin Mini (GE Healthcare). Gene expression was normalized to expression of the GAPDH gene. COX2, A2AR, and A2BR primer sequences are as follows: GAPDH forward: 5′-TGCAGTGGCAAAGTGGAGAT-3′; reverse: 5′-CGTGAGTGGAGTCATACTGGAA-3′; COX2 forward: 5′-GTGGAAAAACCTCGTCC AGA-3′, reverse: 5′-GCTCGGCTTCCAGTATTGAG-3′; A2AR forward: 5′-TTCTTCGCCTGCTTTGTCCT-3′, reverse: 5′-ATACCCGTCACCAAGCCATT-3′; and A2B forward: CTGCTCATAATGCTGGTGATCT, reverse: ATCAGTTCCATGCGCTGA. To CD39 and CD73 expression, total mRNA was extract from DC culture harvested in different periods after LPS stimuli. CD39 forward: AACTCTCTGCAATTCCGTCTCT, reverse: ATGTCCTTGGCCAGTTTCTG; CD73 forward: CAAATCCCACACAACCACTGT and reverse: TATCTCTTGGGCCTCCATA
Statistical analysis
Data are expressed as mean ± SEM and are representative of 2–4 independent experiments. Results of individual experiments were not combined, as they were analyzed individually. The means from different groups were compared by analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was set at P < 0.05.
RESULTS
Systemic treatment with SGE ameliorates CIA
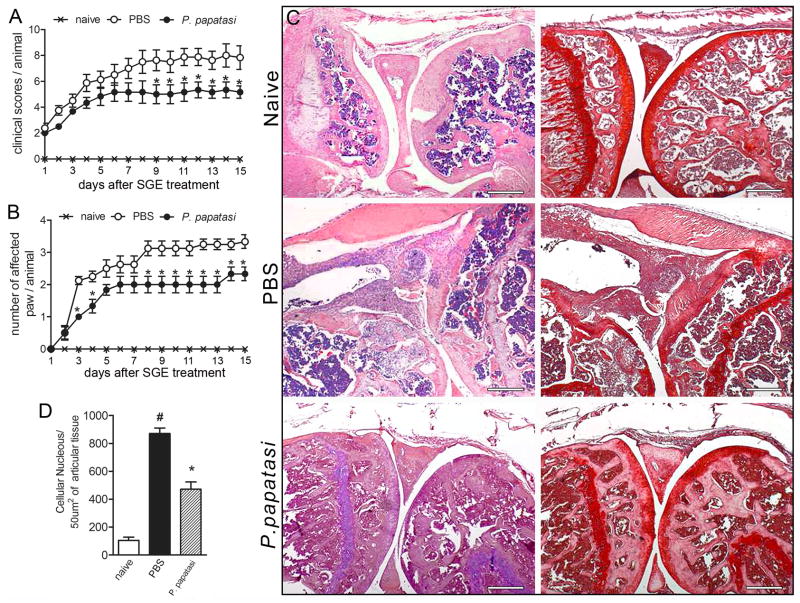
We first investigated the potential therapeutic effect of P. papatasi SGE on the CIA model in DBA/1J mice. The disease was elicited as described in Materials and Methods. Mice were treated with SGE (one salivary gland/animal) by i.v. route daily for 14 d from the first day of clinical manifestation of disease. Collagen-immunized mice treated with PBS developed the expected disease progression, showing a prominent clinical severity score of 8.0 ± 2.23 (Fig. 1A) and a high number of affected paws (3.25 ± 0.51) (Fig. 1B) compared with naïve mice. In contrast, SGE treatment ameliorated disease severity over the entire observation period, displaying a significant reduction of 37.5% in the clinical scores (5.0 ± 1.17) (Fig. 1A) and 38.5% in numbers of arthritic paws (2.0 ± 0.52). Histologic examination of the knees at the end of the monitoring period (14 d of treatment) revealed that untreated mice showed severe cellular infiltration (Fig. 1C, left columns, and Fig.1D) and marked reductions in matrix proteoglycan (Fig. 1C, right columns), suggesting joint cartilage damage. In contrast, these pathologic events were profoundly reduced in SGE-treated animals (Fig. 1C, left columns, and Fig.1D). Together, these data demonstrate that P. papatasi SGE attenuated development of CIA that could be clinically detected, and this activity can prevent progression of articular damage.
Figure 1. SGE from P. papatasi attenuated collagen-induced arthritis.
Naïve (×) or collagen-immunized and challenged DBA/1 mice were injected i.v. daily with PBS (○) or P. papatasi SGE (1 gland/animal) (●) for 14 d. Mice were monitored for disease progression as indicated by clinical scores (A)and number of affected paws(B). On d15 of SGE treatment, mice were euthanized, the articular joints were harvested, and histopathologic analysis was performed. Knee joint sections were stained by H&E (left row) or with safranin-O (right row), a proteoglycan red marker showing profound cartilage damage in PBS-treated mice (absence of proteoglycan) and preservation of cartilage in SGE-treated mice(C). Quantification of cellular infiltrate was performed by ImageJ software (NIH, USA)in 40 fields with 400× magnification for each animal/group (D). Morphometric histologic examination shows markedly less cellular infiltration in the SGE-treated than in the PBS-treated group (black bar vs. striped bar). Results show the mean±SEM, N =4; *, P<0.05 compared with PBS-treated group.
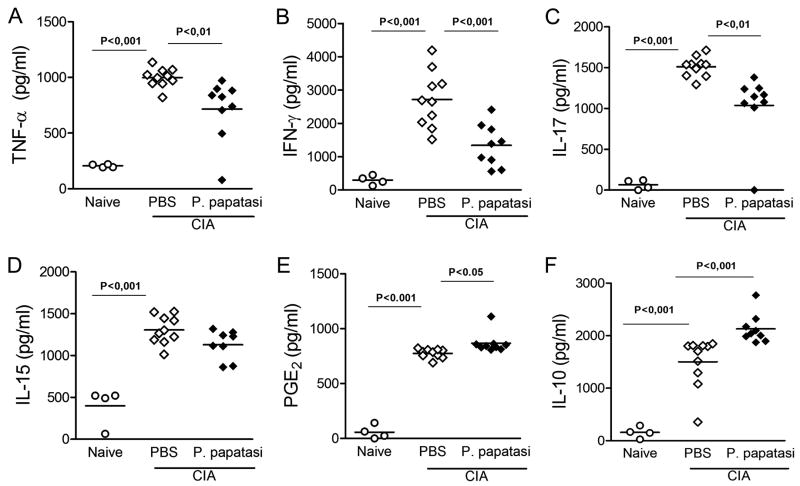
Taking into account that the tissue damage observed in several autoimmune diseases (including rheumatoid arthritis [RA]) is a consequence of pro-inflammatory mediators released into the inflammatory site (31), we investigated the concentration of TNF-α, IFN-γ, IL-17, and IL-15 in affected ankle joints. Paw samples from arthritic mice treated with PBS contained significantly higher concentrations of all above-mentioned inflammatory cytokines compared with those of naïve mice (Fig. 2). On the other hand, mice treated with SGE showed a significant reduction in the levels of TNF- α (Fig. 2A), IFN-γ (Fig. 2B), and IL-17 (Fig. 2C), but no effect on IL-15 levels (Fig. 2D) compared with control PBS-treated arthritic mice.
Figure 2. Decreased inflammatory cytokinesin articular jointsfrom SGE-treated arthritic mice.
Ankle joints from naïve (○)orPBS-(◇) or SGE-(◆) treated arthritic mice were collected for determination of TNF-α (A), IFN-γ (B), IL-17(C), IL-15 (D), and IL-10 (F) levels by ELISA and PGE2 (E) by RIA in the homogenate supernatants. Results are expressed as mean±SEM, N =4 (naïve) and 9–10 (arthritic groups).
We previously demonstrated that PGE2 and IL-10 are involved in the anti-inflammatory activity of P. papatasi SGE in OVA-induced immune peritonitis (19). Thus, we attempted to address whether SGE treatment attenuated CIA by inducing production of these anti-inflammatory mediators. Levels of PGE2 and IL-10 were evaluated in the inflamed paws of CIA mice. Supporting our hypothesis, the treatment of CIA arthritic mice with saliva enhanced the levels of PGE2 (Fig. 2E) and IL-10 (Fig. 2F) production compared with PBS-treated arthritic mice (Figs. 2E and 2F, respectively). These data suggest that sand fly SGE triggers host anti-inflammatory mechanisms that may control progression of arthritis and protects the joint from extreme damage.
Blocking of DC function by SGE treatment inhibits CD4+ T cell activation
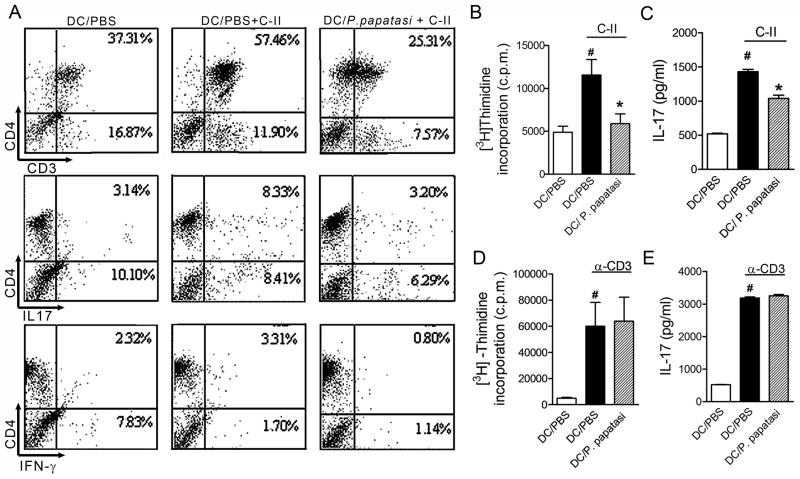
To elucidate the mechanism(s) of successful SGE therapeutic treatment on CIA, we investigated whether blocking the DC ability to present antigen by SGE affects CD4+ T cell activation and, consequently, inhibits pro-inflammatory cytokine release during collagen-induced arthritis. To this purpose, non-adherent inflammatory cells from spleen and draining LNs of arthritic mice were cultured with syngeneic bone marrow-derived DC (BMDC) pretreated overnight with SGE (8 glands/ml) or PBS, followed by collagen (5 μg/ml) or medium stimulation for 96 h, after which the cells were phenotyped.
Collagen-pulsed BMDC markedly triggered an increase of CD4+ T cells and expression of IL-17 and IFN-γ by these cells when compared with BMDCs cultured with PBS only. Pre-incubation of collagen-pulsed BMDC with SGE reduced proliferating CD4+ T cells (56.0% reduction) as well as IL-17 (62.0% reduction) and IFN-γ-positive cells (76.0% reduction) (Fig 3A).
Figure 3. SGE treatment inhibits antigen-specific CD4+ T cell activation.
BMDCs (106 cells/ml) from DBA/1 naïve mice were incubated overnight with P. papatasi SGE (8 glands/ml) or PBS. Non-adherent cells from LNs and spleen cells from CIA mice on d35 of disease were added to BMDC culture, followed by stimulation with or without collagen-II (5 μg/ml) for 96 h. Percentages of CD4+CD3+, CD4+IL-17+, and CD4+IFN-γ+ cells were determined by flow cytometry (A). Purified CD4+T cells (107 cells/ml) from arthritic mice were added to BMDC culture, and lymphoproliferation induced by C-II (5μg/ml) (B) or plate-bound α-CD3 (5μg/ml) (D) for 96h was assessed by [3H]thymidine incorporation. Culture supernatant was harvested to measure IL-17 levels from C-II-(C) or α-CD3-(E) stimulated cultures. The results are expressed as the mean±SEM obtained from two or three independent experiments made in triplicate (N =3 per group). #, P<0.05 when compared with medium; *, P<0.05 when compared with stimuli; *, P <0.05 compared with saliva vehicle (PBS).
To obtain more evidence for the role of SGE on CD4+ T cell activation via DC inhibition, we magnetically purified CD4+ T cells from draining LNs and cultured them with BMDCs pre-incubated with PBS or SGE (8 glands/ml) overnight followed by stimulation with collagen (5 μg/ml), plate-bound anti-CD3 (5 μg/ml), or medium for 96 h. As expected, BMDCs pretreated with PBS and pulsed with collagen induced an intense CD4+ lymphoproliferative response and IL-17 production compared with non-pulsed BMDCs (Figs. 3B and 3C). There was a significant reduction (49.0%; P < 0.05) in antigen-specific CD4+ lymph proliferation when BMDCs were pretreated with SGE (Fig. 3B). Furthermore, IL-17 production was significantly inhibited (27.5% inhibition; P < 0.05) in the supernatant of co-cultures pre-incubated with SGE (Figs. 3B and 3C); however, treatment of DCs with SGE did not alter either proliferative response or IL-17 production after polyclonal anti-CD3 stimulation (Figs. 3D and 3E). Together, these results suggest that SGE suppresses the specific immune response by acting on APC, blocking the antigen presentation process and, as a consequence, inhibiting pro-inflammatory mediator release by CD4+ T cells involved in RA.
Adenosine and adenosine monophosphate are the major anti-inflammatory molecules in P. papatasi SGE
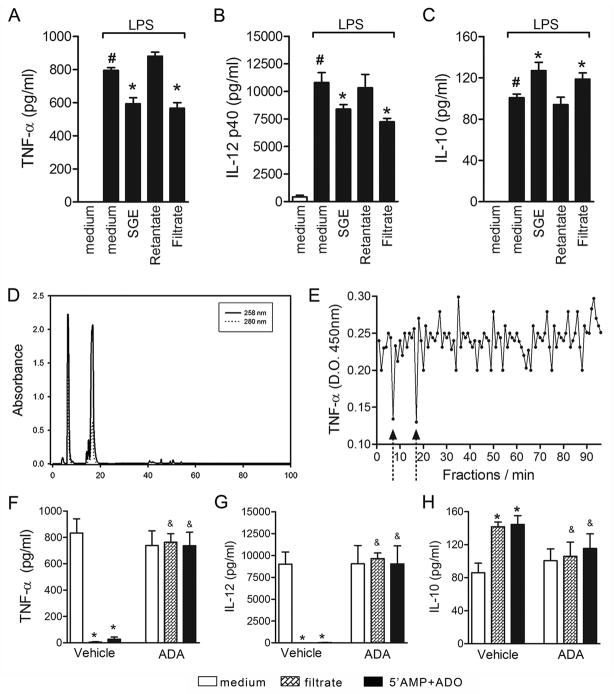
To identify the molecule(s) responsible(s) for the successful therapeutic activity of P. papatasi SGE on CIA, the salivary extract was filtered using Microcon YM3 and separated into filtrate (representing molecules with mass < 3 kDa) or retentate (compounds with mass > 3 kDa). Filtrate, retentate, and SGE (control) samples containing ~16 μg of protein/ml (equivalent to the protein concentration of 8 glands diluted in 1 ml) were tested in LPS-induced DC maturation. Following LPS stimuli, BMDCs produced high levels of both TNF-α and IL-12p40 compared with medium (Fig. 4A and 4B). When cultures were pre-incubated overnight with SGE, significant inhibitions of both pro-inflammatory mediators were observed (Fig. 4A and 4B). Interestingly, similar inhibitions of TNF-α and IL-12p40 synthesis were obtained with the filtrate but not with the retentate, suggesting the presence of active salivary molecule(s) with less than 3 kDa (Fig. 4A and 4B). Anti-inflammatory cytokines such as IL-10 are also induced by LPS and may be upregulated by P. papatasi saliva (19). In fact, LPS-induced IL-10 production was increased upon preincubation of BMDCs with SGE or filtrate, but not with retentate salivary fraction (Fig. 4C).
Figure 4. ADO and 5′AMP are the major suppressive molecules in P. papatasi SGE.
BMDCs were preincubated overnight with SGE (16 μg/ml) or SGE microcon YM-3 fractions: retentate (>3 kDa) and filtrate (<3 kDa) and then stimulated with LPS (50 ng/mL) for 24 h. TNF-α (A), IL-12 p40 (B), and IL-10 (C) production were measured in the culture supernatants. To characterize DC modulator(s), the filtrate fraction was chromatographed by reverse-phase HPLC as described in Materials and Methods (D). The activity of each eluted fractions was evaluated in LPS-induced TNF-α production by BMDCs (E). Filtrate or ADO+5′AMP commercial standards were previously incubated with vehicle (DMSO) or ADA (4.3U) for 3 h before LPS stimulation, and cytokine production (F–H) was measured in the culture supernatant. #, P<0.05 compared with medium; &, P<0.05 when compared with vehicle treatment.
To obtain further information on the biochemical nature of the low molecular weight salivary molecule(s), the filtrate fraction was submitted to reverse-phase HPLC. The absorbance of eluted fractions was monitored at 258 and 280 nm. The chromatogram indicated two major peaks at 7 and 17 min of retention, having a large 258 nm absorption compared with absorption at 280 spectrum with expected retention times of the nucleosides adenosine (ADO) and adenosine monophosphate (5′AMP), respectively (Fig. 4D). These substances were previously described by our group in the salivary glands of P. papatasi (32, 33). Other compounds eluted at forty minutes, although in minor concentrations, some of them also absorbing at 258 nm (Fig. 4D). In fact, the highest inhibition of TNF-α production was achieved using fractions 7 and 17 (Fig. 4E), suggesting that ADO and 5′AMP are the major salivary constituents with modulatory activity on DCs. Confirming this hypothesis, treatment of LPS-stimulated DCs with similar amounts of 5′AMP+ADO mimics the SGE effect, i.e., inhibits TNF-α and IL-12p40 production (Figs. 4F and 4G, respectively). Moreover, treatment of the SGE filtrate fraction or 5′AMP+ADO with adenosine deaminase (ADA), an enzyme that catabolizes ADO (34), prevented the inhibitory effect of SGE and 5′AMP+ADO on TNF-α and IL-12p40 production by LPS-stimulated DCs (Figs. 4F and 4G, respectively). In addition, enhancement of IL-10 by SGE was also abolished by ADA-treated filtrate (Fig. 4H).
ADO and 5′AMP modulate DC maturation
To determine the relative contribution of ADO and 5′AMP in the SGE-dependent anti-inflammatory activity of SGE, a concentration-response curve and time-course inhibitory profile were conducted using standard commercial nucleosides.
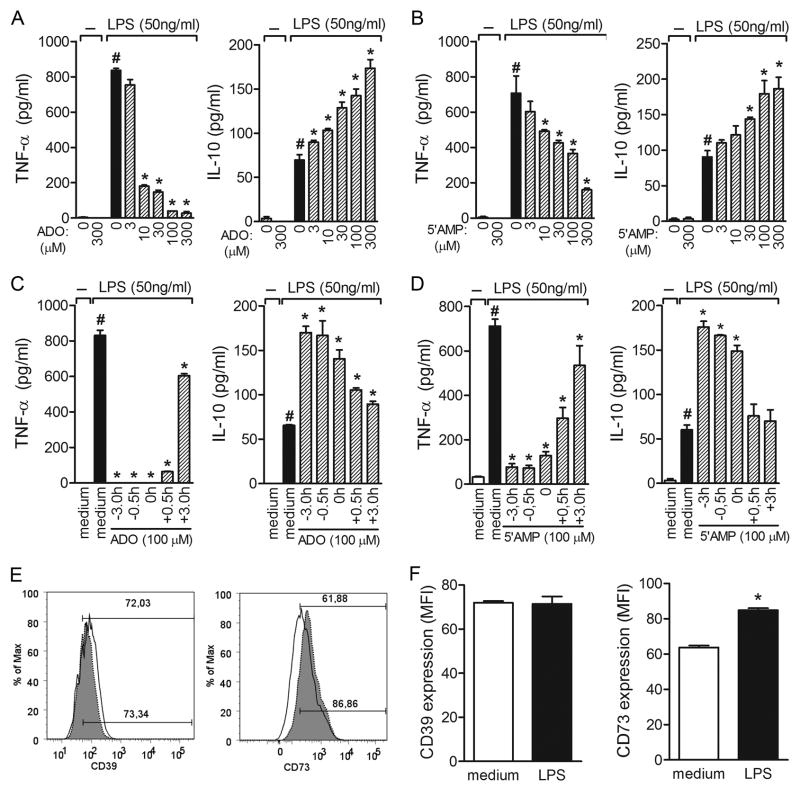
ADO alone had no significant effect on basal cytokines released by BMDC in the absence of stimulus (medium); however, BMDC pretreated overnight with ADO before stimulation with LPS resulted in inhibition of TNF-α production, whereas IL-10 production was stimulated (Fig. 5A). Both effects were observed in a concentration-dependent manner (Fig. 5A). Furthermore, the immunomodulatory effect of ADO was partially observed even when it was added to the culture 3 h after LPS administration into the culture (Fig. 5C).
Figure 5. Effects of ADO and 5′AMP on TNF-α and IL-10 production by LPS-stimulated DCs.
BMDCs were incubated without (open bars) or with (shaded bars) 50 ng/ml LPS for 24 h after overnight pre-incubation with increasing concentrations of ADO (A) or 5′AMP (B) (μM). Cytokine production was measured in the culture supernatants by ELISA. Time-course inhibition of cytokine production by 100μM of ADO (C) or 5′AMP (D) in LPS-stimulated BMDCs. The results are expressed as the mean±SEM obtained from one of three independent experiments made in triplicate (N =3 per group). *, P <0.05 compared with LPS stimuli. Surface molecules were labeled with FITC or PE conjugated with anti-CD11c, anti-CD39, or anti-CD73; representative mean fluorescence intensity (MFI) of BMDC staining from medium or LPS cultures is shown in each box (E). The filled histograms represent cells labeled with the specific mAb; the empty histograms represent the same cell suspension labeled with isotypic control mAb. In (F), the bars display the relative MFI obtained from one of four independent experiments made in quadruplicate (N =4). *, P<0.05 compared with medium.
The impact of ADO on the maturation of DCs was evaluated by flow cytometry. Incubation of BMDCs with ADO alone for 24 h slightly affected MHC-II, CD86, and CD40 basal expression. As expected, exposure to LPS enhanced the expression levels of these surface markers. ADO did not alter the effect of LPS on the surface expression of CD86, although MHC-II and CD40 upregulation was decreased (Supplementary Figure S1).
The anti-inflammatory effect of 5′AMP present in SGE gland could be a consequence of extracellular ADO generation. In fact, similarly to ADO, 5′AMP also inhibited TNF-α production and enhanced IL-10 release in a concentration-dependent manner (Fig. 5B). While the kinetics of TNF-α inhibition in the presence of 5′AMP followed the same pattern as that displayed by ADO, IL-10 enhancement was seen only when 5′AMP was added to DC culture at −3.0, −0.5, and 0 h of LPS administration, but not at later time-points (Fig. 5D), suggesting that some amount of time is required for 5′AMP to generate ADO.
Extracellular generation of ADO is mediated by two sequential enzymes: ecto-ATP disphohydrolase (CD39), which converts ATP and ADP to AMP and, subsequently, ecto5′nucleotidase (CD73), which converts 5′AMP to ADO (35). Recent study demonstrated that regulatory T cells (Treg) express both enzymes and that ATP metabolic disruption by CD39 and CD73 is the mechanism by which these cells mediate immunosuppression (36); however, whether both nucleotidases are expressed in DCs is still unknown. Thus, we analyzed CD39 and CD73 expression in LPS-matured DCs as a putative mechanism by which 5′AMP could generate ADO and mediate a modulatory effect on DCs. Cytometric analysis revealed that both enzymes are expressed in BMDCs. While CD39 expression seems to be constitutive and LPS activation did not alter its expression, CD73 can be upregulated by the maturation process. LPS stimulation enhanced CD73 expression by 34% compared with medium alone (Figs. 5E and 5F). Similarly, expression of CD39 transcripts has not changed upon LPS stimulus, while CD73 expression significantly increased under the same conditions. (Supplementary figure S2A and B). Together, these results clearly demonstrate that ADO, already present in saliva and/or generated by 5′AMP metabolism, most likely accounts for most, if not all, anti-inflammatory activity presented by P. papatasi SGE.
ADO enhances LPS-induced PGE2 production
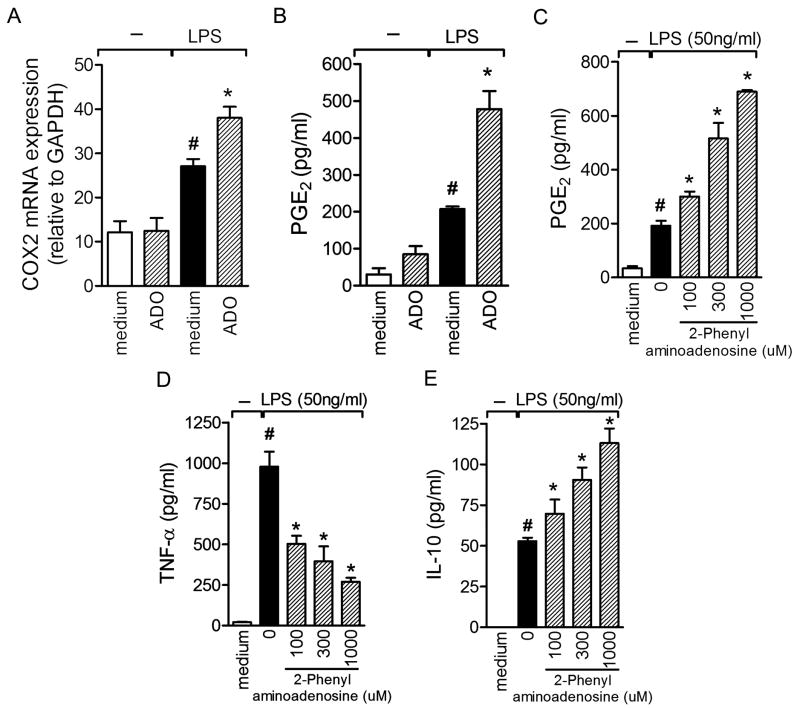
In a previous study, we demonstrated that the inhibitory effect of SGE on an antigen-immune inflammation model depends on sequential production of PGE2 and IL-10 by DCs, which in turn acts in an autocrine manner, reducing the antigen-presenting ability of these cells (19). Taking into account that ADO and 5′AMP are the molecules present on saliva responsible for such anti-inflammatory effects (Fig. 4) and that PGE2 was upregulated in the articular lesions of arthritic mice treated with SGE (Fig. 2D), we next evaluated the impact of ADO on prostanoid production. Because PGE2 is generated by the cyclooxygenase (COX) pathway, we first investigated the impact of incubation of LPS-stimulated DCs with ADO by measuring COX-2 mRNA levels. As expected, LPS induced significant expression of COX-2 mRNA in DCs compared with medium alone. Moreover, preincubation of these cells with ADO significantly enhanced expression of COX-2 transcripts after stimulation (Fig. 6A). In terms of the COX-2 product, PGE2 levels were induced by LPS stimulation, which was potentiated by ADO preincubation (Fig. 6B). ADO alone had no considerable effect on basal prostanoid release compared with medium.
Figure 6. ADO potentiates COX2 mRNA expression and PGE2 production induced by LPS stimulation.
COX2 mRNA quantification (A) and PGE2 production (B) were quantified in BMDCs pre-incubated with ADO or medium with or without LPS-stimulation for 24h. The indicated concentration of adenosinesynthetic analogous (2-phenylaminoadenosine) wasadded into BMDC culture 3h before LPS stimuli. PGE2 (C), TNF-α (D), and IL-10 (E) levels were detected 24 h later. PGE2 was measured by RIA and cytokines by ELISA assay. The results are expressed as the mean±SEM obtained from one of three independent experiments made in triplicate (N =3 per group). #, P<0.05 compared with medium; *, P<0.05 compared with the control (LPS).
To confirm the involvement of ADO in PGE2 production, cells were preincubated with 2-phenilaminoadenosine, a synthetic analog of ADO, for 3 h before LPS stimulation. Potentiation of PGE2 production could be observed by 2-phenylaminoadenosine in a concentration-dependent manner (Fig. 6C). Furthermore, an inhibitory effect on TNF-α production by DCs was also achieved by an ADO synthetic analog (Fig. 6D). Similarly to PGE2, 2-phenilaminoadenosine also potentiated LPS-induced IL-10 production in a concentration-dependent fashion (Fig. 6E). Together, these results indicate that ADO has an important impact on the capacity of DC to generate PGE2.
ADO present in SGE affected DC function during the antigen-presenting process and attenuated collagen-induced arthritis
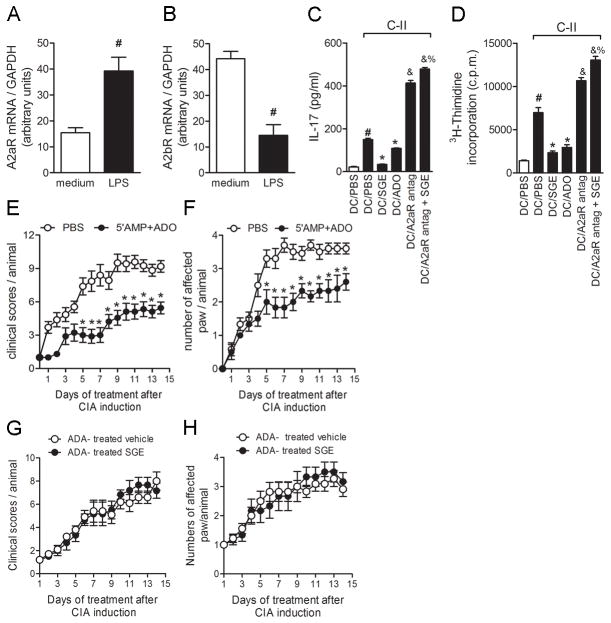
We investigated whether ADO inhibits DC function and determined which ADO receptors were expressed in BMDCs. Transcripts for A2A and A2B receptors (A2AR and A2BR) were detected in unstimulated cells, with A2BR being highly expressed in resting cells (Fig. 7B). After LPS stimulation, the transcript profile completely changed, with significant increase in A2AR (Fig. 7A) and decrease in A2BR expression (Fig. 7B). Given that the immunoregulatory effect of ADO is attributed to its engagement to A2AR (37) and was enhanced after inflammatory stimulation (Fig. 7A), the stable and selective A2AR antagonist (8,3,clorosterylcafeine) was tested on the SGE effect on BMDC-driven CD4+ T cell activation. The A2AR antagonist was added to DC culture 2 h before SGE addition, and DC function was assessed by antigen-induced lymphoproliferative response and IL-17 production. As expected, antigen-induced proliferation and IL-17 production were inhibited by BMDCs treated with SGE. This impaired ability of BMDCs treated with SGE to induce proliferation and IL-17 production by CD4+ T cells was completely prevented by A2AR blockage, suggesting that ADO mediates the immunosuppressive action of SGE on DCs through this receptor (Figs. 7C and 7D, respectively). Corroborating this hypothesis, standard commercial ADO also affected DC function, suppressing antigen proliferative response and IL-17 production (Figs. 7C and 7D). Interestingly, A2AR antagonist potentiated both proliferative response and IL-17 production, even in the absence of SGE, suggesting the participation of endogenous ADO in this process.
Figure 7. BlockingA2AR prevents SGE immunosuppressive effect on DC function and 5′AMP+ADO treatment-attenuated CIA.
BMDCs (106 cells/mL) were incubated ± with ADO (100 μM). Cells were harvested 24h after LPS stimulation for A2AR (A) and A2BR (B) mRNA expression quantification. Purified CD4+T cells (107 cells/ml) from arthritic mice were added into BMDC culture overnight, pretreated with PBS, SGE (8 glands/ml), or ADO (100μM). Specific A2AR-antagonist was added into some wells 2h before nucleoside or saliva treatment. IL-17 production (C) was measured by ELISAand lymphocyte proliferation by [3H]-thymidine incorporation (D) was determined 96h after C-II stimulation (5μg/ml). #, P<0.05 when compared with medium; *, P<0.05 compared with stimuli; &, P<0.05 compared with salivary nucleosides; %P<0.05 compared with A2AR antagonist. Collagen-immunized DBA/1 mice were injected i.v. daily with salivary equimolar concentration of 5′AMP+ADO (20 uM each ones) (●) or PBS (○) for 14d after start of disease. Mice were monitored for disease progression as indicated by clinical scores (E) and numbers of affected paws (F) *, P<0.05 compared with PBS-treated group. Results show the mean±SEM; N =10. In some groups, salivary gland extract or vehicle (PBS) were previously incubated with ADA (4.3 U) for 3 h. Afterwards, collagen-immunized and challenged DBA/1 mice were injected i.v. daily with ADA-treated vehicle (○)or ADA-treated P. papatasi SGE (1gland/animal)(●) for 14 d. Mice were monitored for disease progression as indicated by clinical scores (G)and number of affected paws (H). Results show the mean±SEM, N =5.
We examined the effect of nucleosides on CIA. In this set of experiments, arthritic mice were treated daily with comparable amounts of 5′AMP and ADO present in 1 salivary gland pair(20 uM each ones). Nucleoside treatment was as effective as SGE in ameliorating established CIA (Figs. 7E and 7F). In order to confirm that ADO and 5′AMP are the compounds of P. papatasi SGE with therapeutic effect on CIA, we have depleted both nucleosides by treating SGE with ADA for 3 h. Strikingly, the therapeutic effect of SGE on CIA was completely abolished when SGE was deaminated by ADA (Figs 7G and 7H). Collagen-induced arthritis mice that received ADA-treated SGE display no difference in clinical scores and numbers of affected paw compared to control group (ADA-treated vehicle). Taken together, these results show that ADO and AMP are, in fact, the active constituents present into P. papatasii saliva responsible for anti-inflammatory activity and amelioration of CIA symptoms.
Next, we investigate the effectiveness of nucleosides in two new experiments with different protocols of treatment of arthritic mice. To test whether salivary nucleosides have a therapeutic effect when the disease is already established, arthritic mice were treated when disease score reached 6 (representing a higher disease progression). In this condition, nucleosides treatment was not effective in reducing arthritis severity, since the clinical scores (Supplementary Figure S3A) and numbers of affected paw (Supplementary Figure S3B) were similar to those of vehicle-treaded mice. In the second protocol, mice were treated only during the first week after disease onset. The beneficial effect of the therapy with the nucleosides lasted only for a few days after the treatment was stopped. Once the treatment ended, both, clinical scores (Supplementary Figure S3C) and numbers of affected paw (Supplementary Figure S3D) reached the same level as those of vehicle-treated mice. Thus, salivary nucleosides present a beneficial effect on CIA only when administered on disease onset and maintained during all period of disease.
IL-10 released during treatment contributes to anti-inflammatory activity of nucleosides on CIA
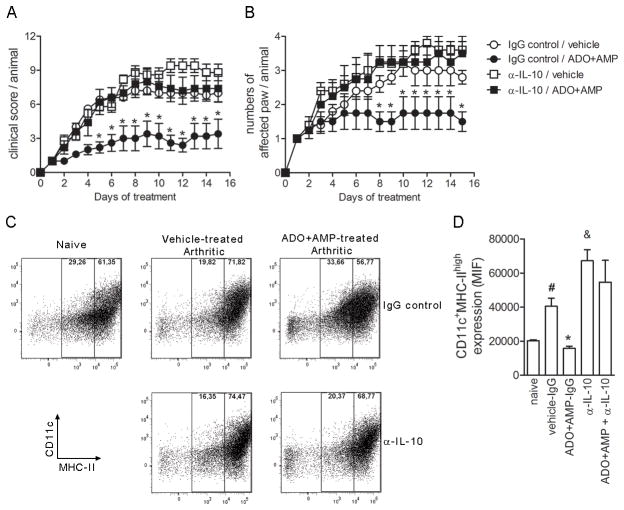
We demonstrated that both PGE2 (Fig. 2E) and IL-10 (Fig. 2F) were increased in the paws of SGE-treated arthritic mice. To assess whether these mediators are responsible for SGE anti-inflammatory activity in vivo, firstly we tested the effect of IL-10 depletion using specific antibody on treated- CIA mice. Treatment of mice with anti-IL-10 antibody initiated at the onset of disease abrogated the animals from protective effect of nucleosides on CIA. The anti-IL-10 antibody concomitantly administered with ADO+AMP (α-IL-10/ADO+AMP) induced in arthritic mice similar evolution of clinical scores that those observed in control groups (vehicle/IgG control and vehicle/αIL-10) (Fig. 8A). In addition, the numbers of affected paw also were comparables to control groups (Fig. 8B). We have attempted to block endogenous PGE2 production using a COX-2 selective inhibitor, Rofecoxibe®. However, mice succumbed within one week of Rofecoxibe treatment, indicating a side effect of the COX2-inhibitor (38).
Figure 8. Suppressive effect of salivary nucleosides in vivo is dependent of IL-10.
At the onset of arthritis symptoms, naïve or collagen-immunized and challenged DBA/1 mice injected i.v. daily vehicle (PBS) or ADO+AMP were concomitantly treated (250ug/mice; i.p. route) with normal rat IgG (IgG control) or rat anti-IL-10 (αIL-10) antibody three times per week. Mice were monitored for disease progression as indicated by clinical scores (A) and number of affected paws (B). Results show the mean±SEM, N =5; *, P<0.05 compared with vehicle/IgG control. On day 15 of treatment, mice were euthanized, the draining lymph nodes were harvested, and DC surface molecules were labeled with anti-CD11c or anti-MHC class II monoclonal antibodies conjugated with FITC and PE. Representative dot plot of DC staining from naïve, vehicle or ADO+5′AMP treated mice injected with αIL-10 or rat IgG control is shown in each box (C). In (D), the bars display the relative MFI and results show the mean±SEM, N =5;#, P<0.05 compared with naïve mice, *, P<0.05 compared with vehicle/IgG control.&, P<0.05 compared with vehicle/IgG control.
We further evaluated DC maturation’ stage in vivo after treatment. Nucleosides (ADO+AMP) or vehicle (PBS) were administered onset of CIA development daily for 14 days. Draining lymph nodes were harvested and DC maturation was analyzed by flow cytometry through of CD11c and MHC-II markers on surface membrane. As expected, naïve mice exhibited low frequency of CD11cMHC-IIhigh and arthritic mice treated with vehicle enhanced the expression levels of these surface markers, suggesting that DC was matured in vivo during disease progression (Fig. 8C and D). Interestingly, similarly to in vitro, intravenous injection of ADO+AMP exhibited a significant down-regulation of dendritic cells maturation with reduction of CD11cMHC-IIhigh that reached the basal levels, similar to naïve group. These findings indicate that DCs exhibit selective defects induced by saliva that was associated with DC maturation. Such inhibitory effect could be, at least in part, dependent of IL-10 release induced by saliva or fractions. Accordingly, the intraperitoneal administration of antibody against IL-10 (α-IL-10) during nucleosides treatment reversed the immunosuppressive effect of salivary fractions, potentiating the expression of CD11cMHC-IIhigh surface markers on DC from ADO+AMP-treated arthritic mice. Taken together, our data suggest that IL-10, and perhaps PGE2, released in the site of inflammation strongly contributes to anti-inflammatory activity of saliva or nucleosides on CIA.
DISCUSSION
In the present study, we report the therapeutic effect of P. papatasi SGE in controlling the pathogenesis of collagen induced arthritis. We purified and identified ADO and 5′-AMP as the active pharmacologic components in SGE responsible for such immunomodulatory activity. Indeed, ADO and 5′AMP blocked antigen presentation by DCs, interfering with Th17 cell activation and consequently suppressing the inflammatory immune response. These data indicate that P. papatasi salivary compounds could be a selective therapy focusing on DC function during the effector phase of the immune response as well as targets to treat CIA.
SGE treatment at the onset of disease attenuated the severity of arthritis and protected joint tissue from degradation. In addition, SGE was associated with a marked decrease of CD4+ Th17 lymphocyte activation. Importantly, IL-17 produced by CD4+ T lymphocytes is a key cytokine implicated in human autoimmune disease such as RA (31). In arthritis models, IL-17 promotes activation of synovial fibroblast and both leukocyte emigration and activation, resulting in the production of several mediators involved in the inflammatory response and tissue lesions, including cytokines (TNF-α, IL-1β), chemokines (MIP-2/CXCL2, KC/CXCL1, IL-8/CXCL8), adhesion molecules (ICAM-1), receptor activator of NF-κB ligand and matrix metalloproteinases (39, 40). The therapeutic efficacy of SGE treatment in collagen-induced rheumatoid arthritis seems to be due to blocking DC antigen presentation and consequently the CD4+ Th17 cell response suppression. Consistently, SGE selectively inhibits specific (i.e., collagen) but not polyclonal stimulation, which directly stimulates lymphocytes, confirming the interference of P. papatasi saliva in DC function. Interestingly, salivary nucleosides present a beneficial effect on CIA only when administered on disease onset and maintained during all period of disease. This finding further supports the notion that in the absence of saliva treatment, DC is constantly activated and contributed to severity of CIA. In agreement with our results, saliva from other blood-feeding arthropods such as Ixodes scapularis ticks (41), the Lyme disease vector, and Ixodes ricinus, (42) also has been demonstrated to block the DC-dependent antigen-presenting process and attenuated experimental autoimmune encephalitis (EAE) (43).
During their evolutionary process, blood-sucking arthropods developed efficient mechanisms to overcome the host immune response and thus successfully complete the blood meal (2). We identified ADO and 5′AMP as the active molecules present in the salivary fraction of P. papatasi that inhibit DC activation. Markedly, the immunomodulatory effects were similarly observed with whole saliva and commercial ADO plus 5′AMP. Moreover, the deamination of ADO with ADA, an enzyme that catabolizes ADO (34), maintains the maturation of BMDCs induced by LPS. Accordingly, ADO has a broad range of effects on inflammatory leukocytes including DCs, downregulating the production of pro-inflammatory mediators and expression of costimulatory markers, which diminish the capacity of DCs to initiate and amplify the inflammatory immune response (44).
ADO effects are mediated by four surface receptors, named A1R, A2AR, A2BR, and A3R, which are members of the G protein-coupled receptor family (45). Whereas A1R and A3R are coupled to Gi (inhibitory) proteins and mediate the inhibition of adenylyl cyclase, A2AR and A2BR interact with Gs (stimulatory proteins) and activate adenylyl cyclase generating the second messenger cyclic ADO monophosphate (cAMP) that downregulates host cell activation (46). Although all four ADO receptors are present on human and mouse peripheral blood monocytes and mature DCs, genetic ablation or pharmacologic blocking of A2AR or A2BR leads to over-exuberant immune responses, indicating that A2A/BR are involved in the anti-inflammatory and immunoregulatory role of ADO (45, 47). Here we show that ADO has little or no measurable effect on cytokine production on resting (nonstimulated) DCs; however, in the context of LPS stimulation, ADO significantly modulates these markers, suggesting that ADO receptors are been modulated along the stages of cellular activation. In fact, LPS-induced DC maturation results in downregulation of A2BR expression, while A2AR is highly expressed. Therefore, it seems that A2AR is the receptor responsible for ADO effects on DCs. Consistent with our results, Panther et al. demonstrated that ADO by itself has only a very limited capacity to modulate DCs whereas this nucleoside provides prominent inhibition of LPS-stimulated DCs through activation of the A2AR (48).
Further exploring the biologic action of salivary nucleosides on DC function, similarly to SGE, ADO showed inhibitory effects on collagen-induced CD4+ T cell response. Interestingly, in the presence of 8,3,cloroesterylcafeine (a selective A2AR antagonist), the inhibitory effect of SGE on CD4+ Th17 activation was prevented; however, such a profile was exacerbated even in the absence of SGE, indicating the involvement of endogenous ADO downregulating the inflammatory process. The exacerbation of inflammation through blocking the A2AR has been demonstrated in a variety of inflammatory models. Treatment of mice with the selective A2AR antagonist ZM241385 potentiated liver injury and inflammation in response to concanavalin A, Pseudomonas aeruginosa, and carbon tetrachloride stimuli (45). Moreover, ZM241385 prevented both anti-inflammatory effects and survival induced by low doses of ketamine, which promotes accumulation of ADO in mice when sepsis is induced by LPS or Escherichia coli (49).
The immunomodulatory effect of 5′AMP present on P. papatasi SGE is due to extracellular ADO generation. Extracellular ADO generation on inflammation foci is mediated via CD39, ecto-ATP diphosphohydrolase, that rapidly converts circulating ATP and ADP to 5′AMP; subsequently, 5′AMP is converted to extracellular ADO by ecto-5′-nucleotidase (CD73) (36). Recent studies show that Tregs express high levels of CD39 and CD73 on their surface, and ADO generation is a mechanism by which Tregs exert their suppressive effects (50). Monocytes also express CD39 and CD73, and these enzymes are active under inflammatory conditions (51, 52). Nevertheless, the expression of both ectonucleotidases on the DC surface has not been demonstrated to date. Our data demonstrate for the first time that both ectonucleotidases are expressed in DCs. While CD39 appears to be constitutive, the expression of CD73 is increased by pro-inflammatory stimuli. Thus, the concomitant expression of both enzymes on the surface of DCs helps to generate ADO in the extracellular compartment by cleaving 5′AMP present in the saliva of P. papatasi, contributing to prevention of the tissue damage observed in CIA. Accordingly, 5′AMP represents a slow-release formulation of ADO, in addition to the pre-formed ADO present in P. papatasi saliva.
Although the increased levels of cAMP seem to be due, at least in part, to interaction and activation of A2AR, triggering a cascade of inhibitory factors of the inflammatory response, this interaction could also lead to an increase in expression of COX-2 (44, 53). Once activated, this enzyme converts arachidonic acid to PGG2, and subsequently to PGH2, which is rapidly metabolized by specific isomerases to the potentially active prostanoids PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (TXA2). Our data show that either ADO or A2R agonist were able to induce enhancement of PGE2 production by LPS-stimulated BMDCs. Prior evidence suggests that incubation of neutrophils with A2AR agonist increases expression of COX-2, leading to production of PGE2 via cAMP stimulation (53). Furthermore, incubation of polymorphonuclear cells with ADA drastically reduces prostanoid production, with downregulation of cAMP and COX2, establishing a direct relationship between ADO and PGE2 (53).
A great body of evidence shows that PGE2 and IL-10 are potent anti-inflammatory mediators, limiting tissue damage on different experimental autoimmune diseases including RA (54, 55). Both mediators were increased in the paws of SGE-treated arthritic mice, and treatment of mice with anti-IL-10 monoclonal antibody blocked the protective effect of nucleosides. Previously we reported that SGE inhibits immune inflammation by sequential production of PGE2/IL-10 acting autocrinally on DC function (19). Thus, it is reasonable that both PGE2 and IL-10 contribute to the therapeutic success of SGE on CIA.
In addition to PGE2 and IL-10 effect, we have showed that DCs from lymph nodes of treated-arthritic mice also present a down regulation of MHC class II molecules on their surface in vivo. Importantly, we also demonstrated that such effect was also dependent on IL-10 production. Although we have not evaluated whether the inhibitory effect of salivary nucleosides on DC affects T cell response in vivo, we have strong evidences that its function is impaired. We have previously published that the in vivo treatment of OVA-immunized mice with SGE reduced the antigen-induced proliferation of splenic T cells (19). Interestingly, saliva affected the proliferation of OVA-specific CD4+ T cells through DC inhibition whereas the incubation of OVA-CD4+ T cells with SGE have not altered their proliferative response (19). The reduction in arthritis progression mediated by impairment of DC function has been demonstrated by others. For instance, treatment of arthritic mice with type II-collagen-pulsed tolerogenic DCs, which presents a semi-mature phenotype (reduced the MHC-II, CD40, CD80 and CD86 coestimulatory molecules on DC surface expression and reduced levels of inflammatory cytokines), decreases the proportion of pathogenic Th17 cells, reducing the severity and progression of arthritis in these animals (56). Moreover, the hyporesponsiveness of CD4+T or CD8+T lymphocytes is due to immunossupressive mediators released by DC (i.e. IL-10, TGF-β and PGE2) (57). Furthermore, DCs down-modulated by innate immunity improve collagen-induced arthritis and induced regulatory T cells in vivo (58). Thus, it is plausible to infer that the intravenous administration of whole extract or nucleosides (adenosine and adenosine monophosphate) present into P. papatasi saliva ameliorates collagen-induced arthritis by inhibiting the effector’s phase of the immune-specific response through inhibition of the DC activation.
It was not the aim of the present study to determine the mechanism that SGE interfere in antigen presenting process. Nonetheless, we cannot rule out the possibility that other salivary components inhibit antigen degradation and/or the displacement of CLIP-peptide associated to MHC class II biding groove, preventing loading of antigenic peptides present on the membrane surface and subsequent affecting presentation to CD4+ T cells. In fact, it has been demonstrated that Sialostatin L, a cysteine protease inhibitor isolated from Ixodes scapularis tick saliva, inhibits LPS-induced maturation of DCs as well as it binds cathepsin S inside lisosomal compartment, thus blocking antigen-specific T cells proliferation (43).
An interesting finding of our study was that the amount of SGE that effectively reduced RA had low amounts of both salivary protein and nucleosides (~2 μg of protein and 20 μM of 5′AMP and ADO). Stressing the relevance of our finding, we demonstrated that treatment of arthritic mice with similar amounts of each nucleoside reduced CIA symptoms. Interestingly, the therapeutic effect of SGE on CIA was completely abolished when SGE was deaminated by ADA. Furthermore, several studies support the hypothesis that the efficacy of methotrexate on experimental and human RA is due to its ability to increase the levels of ADO (59). Indeed, methotrexate induces accumulation of AICAR, a competitive inhibitor of ADA, thus allowing increased levels of ADO (60) and inhibition of chondrocytes (61) and synovial cells (62).
In conclusion, the results presented here indicate that ADO and 5′AMP are present in P. papatasi SGE in pharmacological concentrations and mediate the anti-inflammatory activity on RA. These constituents act through A2AR in the effector phase of the inflammatory process, inhibiting DC ability to present antigen and thus leading to suppression of CD4+ Th17-induced inflammatory immune response. Our data support the idea that A2AR activation could be a novel therapeutic strategy for RA and other destructive chronic disorders.
Supplementary Material
Acknowledgments
We are thankful to o Giuliana Bertozi for help with ELISAs and to NIAID intramural editor Brenda Rae Marshall for assistance.
GRANT SUPPORT
This work was supported by FAPESP, CAPES, CNPq, and FAEPA. David D. Brand was supported by a grant from the Department of Veterans Affairs. J.G. Valenzuela and J.M.C. Ribeiro were supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Because J.G. Valenzuela and J.M.C. Ribeiro are government employees and this is a government work, the work is inthe public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
NONSTANDARD ABBREVIATIONS USED
- ADA
adenosine deaminase
- ADO
adenosine
- BMDC
bone marrow-derived cells
- CIA
collagen-induced arthritis
- PGE2
prostaglandin E2
- SGE
salivary gland extract
Footnotes
DISCLOSURES: The authors have no conflicting financial interests
References
- 1.Ribeiro JM. Role of saliva in blood-feeding by arthropods. Annual review of entomology. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro JM, I, Francischetti M. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual review of entomology. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JM, Weis JJ, Telford SR., 3rd Saliva of the tick Ixodes dammini inhibits neutrophil function. Experimental parasitology. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- 4.Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. The American journal of tropical medicine and hygiene. 1986;35:926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly KB, Lima HC, Titus RG. Histologic characterization of experimental cutaneous leishmaniasis in mice infected with Leishmania braziliensis in the presence or absence of sand fly vector salivary gland lysate. The Journal of parasitology. 1998;84:97–103. [PubMed] [Google Scholar]
- 6.Titus RG, Kelso A, Louis JA. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clinical and experimental immunology. 1984;55:157–165. [PMC free article] [PubMed] [Google Scholar]
- 7.Titus RG, Theodos CM, Shankar AH, Hall LR. Interactions between Leishmania major and macrophages. Immunology series. 1994;60:437–459. [PubMed] [Google Scholar]
- 8.Titus RG. Salivary gland lysate from the sand fly Lutzomyia longipalpis suppresses the immune response of mice to sheep red blood cells in vivo and concanavalin A in vitro. Experimental parasitology. 1998;89:133–136. doi: 10.1006/expr.1998.4272. [DOI] [PubMed] [Google Scholar]
- 9.Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite immunology. 2006;28:131–141. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 10.Urioste S, Hall LR, Telford SR, 3rd, Titus RG. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. The Journal of experimental medicine. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. The Journal of experimental medicine. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waitumbi J, Warburg A. Phlebotomus papatasi saliva inhibits protein phosphatase activity and nitric oxide production by murine macrophages. Infection and immunity. 1998;66:1534–1537. doi: 10.1128/iai.66.4.1534-1537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima HC, Titus RG. Effects of sand fly vector saliva on development of cutaneous lesions and the immune response to Leishmania braziliensis in BALB/c mice. Infection and immunity. 1996;64:5442–5445. doi: 10.1128/iai.64.12.5442-5445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J Immunol. 1998;161:5571–5577. [PubMed] [Google Scholar]
- 15.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. The Journal of experimental medicine. 1998;188:1941–1953. doi: 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunological reviews. 2004;201:318–338. doi: 10.1111/j.0105-2896.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 17.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunological reviews. 2004;201:225–238. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 18.De Freitas LA, Mbow LM, Estay M, Bleyenberg JA, Titus RG. Indomethacin treatment slows disease progression and enhances a Th1 response in susceptible BALB/c mice infected with Leishmania major. Parasite immunology. 1999;21:273–277. doi: 10.1046/j.1365-3024.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- 19.Carregaro V, Valenzuela JG, Cunha TM, Verri WA, Jr, Grespan R, Matsumura G, Ribeiro JM, Elnaiem DE, Silva JS, Cunha FQ. Phlebotomine salivas inhibit immune inflammation-induced neutrophil migration via an autocrine DC-derived PGE2/IL-10 sequential pathway. Journal of leukocyte biology. 2008;84:104–114. doi: 10.1189/jlb.1107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavousanaki M, Makrigiannakis A, Boumpas D, Verginis P. Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy. Arthritis and rheumatism. 62:53–63. doi: 10.1002/art.25037. [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:566–571. doi: 10.1038/nrrheum.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jongbloed SL, Lebre MC, Fraser AR, Gracie JA, Sturrock RD, Tak PP, McInnes IB. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis research & therapy. 2006;8:R15. doi: 10.1186/ar1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebre MC, Jongbloed SL, Tas SW, Smeets TJ, McInnes IB, Tak PP. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. The American journal of pathology. 2008;172:940–950. doi: 10.2353/ajpath.2008.070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chabaud M, Durand JM, Buchs N, Fossiez F, Page G, Frappart L, Miossec P. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis and rheumatism. 1999;42:963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 27.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. The Journal of experimental medicine. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science (New York, N Y) 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 29.Palmer G, Chobaz V, Talabot-Ayer D, Taylor S, So A, Gabay C, Busso N. Assessment of the efficacy of different statins in murine collagen-induced arthritis. Arthritis and rheumatism. 2004;50:4051–4059. doi: 10.1002/art.20673. [DOI] [PubMed] [Google Scholar]
- 30.McKinney MM, Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. Journal of immunological methods. 1987;96:271–278. doi: 10.1016/0022-1759(87)90324-3. [DOI] [PubMed] [Google Scholar]
- 31.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro JM, Katz O, Pannell LK, Waitumbi J, Warburg A. Salivary glands of the sand fly Phlebotomus papatasi contain pharmacologically active amounts of adenosine and 5′-AMP. J Exp Biol. 1999;202:1551–1559. doi: 10.1242/jeb.202.11.1551. [DOI] [PubMed] [Google Scholar]
- 33.Katz O, Waitumbi JN, Zer R, Warburg A. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am J Trop Med Hyg. 2000;62:145–150. doi: 10.4269/ajtmh.2000.62.145. [DOI] [PubMed] [Google Scholar]
- 34.Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007;179:1884–1892. doi: 10.4049/jimmunol.179.3.1884. [DOI] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. The Journal of experimental medicine. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 37.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. The Journal of biological chemistry. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 39.Koenders MI, Joosten LA, van den Berg WB. Potential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritis. Annals of the rheumatic diseases. 2006;65(Suppl 3):iii29–33. doi: 10.1136/ard.2006.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM, Francischetti IM. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- 41.Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol. 2008;180:6186–6192. doi: 10.4049/jimmunol.180.9.6186. [DOI] [PubMed] [Google Scholar]
- 42.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 44.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 45.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 46.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 47.Mazar J, Rogachev B, Shaked G, Ziv NY, Czeiger D, Chaimovitz C, Zlotnik M, Mukmenev I, Byk G, Douvdevani A. Involvement of adenosine in the antiinflammatory action of ketamine. Anesthesiology. 2005;102:1174–1181. doi: 10.1097/00000542-200506000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. The Journal of experimental medicine. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. The Journal of experimental medicine. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of endothelial CD73 by adenosine: paracrine pathway for enhanced endothelial barrier function. J Immunol. 2000;165:5262–5268. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- 51.Pouliot M, Fiset ME, Masse M, Naccache PH, Borgeat P. Adenosine up-regulates cyclooxygenase-2 in human granulocytes: impact on the balance of eicosanoid generation. J Immunol. 2002;169:5279–5286. doi: 10.4049/jimmunol.169.9.5279. [DOI] [PubMed] [Google Scholar]
- 52.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. Journal of leukocyte biology. 2003;73:756–763. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 53.Harizi H, Gualde N. Eicosanoids: an emerging role in dendritic cell biology. Arch Immunol Ther Exp (Warsz) 2004;52:1–5. [PubMed] [Google Scholar]
- 54.Cronstein BN, Naime D, Ostad E. The antiinflammatory effects of methotrexate are mediated by adenosine. Advances in experimental medicine and biology. 1994;370:411–416. doi: 10.1007/978-1-4615-2584-4_89. [DOI] [PubMed] [Google Scholar]
- 55.Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, Cronstein BN. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis and rheumatism. 2003;48:240–247. doi: 10.1002/art.10712. [DOI] [PubMed] [Google Scholar]
- 56.Koolpe M, Pearson D, Benton HP. Expression of both P1 and P2 purine receptor genes by human articular chondrocytes and profile of ligand-mediated prostaglandin E2 release. Arthritis and rheumatism. 1999;42:258–267. doi: 10.1002/1529-0131(199902)42:2<258::AID-ANR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 57.Ralph JA, McEvoy AN, Kane D, Bresnihan B, FitzGerald O, Murphy EP. Modulation of orphan nuclear receptor NURR1 expression by methotrexate in human inflammatory joint disease involves adenosine A2A receptor-mediated responses. J Immunol. 2005;175:555–565. doi: 10.4049/jimmunol.175.1.555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.