Abstract
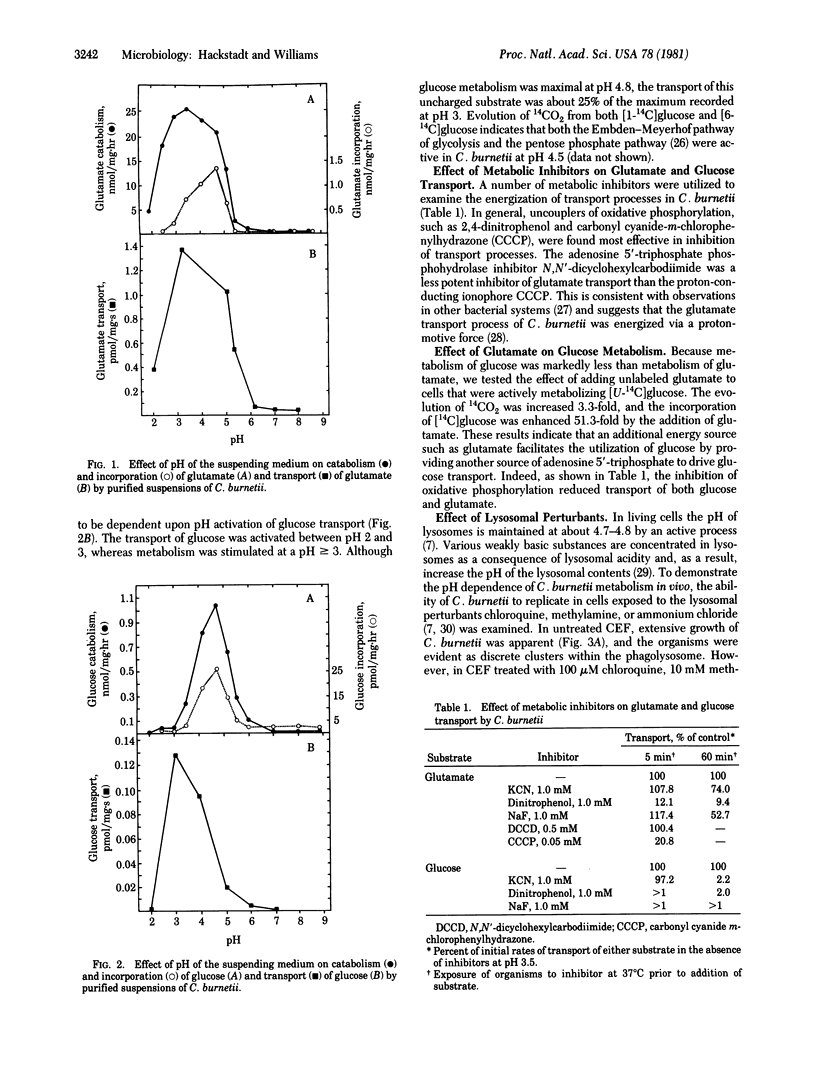
Coxiella burnetti, the etiologic agent of Q fever, is an oligate intracellular parasite of eukaryotes. Unlike the majority of successful bacterial parasites, which escape the bactericidal environment of the phagolysosome by various means, C. burnetii multiplies only in the phagolysosome. In view of the relatively harsh environment inhabited by C. burnetii, we have examined (i) the in vitro metabolism of glucose and glutamate by whole cells of C. burnetii under conditions designed to approximate the pH within the phagolysosome and (ii) the effect of manipulation of the phagolysosomal pH by lysosomotropic amines on the replication of C. burnetii in chicken embryo fibroblasts. The transport, catabolism, and incorporation of both glucose and glutamate were found to be highly stimulated by acidic conditions, whereas at pH 7.0 metabolism of these substrates was minimal. The transport processes were shown to be energy dependent and highly sensitive to inhibition by uncouplers of oxidative phosphorylation. Increasing the phagolysosomal pH of infected chicken embryo fibroblasts by use of the lysosomotropic agents chloroquine, methylamine, or ammonium chloride inhibited the multiplication of C. burnetii, thus demonstrating the in vivo requirement for the acidic conditions of the phagolysosome. This apparent dependence upon phagosome--lysosome fusion to generate pH conditions favorable to C. burnetii replication suggests a unique biochemical mechanism of parasite activation. A pathogenic mechanism based on regulation of microbial metabolism by H+-dependent stimulation of cell function is proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. R., Kordová N., Paretsky D. Electron microscopic studies of the rickettsia Coxiella burneti: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can J Microbiol. 1971 Feb;17(2):143–150. doi: 10.1139/m71-025. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Welsh R. M., Paretsky D. Some ultrastructural effects of persistent infections by the rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978 Aug;21(2):556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSIGLI R. A., PARETSKY D. Oxidation of glucose 6-phosphate and isocitrate by Coxiella burnetii. J Bacteriol. 1962 Jan;83:206–207. doi: 10.1128/jb.83.1.206-207.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978 Feb 1;147(2):515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Coolbaugh J. C., Progar J. J., Weiss E. Enzymatic activities of cell-free extracts of Rickettsia typhi. Infect Immun. 1976 Jul;14(1):298–305. doi: 10.1128/iai.14.1.298-305.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Brenchley G. V., Hales C. N. The permeability of rat liver lysosomes to sugars. Evidence for carrier-mediated facilitated diffusion. Biochem J. 1979 Feb 15;178(2):361–366. doi: 10.1042/bj1780361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brunschede G. Y., Brown M. S. Inhibition of proteolytic degradation of low density lipoprotein in human fibroblasts by chloroquine, concanavalin A, and Triton WR 1339. J Biol Chem. 1975 Oct 10;250(19):7854–7862. [PubMed] [Google Scholar]
- Gordon A. H., Hart P. D., Young M. R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980 Jul 3;286(5768):79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Hanks J. H. Host-dependent microbes. Bacteriol Rev. 1966 Mar;30(1):114–135. doi: 10.1128/br.30.1.114-135.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Manipulations of the phagosome-lysosome fusion response in cultured macrophages. Enhancement of fusion by chloroquine and other amines. Exp Cell Res. 1978 Jul;114(2):486–490. doi: 10.1016/0014-4827(78)90516-5. [DOI] [PubMed] [Google Scholar]
- IGNATOVICH V. F. [Certain die-out rules of Rickettsia burneti in liquid media]. Zh Mikrobiol Epidemiol Immunobiol. 1959 Sep;30:111–116. [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C. Macrophages and intracellular parasitism. J Reticuloendothel Soc. 1974 May;15(5):439–450. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- Kress Y., Tanowitz H., Bloom B., Wittner M. Trypanosoma cruzi: infection of normal and activated mouse macrophages. Exp Parasitol. 1977 Apr;41(2):385–396. doi: 10.1016/0014-4894(77)90110-2. [DOI] [PubMed] [Google Scholar]
- LITWIN J. The growth cycle of the psittacosis group of micro-organisms. J Infect Dis. 1959 Sep-Oct;105:129–160. doi: 10.1093/infdis/105.2.129. [DOI] [PubMed] [Google Scholar]
- Mallavia L. P., Paretsky D. Physiology of rickettsiae. VII. Amino acid incorporation by Coxiella burnetii and by infected hosts. J Bacteriol. 1967 May;93(5):1479–1483. doi: 10.1128/jb.93.5.1479-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia L. P., Weiss E. Catabolic activities of Neisseria meningitidis: utilization of glutamate. J Bacteriol. 1970 Jan;101(1):127–132. doi: 10.1128/jb.101.1.127-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. L., Mallavia L. Biochemistry of Coxiella burnetii: 6-phosphogluconic acid dehydrogenase. J Bacteriol. 1970 Apr;102(1):1–5. doi: 10.1128/jb.102.1.1-5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. L., Mallavia L. Biochemistry of Coxiella burnetii: Embden-Meyerhof pathway. J Bacteriol. 1971 Sep;107(3):864–869. doi: 10.1128/jb.107.3.864-869.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., PEACOCK M. G. METABOLIC ACTIVITY IN COXIELLA BURNETII. J Bacteriol. 1964 Nov;88:1205–1210. doi: 10.1128/jb.88.5.1205-1210.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., WEISS E. TRACHOMA AGENT: GLUCOSE UTILIZATION BY PURIFIED SUSPENSIONS. Science. 1963 Nov 22;142(3595):1077–1078. doi: 10.1126/science.142.3595.1077. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormsbee R. A. Rickettsiae (as organisms). Annu Rev Microbiol. 1969;23:275–292. doi: 10.1146/annurev.mi.23.100169.001423. [DOI] [PubMed] [Google Scholar]
- PARETSKY D., CONSIGLI R. A., DOWNS C. M. Studies on the physiology of rickettsiae. III. Glucose phosphorylation and hexokinase activity in Coxiella burnetii. J Bacteriol. 1962 Mar;83:538–543. doi: 10.1128/jb.83.3.538-543.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARETSKY D., DOWNS C. M., CONSIGLI R. A., JOYCE B. K. Studies on the physiology of rickettsiae. I. Some enzyme systems of Coxiella burnetii. J Infect Dis. 1958 Jul-Aug;103(1):6–11. doi: 10.1093/infdis/103.1.6. [DOI] [PubMed] [Google Scholar]
- Paretsky D. Biochemistry of rickettsiae and their infected hosts, with special reference to Coxiella burneti. Zentralbl Bakteriol Orig. 1968 Apr;206(3):283–291. [PubMed] [Google Scholar]
- Peacock M., Burgdorfer W., Ormsbee R. A. Rapid fluorescent-antibody conjugation procedure. Infect Immun. 1971 Feb;3(2):355–357. doi: 10.1128/iai.3.2.355-357.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C., Hall M. J. Influence of metal ions on the formation of mycobactin and salicylic acid in Mycobacterium smegmatis grown in static culture. J Bacteriol. 1971 Oct;108(1):314–319. doi: 10.1128/jb.108.1.314-319.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijngoud D. J., Oud P. S., Kás J., Tager J. M. Relationship between medium pH and that of the lysosomal matrix as studied by two independent methods. Biochim Biophys Acta. 1976 Oct 5;448(2):290–302. doi: 10.1016/0005-2736(76)90243-1. [DOI] [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. Chloroquine accumulation in isolated rat liver lysosomes. FEBS Lett. 1976 Apr 15;64(1):231–235. doi: 10.1016/0014-5793(76)80290-6. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J Exp Med. 1979 Sep 19;150(3):703–708. doi: 10.1084/jem.150.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Voytek A., Bruskin A. M. Energization of glucose transport by Pseudomonas fluorescens. J Bacteriol. 1980 Jun;142(3):755–762. doi: 10.1128/jb.142.3.755-762.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Interactions between Rickettsia prowazekii and rabbit polymorphonuclear leukocytes: rickettsiacidal and leukotoxic activities. Infect Immun. 1981 Jan;31(1):289–296. doi: 10.1128/iai.31.1.289-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Weiss E. Energy metabolism of Rickettsia typhi: pools of adenine nucleotides and energy charge in the presence and absence of glutamate. J Bacteriol. 1978 Jun;134(3):884–892. doi: 10.1128/jb.134.3.884-892.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]



