Abstract
Background
This study compared diurnal variation in mood and regional cerebral metabolic rate of glucose (rCMRglc) in depressed and healthy subjects.
Methods
Depressed and healthy subjects were investigated using [18F]-fluoro-deoxyglucose positron emission tomography scans during morning and evening wakefulness. All subjects completed subjective mood ratings at both times of day. Statistical parametric mapping was used to compare rCMRglc between the two groups across time of day.
Results
Depressed patients showed evening mood improvements compared with healthy subjects. Compared with healthy subjects, depressed patients showed smaller increases in rCMRglc during evening relative to morning wakefulness in lingual and fusiform cortices, midbrain reticular formation, and locus coeruleus and greater increases in rCMRglc in parietal and temporal cortices. Depressed patients had hypermetabolism in limbic-paralimbic regions and hypometabolism in frontal and parietal cortex at both times of day compared with healthy subjects.
Conclusions
Variation in rCMRglc differs across times of day in depressed and healthy subjects. In depressed patients, evening mood improvements were associated with increased metabolic activity in ventral limbic-paralimbic, parietal, temporal, and frontal regions and in the cerebellum. This increased metabolic pattern during evening wakefulness may reflect partial normalization of primary and compensatory neural systems involved in affect production and regulation.
Keywords: Depression, morning and evening wakefulness, positron emission tomography, regional cerebral metabolic rate of glucose
Diurnal variation in mood characterizes many depressed patients, with evening mood improvement relative to the morning mood (Gordijn et al. 1994; Leibenluft et al. 1992; Tolle 1987). This diurnal variation appears to be mediated by subjective and possibly autonomic arousal (Bouhuys et al. 1990; Kuhs and Tölle 1991; Rechlin et al. 1995; Szuba et al. 1991). Increased magnitude of ultradian (i.e., within-day) mood variability also characterizes depressed patients compared with nondepressed subjects (Cowdry et al. 1991; Hall et al. 1991; Wefelmeyer and Kuhs 1996). Diurnal mood variation in depression may relate to regional cerebral metabolic changes during evening relative to morning wakefulness. However, regional brain metabolic correlates of diurnal mood variation have not been examined.
The primary focus of the present study was to investigate the regional changes in brain activity that correlate with diurnal mood variation in depressed compared with healthy subjects. Diurnal mood variation in depression may relate to functional changes in components of the ventral and dorsal emotion neural systems (Phillips et al. 2003a). The ventral emotion neural system is believed to subserve affect production and includes the amygdala, ventral anterior cingulate and orbitofrontal cortex, ventral striatum, and insula. The dorsal emotion neural system is involved in effortful affect regulation and includes the hippocampus, dorsal cingulate, and prefrontal cortex (Phillips et al. 2003a). Functional anomalies in either or both of these neural systems underlie abnormal affect and emotion regulation (Phillips et al. 2003b).
Findings from clinical and experimental neuroimaging studies in depressed patients and healthy subjects support the notion that diurnal mood variation may relate to functional changes in either or both ventral and dorsal emotion neural systems. Depression severity has been shown to correlate positively with blood flow and glucose metabolism in the amygdala (Drevets et al. 2002b) and negatively with blood flow or glucose metabolism in the prefrontal, cingulate, and temporoparietal cortex (Baxter et al. 1989). In healthy subjects, induced sadness is associated with increased blood flow in ventral cerebral regions, including the subgenual cingulate and insula, and reduced blood flow in frontal and parietal regions (Mayberg et al. 1999; Phan et al. 2002). In depressed patients, pretreatment to posttreatment improvements in mood ratings have been associated with changes in blood flow and glucose metabolism in the amygdala, dorsolateral frontal cortices, anterior cingulate cortex, and orbital frontal cortex (e.g., Brody et al. 1999; Drevets et al. 2002b; Goldapple et al. 2004; Mayberg et al. 1999). Changes in regional cerebral metabolic activity in components of the ventral and dorsal emotion neural system may parallel mood changes and may be associated with diurnal mood variation in depression. Therefore, we first hypothesized that group differences in morning-to-evening mood ratings would parallel group differences in relative regional cerebral metabolic rate of glucose (rCMRglc) during morning and evening wakefulness. Specifically, we hypothesized that evening mood improvements in depressed patients would be accompanied by a reduction in rCMRglc in components of the ventral emotion neural system and/or an increase in rCMRglc in components of the dorsal emotion neural system during evening relative to morning wakefulness compared with healthy subjects.
A secondary goal of the study was to examine whether patterns of morning-to-evening changes in rCMRglc differed in depressed and healthy subjects in brain systems that promote wakefulness. Using [18F]-fluoro-deoxyglucose ([18F]-FDG) positron emission tomography (PET) in healthy adults, we have previously shown that relative rCMRglc increases during evening compared with morning wakefulness in wake-promoting brainstem and hypothalamic regions (Buysse et al. 2004). We suggested that this pattern may reflect input from the circadian timing system to promote wakefulness in the face of increasing sleep pressure. Based on the notion that circadian rhythms are blunted in depression (Schulz and Lund 1983), we hypothesized that diurnal variations in rCMRglc in brainstem and hypothalamic areas may be blunted in depressed compared with healthy individuals.
Methods and Materials
Study Design
Studies were conducted in the University of Pittsburgh General Clinical Research Center (GCRC) and PET Facility. These results were part of a larger project investigating relative regional glucose metabolism during wakefulness and sleep (MH 24652). As part of this study, each subject completed PET studies using the [18F]-FDG method to assess regional cerebral glucose metabolism: one phase was conducted during morning wakefulness, and one was conducted during evening wakefulness at the subject's usual bedtime. Sleep-wake state was monitored by continuous electroencephalographic (EEG) recordings during each of the [18F]-FDG PET studies.
Participants
All participants signed written informed consent according to guidelines of the University of Pittsburgh Institutional Review Board. Twelve right-handed depressed patients (10 women, 2 men; mean age = 38.1, SD = 12.6) and 13 age- and sex-matched healthy participants (10 women, 3 men; mean age = 37.3, SD = 11.5) participated in this study. Data collected in this sample of healthy participants have been reported elsewhere (Buysse et al. 2004). Depressed participants met research diagnostic criteria (Spitzer et al. 1978) for major depression on the basis of the Structured Clinical Interview for DMS-III-R (SCID) (Spitzer et al. 1992). All depressed patients had a minimum score of 15 on the Hamilton Rating Scale for Depression, 25-item version (Hamilton 1960). Only participants who were not medicated at the time of the intake assessments were recruited and included in this study. There was no protocol to change or discontinue medications for enrolled participants. All were free of medication for at least 2 weeks (8 weeks for fluoxetine) prior to the EEG sleep and PET studies. A nightly urine drug screen confirmed that all participants were free of alcohol and recreational drugs during the studies. None of the healthy subjects had current or past medical or psychiatric conditions, as determined by the SCID. Medical histories, physical examinations, and laboratory tests were conducted on all subjects at entry into the study. Subjects with an apnea/hypopnea index >10 or with an index of periodic leg movement with arousal >10 on night 1 on the screening night were excluded from further study. All had stable sleep-wake patterns as verified by 2-week diaries prior to the study. All female subjects had negative serum pregnancy tests (obtained within 48 hours prior to PET scans). During screening evaluation, participants completed the Hamilton Rating Scale for Depression (HRSD) (Hamilton 1960), the Raskin Severity of Depression and Mania Scale (Raskin 1988), and the Pittsburgh Sleep Quality Index (Buysse et al. 1989). Participants also completed the self-report Circadian Type Questionnaire (CTQ) (Folkard et al. 1979), which includes a morningness-eveningness subscale. On the latter subscale, a score of 1 indicates a preference for eveningness, whereas a score of 100 indicates a preference for morningness.
An initial polysomnographic (PSG) screening study was conducted at subjects' usual sleep times to rule out sleep disorders and to serve as an accommodation night. A second night of sleep studies for baseline sleep measures was conducted the following night. On the third morning, 2 to 4 hours after awakening, subjects underwent the morning [18F]-FDG PET study. On the evening of the third night, subjects completed the waking study. The 2- to 4-hour window was selected to minimize the effects of sleep inertia on brain activity (Balkin et al. 2002) and to optimize alertness during the scanning procedures.
EEG Studies
During the morning and evening [18F]-FDG uptake periods, a technologist constantly monitored the EEG for signs of drowsiness and incipient sleep (e.g., slow rolling eye movements, increased EEG amplitude, and reduced EEG frequency) and called the subject's name or tapped a pencil to alert the subject if such signs occurred. Procedures for EEG sleep recording, monitoring, and data processing and definitions for visually scored sleep variables have been provided elsewhere (Doman et al. 1995).
Magnetic Resonance and PET Study Procedures
All subjects received a brain magnetic resonance (MR) scan prior to PET studies to screen for pathology and to provide a high-resolution anatomical image for co-registration with PET scan images. Magnetic resonance imaging was performed at the University of Pittsburgh MR Center using a GE Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, Wisconsin). Subjects were positioned in a standard head coil and a brief scout T1-weighted image was obtained. The following axial series oriented to the plane connection the anterior commissure-posterior commissure line (AC-PC line) will be acquired to screen subjects for unexpected pathology: fast spin-echo T2-weighted (effective echo time [TE] = 17, repetition time [TR] = 2500, number of excitations [NEX] = 1, slice thickness = 5 mm/1 mm interslice). A volumetric spoiled gradient recalled echo (SPGR) sequence with parameters optimized for maximal contrast among gray matter, white matter, and cerebrospinal fluid (CSF) was acquired in the coronal plane (TE = 5, TR = 25, flip angle = 40°, NEX = 1, slice thickness = 5 mm/0 mm interslice). A field of view of 24 cm and image matrix of 256 × 192 pixels was used for all axial MR series.
Morning and evening waking [18F]-FDG PET scans used identical methodology except for time of day. Prior to each scan, subjects completed rating scales which included a rating of subjective mood and alertness using either a 100 mm Visual Analog Scale (VAS) (prior to morning scans) or Likert scales from the Profile of Mood States (POMS) (prior to evening scans) (McNair et al. 1971). The depression item of the POMS was used to assess mood at the two times of day. Data from the Visual Analog Scales for alertness and depression were converted to Likert scales to allow for comparison between morning and evening subjective ratings as follows: VAS score 0–20 = 0; VAS score 21–40 = 1; VAS score 41–60 = 2; VAS score 61–80 = 3; VAS score 81–100 = 4, allowing assessment of the direction and magnitude of self-reported mood and alertness changes in the evening relative to the morning. To investigate group × time of day interactions on subjective ratings of mood and alertness, data from both scales were converted to z-scores.
Each PET study began with a 20-minute accommodation period, during which subjects sat quietly in a comfortable chair with eyes closed, ears open, and no specific cognitive task, while EEG was monitored for wakefulness. A 5 mCi dose of [18F]-FDG was then injected via an indwelling venous catheter, with continued EEG monitoring for wakefulness during a 20-minute uptake period. Twenty minutes following [18F]-FDG injection, subjects were transported to the PET Facility (see Buysse et al. 2004; Nofzinger et al. 2004 for details on scanning procedures). Positron emission tomography imaging was performed on an ECAT HR+ tomograph (CTI PET Systems, Knoxville, Tennessee) in three-dimensional (3-D) mode (septa retracted). Emission data were corrected for scatter and for attenuation (by transmission).
Statistical and Image Analysis
Group differences on clinical data were investigated using t tests. Group × time of day differences on mood, alertness, and time spent awake during scans were computed using analysis of variance (ANOVA) with time of day as the repeated measure. Spearman correlations were conducted to assess the relationship between the changes in absolute cerebral metabolic rate of glucose in morning and evening in depressed patients and differences in mood scores.
Positron emission tomography image analyses included evaluation of morning-evening differences in relative rCMRglc. Morning-evening differences were first investigated in the depressed sample only. Two interaction models were then conducted using Statistical Parametric Mapping, 1999 version (SPM 99) (Friston et al. 1990, 1991, 1995) to provide whole-brain voxel-by-voxel analyses of between-group differences during morning and evening waking scans. Image processing, spatial normalization, smoothing, and MR-PET co-registration were conducted according to standard methods (Minoshima et al. 1993; Wiseman et al. 1996; Woods et al. 1992, 1993). Post hoc analyses were also conducted to assess group differences within each state. Changes in rCMRglc were considered significant if the corrected p value was < .05. Morning and evening scans were corrected for global metabolism using analysis of covariance (ANCOVA). Statistical images across all 63 brain slices, consisting of at score at every voxel, were created for the contrasts of interest. Statistical inferences were based on p values corrected for multiple comparisons (Germain et al. 2004; Nofzinger et al. 1999, 2002, 2004). In addition to global analysis, volume-of-interest analyses using small volume corrections were conducted for the interaction and post hoc tests on regions of the dorsal and ventral emotion systems (Phillips et al. 2003a) and wake-promoting areas (Buysse et al. 2004) using previously reported Tailarach coordinates (Buysse et al. 2004; Mayberg et al. 1999). The Tailarach coordinates of these regions are indicated in Table 1. Volume-of-interest analyses were considered significant if the corrected cluster-level p value was < .05.
Table 1. Tailarach Coordinates for Midpoints of Each Volume of Interest.
| Structure | Talairach Coordinates of Volume of Interest (x, y, z; Right/Left) |
|---|---|
| Emotion Systema | |
| Amygdala | 22, −6, −14/−22, −6, −14 |
| Subgenual Cingulate | 2, 6, −6/−2, 6, −6 |
| Anterior Ventral Insula | 30, 18, −8/−16, 18, −16 |
| Mid-anterior Insula | 30, −2, 8/−26, −4, 8 |
| Posterior Insula | 38, −18, 20/−34, −14, 20 |
| Lateral Frontal Cortex | 42, 14, 20/−42, 14, 20 |
| Dorsolateral Prefrontal Cortex | 28, 12, 38/−28, 12, 38 |
| Anterior Cingulate Cortex | 10, 24, 24/−10, 24, 24 |
| Inferior Parietal Cortex | 42, −34, 34/−40, −46, 34 |
| Wakefulness Promoting Regions | |
| Posterior Hypothalamus | 0, −6, −6 |
| Midbrain Reticular Formation | 0, −26, −8 |
| Pontine Reticular Formation | 0, −28, −24 |
| Raphe Nuclei | 0, −34, −12 |
| Locus Coeruleus (right/left) | 10, −38, −20/−10, −38, −20 |
Volumes of interest measured 10 × 10×8 mm.
Coordinates derived from Mayberg et al. (1999), except for the amygdala (from Tailarach and Tournoux 1988).
Results
Mean HRSD scores for depressed and healthy participants were 24.42 (SD = 4.87) and .3 (SD = .5), respectively [t(16) = 16.68, p < .001]. Depressed patients were not on lithium prior to the study. Only participants who were not medicated at the time of the intake assessments were recruited and included in this study. There was no protocol to change or discontinue medications for enrolled participants. All included depressed patients were experiencing a recurrent major depressive episode. Mean duration of the current major depressive episode was 50.73 weeks (SD = 41.25 weeks; range = 1 to 124). On average, patients had 4.22 prior major depressive episodes (SD = 1.92; range 1 to 6). None of the healthy subjects presented with diurnal mood variation, as rated on the HRSD. One of the 12 depressed patients demonstrated obvious changes in mood across time of day on the HRSD, as determined by a score of 2 on the diurnal variability item of the HRSD. Five depressed patients were rated as showing mild and infrequent changes in mood variation (HRSD item score = 1), and six presented no diurnal variation in mood on the HRSD (HRSD item score = 0). Also based on HRSD, five depressed patients showed no current melancholic features (HRSD item score = 0), five showed some current melancholic feature (HRSD item score = 1), and one showed definite current melancholic features (HRSD item score = 2). This information was not available for the remaining patient.
Self-reported diurnal variation in subjective mood ratings improved in the evening relative to morning ratings in six patients, remained unchanged in five patients, and worsened in one patient. Data from the morningness subscale of the Circadian Type Questionnaire (Folkard et al. 1979) were available for 11 healthy subjects and 9 depressed subjects. The mean morningness score for healthy subjects was 57.27 ± 13.85 (range = 37–90), and the mean morningness score for depressed patients was 57.11 ± 13.34 (range = 27–73). Mean scores did not differ between the two groups, t(18) = .26, ns. The Spearman correlation between the score on the HRSD item on diurnal variation and the morningness subscale of the CTQ in depressed patients did not reach statistical significance (rho = .61, p = .08).
The repeated measure ANOVA revealed a significant group × time of day interaction for the mood scale [F(1,21) = 11.56, p = .003]. Depressed patients showed improvements in mood ratings in the evening relative to morning, whereas healthy participants showed lower mood ratings in the evening compared with the morning (Figure 1A). There was no group (healthy vs. depressed) × time of day (morning vs. evening) interaction on alertness ratings [F(1,21) = .12, ns], no main effect of group [F(1,21) = 4.07, ns), and no main effect of time of day [F(1,21) = .04, ns; Figure 1B).
Figure 1.
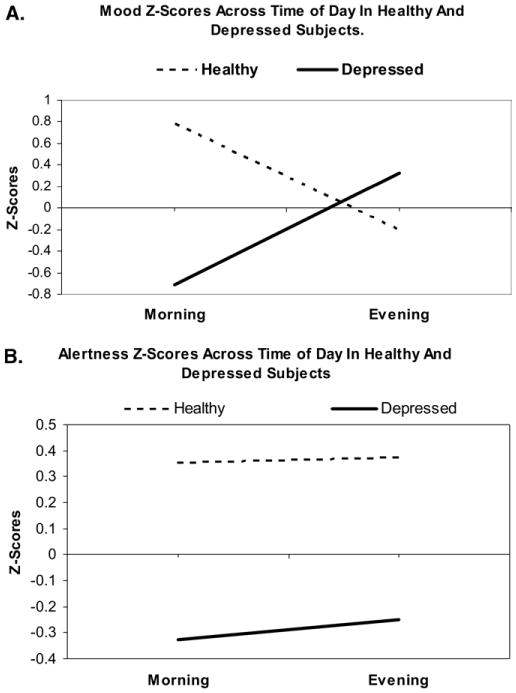
Z-score changes in morning and evening ratings for the mood (A) and alertness (B) scales in healthy and depressed subjects.
Absolute glucose metabolism data were complete and available for 11 depressed patients and 8 healthy subjects. There was no correlation between changes in absolute glucose metabolism and changes in mood scores in depressed patients (Spearman rho = .08, ns). Similarly, there was no significant correlation between these measures in healthy subjects (Spearman rho = .06, ns).
Electroencephalographic studies showed that depressed participants remained awake for a mean time of 18.58 minutes (SD = 2.20) during the morning uptake period and 19.64 minutes (SD = .96) during the evening uptake period. Healthy subjects remained awake for a mean of 19.42 minutes (SD = 1.34) of the 20-minute [18F]-FDG uptake period during the morning study and for a mean of 19.73 minutes (SD = 0.66) during the evening study. Time spent awake during morning and evening scans did not differ between groups or time of day [i.e., no significant group × time of day interaction; F(1,20) = .49, p = .49], confirming that the PET scans represent brain metabolic activity during wakefulness rather than sleep in both groups.
Evening-Morning Differences in rCMRglc in Depressed Patients
In depressed patients, relative rCMRglc was significantly greater during evening wakefulness compared with morning wakefulness in two areas (Figure 2). The first area (Tailarach coordinates x, y, z of voxel of maximum = 28, 36, 8; extent: 4829 contiguous voxels; z = 4.27, p = .001) included midline medial prefrontal cortex, dorsal anterior and pregenual cingulate cortices, basal ganglia (striatum and globus pallidus), thalamus, and hypothalamus and extended into the left medial temporal regions including hippocampus, parahippocampal cortex, amygdala, uncal gyrus, fusiform gyrus, and the cerebellum. The second area was composed of 1282 voxels (voxel of maximum significance = 34, 0, -16; z = 4.48, p < .001). This area included the right superior temporal gyrus and extended into the fusiform and parahippocampal gyrus, hippocampus, and cerebellum.
Figure 2.
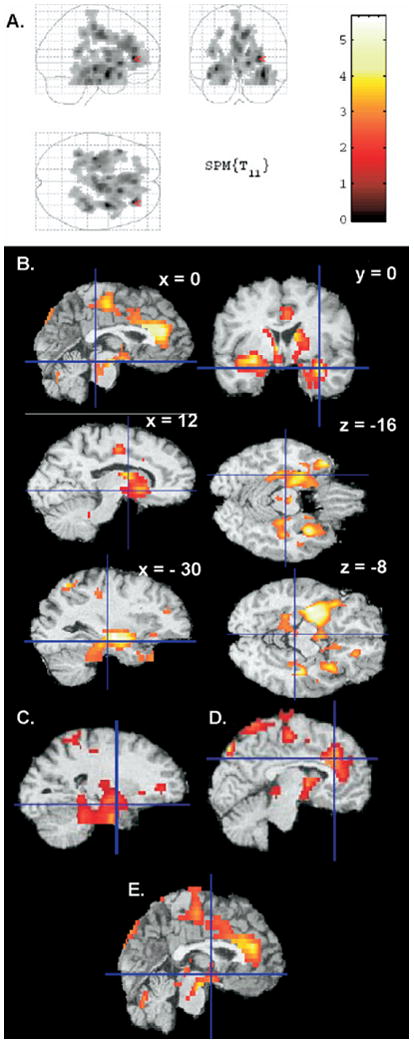
Areas with greater relative glucose metabolism during evening wakefulness than morning wakefulness in the depressed sample only (p < .05 at the corrected cluster level), projected onto a glass brain (A) and transverse sections (B). The volumes of interest corresponding to left amygdala (C), anterior cingulate cortex (D), and posterior hypothalamus (E) are also presented. The color scale on the right depicts t values for the evening-morning contrast.
Volume-of-interest analyses confirmed that rCMRglc was greater during evening wakefulness compared with morning wakefulness in the left amygdala (z = 3.20, p = .001; Figure 2C), left anterior cingulate gyrus (z = 3.22, p = .02; Figure 2D), and posterior hypothalamus (z = 2.93, p = .006; Figure 2E).
Group × Time of Day Interaction Analyses
Depressed patients showed a statistically significant smaller morning-to-evening increase in relative rCMRglc compared with healthy subjects in one cluster. This cluster was composed of 1647 contiguous pixels (voxel of maximal significance at Taila-rach coordinates x, y, z = -20, -76, -4; z = 3.97, p < .001) and included bilaterally the lingual and fusiform gyri. Figures 3A and 3B depict this area onto glass brains and rendered images. Two volumes of interest also achieved statistical significance: the right midbrain reticular formation (Figure 3C; z = 2.26, p = .02), and the left locus coeruleus (Figure 3D; z = 2.07, p = .03). None of the other volumes of interest showed smaller increases in rCMRglc during evening relative to morning wakefulness in depressed compared with healthy subjects.
Figure 3.
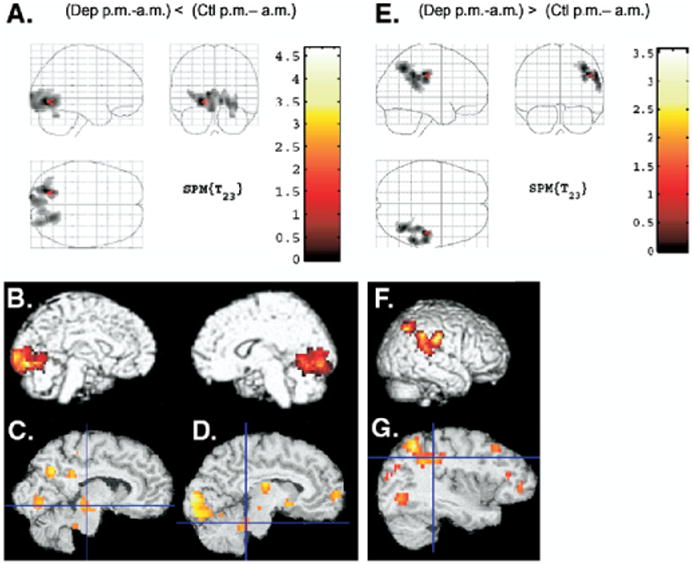
Areas where depressed patients significantly differed from healthy subjects during evening relative to morning wakefulness projected onto glass brains (A) and (E) and rendered images (B) and (F). The color scale depicts t values for the morning-evening contrast. Areas where depressed patients showed smaller increases in rCMRglc during evening relative to morning wakefulness compared with healthy subjects are presented in (A) and (B). Areas where depressed patients showed greater increases in rCMRglc during evening relative to morning wakefulness compared with healthy subjects are presented in (E) and (F). Volumes of interest corresponding to the midbrain reticular formation volume (C), left locus coeruleus (D), and inferior parietal cortex (G) are also presented. rCMRglc, regional cerebral metabolic rate of glucose.
One area of 726 contiguous voxels reached statistical significance (voxel of maximal significance at Tailarach coordinates x, y, z = 42, -26, 36; z = 3.13, p = .04) for the interaction analysis conducted to investigate areas where depressed patients showed greater increase in rCMRglc during evening wakefulness relative to morning wakefulness compared with healthy subjects (Figure 3E and 3F). This area included the central postcentral gyrus and superior and inferior parietal cortices and extended into the superior temporal gyrus. Small volume correction analyses revealed that rCMRglc was significantly greater in depressed patients during evening wakefulness relative to morning wakefulness compared with healthy subjects in the right inferior parietal cortex only (z = 2.72, p = .01; Figure 3G).
Post Hoc Group Differences During Morning and Evening Wakefulness
Post hoc analyses were conducted to identify between-group differences during each time of day (Figure 4). During morning wakefulness, depressed patients showed lower rCMRglc than healthy subjects bilaterally in the superior and middle frontal gyrus, medial frontal gyrus, and left central and superior parietal gyri and precuneus (3238 voxels; voxel of maximal significance at coordinates x, y, z = 34, 8, 44; z = 3.79; p < .001; Figure 4A). The volume of interest corresponding to the right dorsolateral prefrontal cortex also showed significantly lower rCMRglc during morning wakefulness in depressed patients compared with healthy subjects (z = 3.44, p = .03). None of the other volumes of interest showed significantly reduced rCMRglc during morning wakefulness in depressed compared with healthy subjects. A similar pattern persisted during evening wakefulness. Specifically, depressed patients showed lower rCMRglc bilaterally in the medial dorsal anterior cingulate cortex, medial frontal cortex, and middle and superior frontal gyri during evening wakefulness compared with healthy subjects (Germain et al. 2004) (Figure 4C). Additionally, relative rCMRglc did not differ between groups during evening wakefulness in any of the volumes of interest.
Figure 4.
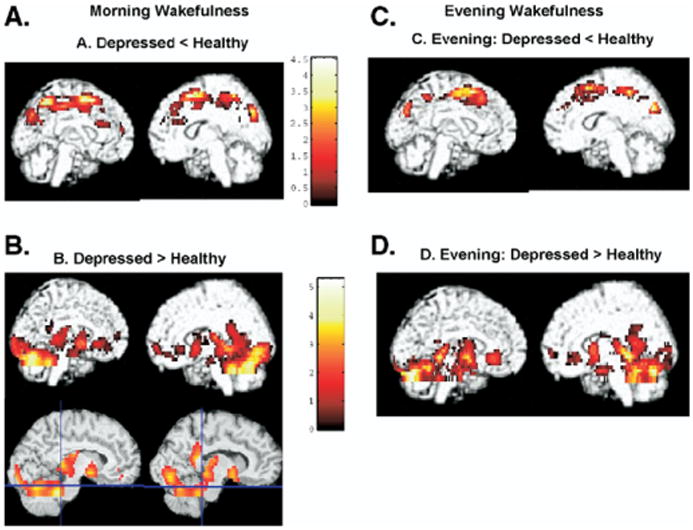
Between-group differences during morning and evening wakefulness. Areas where rCMRglc is greater or smaller in depressed patients compared with healthy subjects during morning wakefulness presented onto rendered images. Blue lines in the lower two images included in (B) intersect at Tailarach coordinates corresponding to left and right locus coereleus. rCMRglc, regional cerebral metabolic rate of glucose.
Depressed patients showed greater rCMRglc in two regions compared with healthy subjects (Figure 4B) during morning wakefulness. The first area (5347 contiguous pixels) included the right inferior occipital gyrus; parahippocampal, lingual, and fusiform gyri; hippocampus; thalamus; and striatum (voxel of maximal significance at coordinates x, y, z = 32, -86, 0; z = 4.22, p < .001). The second cluster included the left parahippocampal and lingual gyri, striatum, thalamus, insula, uncus, and amygdala (1836 contiguous voxels; coordinates x, y, z for voxel of maximum significance = -14, -38, 4; z = 3.54, p = .02). Volume-of-interest analyses also revealed that depressed patients showed greater rCMRglc than healthy participants in morning wakefulness in left amygdala (z = 2.46, p = .01), left (z = 2.92, p = .04) and right (z = 2.35, p = .03) subgenual cingulate cortex, right (z = 2.19, p = .01) and left (z = 2.72, p = .002) mid-anterior insula, and left (z = 3.36, p = .006) and right (z = 2.96, p = .005) locus coeruleus. During evening wakefulness, relative rCMRglc was greater in depressed compared with healthy subjects in a large area composed of 5235 contiguous pixels (voxel of maximum significance at x, y, z coordinates = -26, -20, -16; z = 4.22, p < .001) that included bilaterally the cerebellum, fusiform and parahippocampal gyri, and hippocampus and extended into the uncus, striatum, and pulvinar (Figure 4D). None of the volumes of interest showed greater rCMRglc in depressed patients compared with healthy participants during evening wakefulness.
Discussion
Differences in diurnal mood variations have been described in depressed and healthy subjects. In this study, mood ratings improved across time of day in depressed patients compared with healthy subjects and were associated with different patterns of morning-to-evening changes in rCMRglc in the two groups. Consistent with previous studies, hypometabolism in frontal cortical areas and hypermetabolism in subcortical and limibic-paralimbic structures (e.g., striatum, amygdala, insula, uncus) characterized depressed patients and persisted across time of day. Nevertheless, evening improvements in mood in major depressive disorder (MDD) patients were paralleled by increases in rCMGglc in the parietal and temporal cortices, basal ganglia, and cerebellum. To our knowledge, this is the first study to provide preliminary indications that regional metabolic changes associated with depression show diurnal variations and that metabolic changes relative to healthy subjects persist across time of day. The findings also suggest that potential compensatory systems, which may be involved in preserving emotional homeostasis (Mayberg 2003) and which include the parietal and temporal cortices, basal ganglia, and cerebellum, may contribute to evening mood improvements in depressed patients.
The findings partially supported the initial hypothesis that evening mood improvements in depressed patients would be associated with inverse changes in rCMRglc in components of the ventral and dorsal emotion systems as suggested by Phillips et al. (2003a). Concomitant with evening mood improvements, depressed patients demonstrated greater increases in central, parietal, and temporal cortices during evening wakefulness relative to morning wakefulness compared with healthy subjects. However, post hoc analyses indicated that depressed patients nevertheless remained hypometabolic in frontal, central, and parietal cortices across time of day compared with healthy subjects. This is consistent with previously reported hypofrontality characterizing depression across the sleep-wake cycle (Baxter et al. 1989; Buchsbaum et al. 1986; Germain et al. 2004; Nofzinger et al. 2004). Post hoc analyses also revealed that depressed patients continue to have hypermetabolism in ventral regions (i.e., pre-genual cingulate cortex, striatum, thalamus, amygdala, and uncal gyrus) during both morning and evening wakefulness compared with healthy subjects. Together, these observations suggest that evening mood improvements in depression may reflect partial normalization of the balance between the ventral and dorsal emotion neural systems (Phillips et al. 2003a, 2003b).
These preliminary results also support the hypothesis that compensatory mechanisms or neural networks that may be involved in preserving the emotional homeostasis (Mayberg 2003) become activated during evening wakefulness and parallel mood improvements. Specifically, this hypothesis suggests that depression is a multidimensional disorder characterized by a set of dysfunctions in brain regions and neurochemical systems, as well as with a failure of remaining systems to maintain emotional homeostasis under stress. However, the specific neurobiological and neurochemical mechanisms underlying this failure remain undetermined. In the present study, evening mood improvements paralleled increased metabolic activity in frontal regions in depressed patients. The observed heightened limbic-paralimbic metabolic activity observed during evening relative to morning wakefulness in depressed patients is consistent with the hypothesis that increased activation in this ventral network may maintain frontal activation and mood improvement (Mayberg 2003). Increased metabolism in parietal and temporal cortices during evening wakefulness may also reflect the recruitment of cortical compensatory mechanisms by heightened limbic-paralimbic activation. Studies comparing morning and evening rCRMglc patterns in depressed patients with and without diurnal mood variation are necessary to determine the presence and specific contribution of these hypothesized compensatory mechanisms.
In addition, other components of a compensatory network may fail during morning wakefulness in depressed patients. Specifically, evening improvements in mood in depressed patients were paralleled by further increases in rCMRglc in the basal ganglia and by significantly greater rCMRglc in the cerebellum. Both the basal ganglia and the cerebellum have been implicated in the regulation of cognitive and affective processes (Afifi 2003; Konarski et al. 2005; Schmahmann and Caplan 2006). Neurological disorders affecting the basal ganglia or the cerebellum are associated with depressive symptoms (Afifi 2003; Konarski et al. 2005). Hypometabolism in the basal ganglia has been reported in depressed patients (Videbech et al. 2002). Hypermetabolism and increased perfusion in the cerebellum have been reported in healthy subjects during induced sadness paradigms, as well as in depressed patients during resting states (Videbech et al. 2002). In depressed patients, response to antidepressant treatment has been associated with changes in blood flow and metabolic activity in the cerebellum (Holthoff et al. 2004; Mayberg et al. 2000). Recently, metabolic activity in the basal ganglia and cerebellum has been associated with the severity of insomnia complaints assessed on the Hamilton Rating Scale for Depression (Milak et al. 2005). Increased metabolic activity in the basal ganglia and the cerebellum during evening wakefulness may also contribute to stimulate and maintain increased frontal metabolism concomitant with mood improvements. Specifically, heightened activity of the cerebellum, the basal ganglia, and limbic system during evening wakefulness may stimulate the frontal cortices and may relate to evening mood improvements. A similar global hyperactivation process may underlie the mood-improving effects of prolonged wakefulness by sleep deprivation in depressed patients.
Of note, the pattern of increased metabolic activity in the ventral emotional network observed during evening wakefulness closely resembles the metabolic pattern previously reported during nonrapid eye movement sleep (Nofzinger et al. 2005). Therefore, heightened activity in ventral structures observed during evening wakefulness in depressed patients and which may improve mood via activation of the frontal cortices may also directly promote arousal via connections with the brainstem, basal forebrain, and hypothalamus and adversely affect sleep. Further investigation of the neurobiological effects underlying the effects of pharmacological or psychological antidepressant treatment across the sleep-wake cycle are required to investigate the interrelations between mood regulation compensatory mechanisms and sleep regulation mechanisms in depression.
Despite the lack of group × time differences in subjective ratings of alertness, patterns of changes in rCMRlgc during morning and evening wakefulness differed in depressed and healthy subjects in wake-promoting areas. Consistent with the second hypothesis, depressed patients showed blunted increases in rCRMglc during evening relative to morning wakefulness in the posterior cortex, midbrain reticular formation, and left locus coeruleus compared with healthy subjects. Post hoc analyses indicated that these observations may reflect ceiling effects, i.e., a lack of rCMRglc increase during evening wakefulness was related to elevated rCMRglc during morning wakefulness in depressed patients compared with healthy subjects, which persisted during evening wakefulness. Alternatively, depressed patients may show normalization of increased arousal in the evening. Larger samples are required to further elaborate on the possible clinical correlates of these differences.
Limitations of the study must be acknowledged. First, the subjects encompassed a fairly wide age range and included both men and women. It seems plausible that age, sex, or both could influence the study findings. Second, we investigated only two time points during the waking day and therefore do not have a detailed analysis of the time course of regional brain activation patterns. Studies using other radioligands such as 15O-H2O would potentially provide a more detailed temporal analysis but would not have equivalent spatial resolution to the [18F]-FDG technique. Larger samples are required to investigate the clinical correlates of diurnal variations in rCMRglc and to compare patients with and without marked diurnal mood variation. Nevertheless, the present findings suggest that diurnal mood variation in depression may relate to changes in rCMRglc across time of day. More generally, the present findings are consistent with the hypothesis that a dysfunctional limbic-cortical network underlies depression and that functional changes in the components of this network underlies mood improvements (Mayberg 2003).
Acknowledgments
This research was supported by National Institutes of Health (NIH) Grants MH24652, MH30915, MH66227, MH61566, MH 01414, RR 00056, AG 00972, and the Canadian Institutes of Health Research.
We thank the staff of the Sleep and Chronobiology Program and the General Clinical Research Center for their dedication in completing these studies. Special thanks to Professor Mary L. Phillips, MA, M.Sc, MRCPsych, M.D., at the Institute of Psychiatry and Guy's, Kings, and St. Thomas' School of Medicine for her valuable editing comments.
References
- Afifi AK. The basal ganglia: A neural network with more than motor function. Semin Pediatr Neurol. 2003;10:3–10. doi: 10.1016/s1071-9091(02)00003-7. [DOI] [PubMed] [Google Scholar]
- BalkinTJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, et al. The process of awakening: A PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Flentge F, van den Hoofdakker RH. Effects of total sleep deprivation on urinary cortisol, self-rated arousal, and mood in depressed patients. Psychiatry Res. 1990;34:149–162. doi: 10.1016/0165-1781(90)90016-x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, et al. Brain metabolic changes in major depressive disorder from pre-to post- treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Wu J, DeLisi LE. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with 18F-2-deoxyglucose in affective illness. J Affect Disord. 1986;10:137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, et al. Regional brain glucose metabolism during morning and evening wakefulness in humans: Preliminary findings. Sleep. 2004;27:1245–1254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index (PSQI): A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cowdry RW, Gardner DL, O'Leary KM, Leibenluft E, Rubinow DR. Mood variability: a study of four groups. Am J Psychiatry. 1991;148:1505–1511. doi: 10.1176/ajp.148.11.1505. [DOI] [PubMed] [Google Scholar]
- Doman J, Detka C, Hoffman T, Kesicki D, Monahan JP, Buysse DJ, et al. Automating the sleep laboratory: Implementation and validation of digital recording and analysis. Int J Biomed Comput. 1995;38:277–290. doi: 10.1016/s0020-7101(05)80010-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neupsychopharmacol. 2002a;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002b;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Folkard S, Monk TH, Lobban MC. Towards a predictive test of adjustment to shift work. Ergonomics. 1979;22:79–91. doi: 10.1080/00140137908924591. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: The assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Germain A, Nofzinger EA, Kupfer DJ, Buysse DJ. Neurobiology of non-REM sleep in depression: Further evidence for hypofrontality and thalamic dysregulation. Am J Psychiatry. 2004;161:1856–1863. doi: 10.1176/ajp.161.10.1856. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gordijn MC, Beersma DG, Bouhuys AL, Reinink E, Van den Hoofdakker RH. A longitudinal study of diurnal mood variation in depression: Characteristics and significance. J Affect Disord. 1994;31:261–273. doi: 10.1016/0165-0327(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Hall DP, Jr, Sing HC, Romanoski AJ. Identification and characterization of greater mood variance in depression. Am J Psychiatry. 1991;148:1341–1345. doi: 10.1176/ajp.148.10.1341. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthoff VA, Beuthien-Baumann B, Zundorf G, Triemer A, Ludecke S, Winiecki P, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–194. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Kuhs H, Tölle R. Sleep deprivation therapy. Biol Psychiatry. 1991;29:1129–1148. doi: 10.1016/0006-3223(91)90255-k. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Noonan BM, Wehr TA. Diurnal variation: Reliability of measurement and relationship to typical and atypical symptoms of depression. J Affect Disord. 1992;26:199–204. doi: 10.1016/0165-0327(92)90016-y. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, Mcginnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, Mcginnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA. Automated detection of the intercommissural line for stereotactic localization of functional brain. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Carter CS, Luna B, Price JC, et al. Increased activation of anterior paralimbic and executive cortex from waking to rapid eye movement sleep in depression. Arch Gen Psychiatry. 2004;61:695–702. doi: 10.1001/archpsyc.61.7.695. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Meltzer CC, Miewald JM, et al. Alterations in regional cerebral glucose metabolism across waking and non-rapid eye movement sleep in depression. Arch Gen Psychiatry. 2005;62:387–396. doi: 10.1001/archpsyc.62.4.387. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Nichols TE, Meltzer CC, Price J, Steppe DA, Miewald JM, et al. Changes in forebrain function from waking to REM sleep in depression: Preliminary analyses of [18F] FDG PET studies. Psychiatry Res. 1999;91:59–78. doi: 10.1016/s0925-4927(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Ombao H, Buysse DJ, Meltzer CC, Price JC, Reynolds CF, et al. Relationships between NREM sleep delta EEG specral power and morning waking regional ceregral clucose metabolism in adult humans: Age-dependent and age-independent effects. Sleep. 2002;25(abstract suppl):A103–A104. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Raskin A. Three-Area Severity of Depression Scale. In: Bellack AS, Herson M, editors. Dictionary of Behavioral Assessment Techniques. New York: Pergamon; 1988. [Google Scholar]
- Rechlin T, Weis M, Kaschka WP. Is diurnal variation of mood associated with parasympathetic activity? J Affect Disord. 1995;34:249–255. doi: 10.1016/0165-0327(95)00011-b. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schulz H, Lund R. Sleep onset REM episodes are associated with circadian parameters of body temperature. A study in depressed patients and normal controls. Biol Psychiatry. 1983;18:1411–1426. [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Baxter LR, Fairbanks LA, Guze BH, Schwartz JM. Effects of partial sleep deprivation on the diurnal variation of mood and motor activity in major depression. Biol Psychiatry. 1991;30:817–829. doi: 10.1016/0006-3223(91)90237-g. [DOI] [PubMed] [Google Scholar]
- Tailarach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Tolle R, Goetze U. On the daily rhythm of depression symptomatology. Psychopathology. 1987;20:249. doi: 10.1159/000284507. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, Egander A, Clemmensen K, et al. The Danish PET/depression project: Clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Wefelmeyer T, Kuhs H. Diurnal mood variation in melancholic patients and healthy controls. Psychopathology. 1996;29:184–192. doi: 10.1159/000284990. [DOI] [PubMed] [Google Scholar]
- Wiseman MB, Nichols TE, Dachille MA, Mintun MA. Working towards an automatic and accurate method for PET-MR alignment. J Nucl Med. 1996;37(5):224. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. Automated image registration. In: Uemura K, Jones T, Lassen NA, Kanno I, editors. Quantification of Brain Function. Tracer Kinetics and Image Analysis in Brain PET. Amsterdam: Elsevier Science Publishers; 1993. pp. 391–398. [Google Scholar]


