Abstract
Rationale
Previous studies have shown that female rats exhibit enhanced cocaine-seeking across several phases of the addiction cycle when compared to males. Drug-seeking in females is also estrous cycle dependent and inversely associated with plasma progesterone. Although sex and estrous cycle-dependent differences have been reported in the reinstatement of cocaine-seeking triggered by cocaine injections or drug-paired cues, it is not yet known what role the estrous cycle may have on stress-induced reinstatement, either alone or in combination with drug-paired cues.
Objectives
Here, we examined male and female rats for reinstatement of extinguished cocaine-seeking produced by cocaine-paired cues or the stress-activating drug, yohimbine.
Methods
Male and female Sprague-Dawley rats self-administered intravenous cocaine (0.5 mg/kg/infusion) paired with a light+tone stimulus for 10–14 days. Lever responding was then allowed to extinguish, with subsequent reinstatement testing occurring 30 min following an injection of yohimbine (1.25 or 2.5 mg/kg, intraperitoneal) or vehicle either in the presence or absence of the conditioned stimulus.
Results
While males and females showed similar cue- and yohimbine-induced reinstatement (3–4 times over “No Cue”-vehicle responding), combining these stimuli resulted in a robust enhancement in cocaine-seeking in both groups, with a greater increase in females (10–12 vs 14–15 times over “No Cue”-vehicle responding for the males and females, respectively). When examined as a function of the estrous cycle, females in proestrus demonstrated higher levels of responding during yohimbine + cues reinstatement.
Conclusions
This cycle-dependent enhanced sensitivity to stress enhancement of cocaine-paired cues may generalize to greater relapse susceptibility under stressful conditions.
Keywords: sex differences, cocaine, relapse, reinstatement, stress, yohimbine, cues, estrous cycle
Introduction
Abundant evidence has shown that significant gender differences exist in cocaine addiction. Although men are more likely to have a cocaine abuse or dependence disorder (see (Brady and Randall 1999), for review), women begin using cocaine at an earlier age (Chen and Kandel 2002; Weiss et al. 1997) and progress more rapidly from casual use to the development of a dependence disorder (O'Brien and Anthony 2005; Westermeyer and Boedicker 2000). Differences also exist between men and women in terms of relapse to cocaine use. For example, women tend to have shorter cocaine-free periods (Griffin et al. 1989; Kosten et al. 1996), with some reports indicating an enhanced likelihood to relapse (Hyman et al. 2008; McKay et al. 1996) following exposure to stressful stimuli/life events or depression, factors that have been implicated as the leading cause of relapse in humans (see Sinha 2009, for review). However, reports of gender differences in relapse in abstinent addicts following exposure to cocaine-associated environmental stimuli have been mixed, with some reports indicating more (Robbins et al. 1999), less (Avants et al. 1995), or equal (Negrete and Emil 1992) susceptibility in women relative to men. Neurophysiological differences have also been demonstrated in men and women following exposure to stressful stimuli (Li et al. 2005) or cocaine-paired cues (Kilts et al. 2004).
Similar to clinical findings, sex differences have been reported for cocaine-related behaviors in laboratory animals. Relative to males, female rats demonstrate enhanced locomotor activity to acute cocaine injections and greater behavioral sensitization after repeated cocaine administration (Hu and Becker 2003; van Haaren and Meyer 1991). Moreover, females acquire conditioned place preference (CPP) more rapidly and at lower cocaine doses than male rats (Russo et al. 2003). In self-administration studies, females not only more readily acquire cocaine self-administration, they also demonstrate faster rates of acquisition than males (Hu et al. 2004; Lynch and Carroll 1999). Greater motivation for cocaine reward is also evident in that females exhibit higher responding (but not intake) than males on short access (2 h/day) schedules of reinforcement (Fuchs et al. 2005b; Kippin et al. 2005), enhanced cocaine intake on long access (6 h/day) schedules of reinforcement (Lynch and Taylor 2004; Roth and Carroll 2004), and higher breakpoints on progressive ratio schedules of reinforcement (Mello et al. 2007; Roberts et al. 1989). In animal models of relapse, females exhibit greater cocaine-seeking not only during early withdrawal (Anker and Carroll 2010; Fuchs et al. 2005b; Kippin et al. 2005), but also following protracted forced abstinence (Kerstetter et al. 2008). While female rats reinstate slightly less or equal to males following exposure to cocaine-associated cues (Fuchs et al. 2005a; Kerstetter et al. 2008), females show greater cocaine-primed (Kippin et al. 2005; Lynch and Carroll 2000) and stress-induced reinstatement (Anker and Carroll 2010) than males. Taken together, these data suggest that females may exhibit greater motivation for cocaine than males across all phases of the addiction cycle (see Lynch et al. 2002, for review).
Growing evidence suggests that sex differences may be mediated at least in part by cyclic ovarian hormone changes across the menstrual cycle in human and non-human primates (see Evans and Foltin 2010, for review) or the estrous cycle in rodents (see Festa and Quinones-Jenab 2004, for review). Relative to women during the luteal phase, women in the follicular phase report an enhancement in the subjective effects of cocaine (Evans et al. 2002; Sofuoglu et al. 1999) and d-amphetamine (Justice and de Wit 1999; White et al. 2002). An enhancement in motivation for cocaine as seen by higher progressive ratio breakpoints has been reported in female monkeys during the follicular phase, relative to the late luteal phase (Mello et al. 2007). In rats, females in vaginal estrus exhibit enhanced cocaine-seeking during self-administration (Feltenstein and See 2007; Lynch et al. 2000; Roberts et al. 1989), early extinction (Feltenstein and See 2007), protracted withdrawal (Kerstetter et al. 2008), and reinstatement (Feltenstein et al. 2009; Feltenstein and See 2007; Kerstetter et al. 2008; Kippin et al. 2005).
Despite evidence showing sex differences and the impact of rodent estrous cycle stages in mediating relapse behaviors, limited data exists on how these factors may influence drug-seeking following exposure to multiple risk factors (e.g., drug-associated stimuli and stress). While evidence in male rats has demonstrated that exposure to a stressor potentiates cue-induced reinstatement of ethanol- (Liu and Weiss 2002), heroin- (Banna et al. 2010), and cocaine-seeking (Buffalari and See 2009; Feltenstein and See 2006), no studies to date have examined stress and cue interactions in female rats. Here, we examined the separate and interactive effects of previously cocaine-paired stimuli and a pharmacological stressor (yohimbine) on the reinstatement of cocaine-seeking in freely cycling female rats. Yohimbine is a norepinephrine (NE) α2 receptor antagonist that has been shown to produce stress-like responses (e.g., increases in subjective anxiety, blood pressure, and sympathetic symptoms) in humans (Charney et al. 1983; Holmberg and Gershon 1961) and to induce drug-craving in abstinent drug-dependent subjects (Stine et al. 2002). Similarly, yohimbine produces stress activation (e.g., increases in plasma corticosterone, arterial blood pressure, heart rate, and potentiated startle response) in animals (Davis et al. 1979; Lang and Gershon 1963; Suemaru et al. 1989) and reinstates drug-seeking for heroin (Banna et al. 2010), alcohol (Gass and Olive 2007; Le et al. 2005), methamphetamine (Shepard et al. 2004), and cocaine (Anker and Carroll 2010; Bongiovanni and See 2008; Brown et al. 2009; Feltenstein and See 2006). We predicted that female rats would show a greater yohimbine-stress enhancement of cue reactivity than males and that this response would vary across the estrous cycle.
Materials and methods
Subjects
Aged-matched male (n=14; initial weight 250–300 g, P59-67) and female (n=47; initial weight 175–225 g, P57-74) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were individually housed in a temperature- and humidity-controlled vivarium on a normal 12 h light-dark cycle with the lights on at 06:00. All experimental procedures (drug self-administration, extinction, and reinstatement testing) occurred between 09:00 and 17:00. Data for the male rats consisted of a subset of animals from a previously published study (Feltenstein and See 2006) that were tested using the same protocols used for the females and included here for comparison of sex differences. Animals were given water ad libitum and maintained on 20–25 g of standard rat chow (Harlan, Indianapolis, IN, USA) per day for the duration of each experiment. Rats were habituated to the vivarium for at least 4 days prior to the start of the experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and conformed to federal guidelines as described in the “Guide for the Care and Use of Laboratory Rats” of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP), followed by equithesin (sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution, IP) and chronic indwelling catheters were implanted into the right jugular vein using previously described methods (Feltenstein and See 2006). Catheter patency was maintained by flushing with 0.1 ml of 10 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ, USA) immediately prior to self-administration sessions with a 0.1 ml antibiotic solution of cefazolin (Schein Pharmaceuticals, Florham Park, NJ, USA; 10 mg/ml dissolved in 70 U/ml heparinized saline) and 0.1 ml 70 U/ml heparinized saline regimen following each session. Stylets were inserted into the catheters when the rats were not connected to the infusion pumps. For assessment of catheter patency, rats occasionally received a 0.1 ml intravenous (IV) infusion of methohexital sodium (Eli Lilly, Indianapolis, IN, USA; 10 mg/ml dissolved in 0.9% physiological saline), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
Cocaine self-administration
Rats lever pressed for cocaine in standard self-administration chambers linked to a computerized data collection program (MED-PC, Med Associates Inc., St. Albans, VT, USA). The chambers were equipped with two retractable levers, a white stimulus light above each lever, a tone generator, and a white house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% physiological saline; cocaine provided by the National Institute on Drug Abuse, Research Park Triangle, NC, USA) during daily 2-h sessions according to an FR1 schedule of reinforcement. At the start of each session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active (i.e., cocaine-paired) lever resulted in a 2-s activation of the infusion pump (0.15 mg and 0.125 mg cocaine per 50 µl infusion for the males and females, respectively, resulting in approximately 0.5 mg/kg/infusion) and a 5-s presentation of a stimulus complex, consisting of illumination of the white stimulus light above the active lever and activation of the tone generator (4.5 kHz, 78 dB). After each infusion, responses on the active lever were recorded, but resulted in no consequences during a 20-s time-out period. Inactive lever responses were also recorded, but had no programmed consequences. All self-administration sessions were conducted 6 days per week to criterion (i.e., ≥ 10 infusions per session) for a total period of 10–14 days.
Extinction and reinstatement of cocaine-seeking
Following chronic self-administration and before the first reinstatement test, animals underwent daily 2-h extinction sessions. During each session, responses on both levers were recorded, but had no consequences. Once active lever responding extinguished to criterion (i.e., a minimum of seven extinction sessions with ≤ 25 active lever responses per session for two consecutive days), each animal underwent six separate within subjects tests to examine yohimbine and cue-induced reinstatement of responding. We have previously found no decrement in responding when the number of tests is limited to a total of three for a given reinstatement condition (Feltenstein et al. 2007; Feltenstein and See 2006; Kippin et al. 2005). Thirty min prior to a reinstatement test, each rat received an IP injection of sterile distilled water (vehicle) or yohimbine hydrochloride (1.25 or 2.5 mg/kg; Sigma-Aldrich, Saint Louis, MO, USA). The doses were selected based on previous studies in rats (Feltenstein and See 2006; Le et al. 2005; Shepard et al. 2004) and were given in a counterbalanced order based on active lever responding during the last three days of cocaine self-administration. For half the animals, responses on the active lever for the first three reinstatement tests resulted in 5 s presentation of the previously cocaine-paired light + tone cues in the absence of cocaine reinforcement (i.e., “Cue” reinstatement tests), while active lever responding for the last three reinstatement tests had no programmed consequences (i.e., “No Cue” reinstatement tests). For the remaining animals, the situation was reversed. The order of yohimbine treatment for each animal was the same for both sets of reinstatement tests and animals underwent extinction sessions between reinstatement tests until they reached criterion (i.e., ≤ 25 active lever responses per session for two consecutive days).
Estrous cycle monitoring
In order to ascertain estrous cycle stages, vaginal lumen samples were collected immediately prior to and following each reinstatement test. However, to habituate females to the vaginal cytology procedure, samples were taken daily across the entire experiment. Vaginal lumen samples were collected by gently flushing 30 µl of double distilled water and extracting the sample using a micropipette and 100 µl pipette tips. Samples were placed on glass slides, stained using Quick-Dip Hematology Stain (Mercedes Medical, FL, USA), examined using a light microscope set to 10× magnification, and classified according to previously published criteria (Marcondes et al. 2002). The proestrus phase was defined as the presence of more than 75% nucleated epithelial cells. The estrous phase (note: vaginal estrus as opposed to behavioral estrus) was defined as the presence of more than 75% anucleated cornified epithelial cells. The diestrus I (also known as metestrus) phase was defined as the presence of approximately equal proportions of nucleated epithelial cells, anucleated cornified epithelial cells and leukocytes. The diestrus II phase was defined as containing a majority of leukocytes, a minimum amount of cells, and occasional epithelia. Due to the lower number of animals from which diestrus smears were obtained and the lack of behavioral differences between females in the diestrus I and II states, females in the two diestrus phases were combined in statistical analyses and are hereafter referred to as diestrus I/II.
Data analysis
To determine if any pre-existing sex differences existed, independent t tests were used to analyze lever responses and cocaine intake (mg/kg) during the last three days of cocaine self-administration, as well as the number of days of cocaine self-administration and extinction, with sex as the between-subjects factor. Mixed factors repeated-measures analyses of variance (ANOVA) were used to analyze lever responses during extinction with sex and session as the between- and within-subjects factors, respectively. For reinstatement testing, three-way ANOVAs were used to analyze lever responses with sex, reinstatement test condition, yohimbine dose, and estrous phase as factors, where appropriate. Significant interactions were further investigated using ANOVAs or independent t tests, where appropriate. Data points were eliminated if they were 2.5 standard deviations beyond the group mean. All post hoc analyses were conducted using Student-Newman-Keuls with the alpha set at 0.05, and only significant F or t values are presented.
Results
Cocaine self-administration and extinction
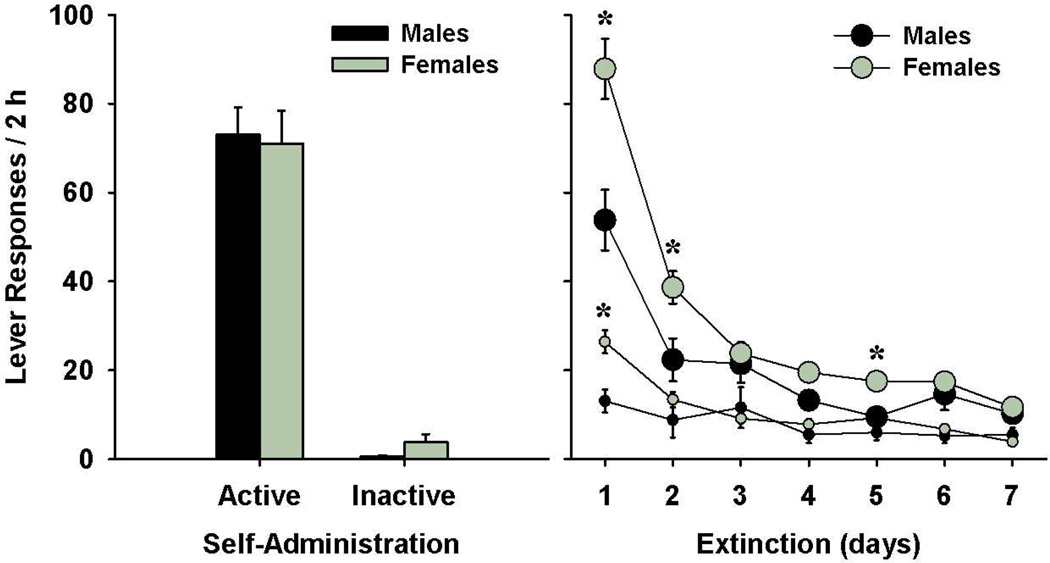
Rats readily acquired cocaine self-administration, responded preferentially on the cocaine-paired lever, and displayed stable lever responding and cocaine intake. No pre-existing differences were found between the male and female rats for active and inactive lever responding (Fig. 1) and the mean cocaine intake (mg/kg) across the last 3 days of cocaine self-administration, as well as total number of days of cocaine self-administration (Table 1). Inactive lever responding was uniformly very low in both males and females across all phases of the study and failed to demonstrate any substantial changes in response to yohimbine and/or cues. Thus, while inactive lever responding is shown in the figures for comparison purposes, no statistical analyses are presented.
Fig. 1.
Cocaine self-administration and extinction for male (n = 14) and female (n = 47) rats. Left panel: Average active (top) and inactive lever responding (mean ± SEM) for the last three days of cocaine self-administration. Right panel: Active (large symbols) and inactive (small symbols) lever responding for the first seven days of extinction. Significant sex differences in responding are indicated (*p<0.05).
Table 1.
Average cocaine intake across the last three days of cocaine self-administration, the total number of cocaine self-administration sessions, and the total number of extinction sessions prior to reinstatement testing. Data are expressed as mean ± SEM.
| Self-administration | Extinction | ||
|---|---|---|---|
| Cocaine intake (mg/kg) | Total # of sessions | Total # of sessions | |
| Group | |||
| Males (n=14) | 19.54 ± 0.92 | 10.79 ± 0.32 | 7.12 ± 0.11 |
| Females (n=47) | 20.90 ± 0.78 | 11.51 ± 0.23 | 7.63 ± 0.16 |
Following the removal of cocaine reinforcement, lever responding in both males and females decreased steadily over the course of daily extinction sessions (Fig. 1). Similar to previous studies (Fuchs et al. 2005b; Kippin et al. 2005), female rats exhibited more responding on the active lever than males during early extinction; however, there was no difference between the two groups in the number of sessions required to reach the extinction criterion (Table 1). A 2 × 7 ANOVA of active lever responding during the first seven extinction sessions revealed significant main effects for sex (F(1,59)=7.57, p<0.01), session (F(6,354)=52.98, p<0.001), as well as a significant sex by session interaction (F(6,354)=4.16, p<0.001). Further analyses showed that female rats responded more on the active lever than males during the first, second and fifth extinction sessions (ts(59)=2.25–2.61, ps<0.05). Moreover, one-way simple effects ANOVAs revealed a significant effect of session for the males (F(6,91)=14.96, p<0.001) and females (F(6,322)=63.97, p<0.001), with greater active lever responding during the first session for both groups and for the second session for females (ps<0.05).
Reinstatement of cocaine-seeking
Data for both males and females were examined for possible order effects of reinstatement testing or drug treatment. Analyses failed to reveal any significant order effects of reinstatement testing (“No Cue”, “Cue”) or dose treatment (0, 1.25, 2.5 mg/kg yohimbine). As such, the data were collapsed within sex group and across test day order.
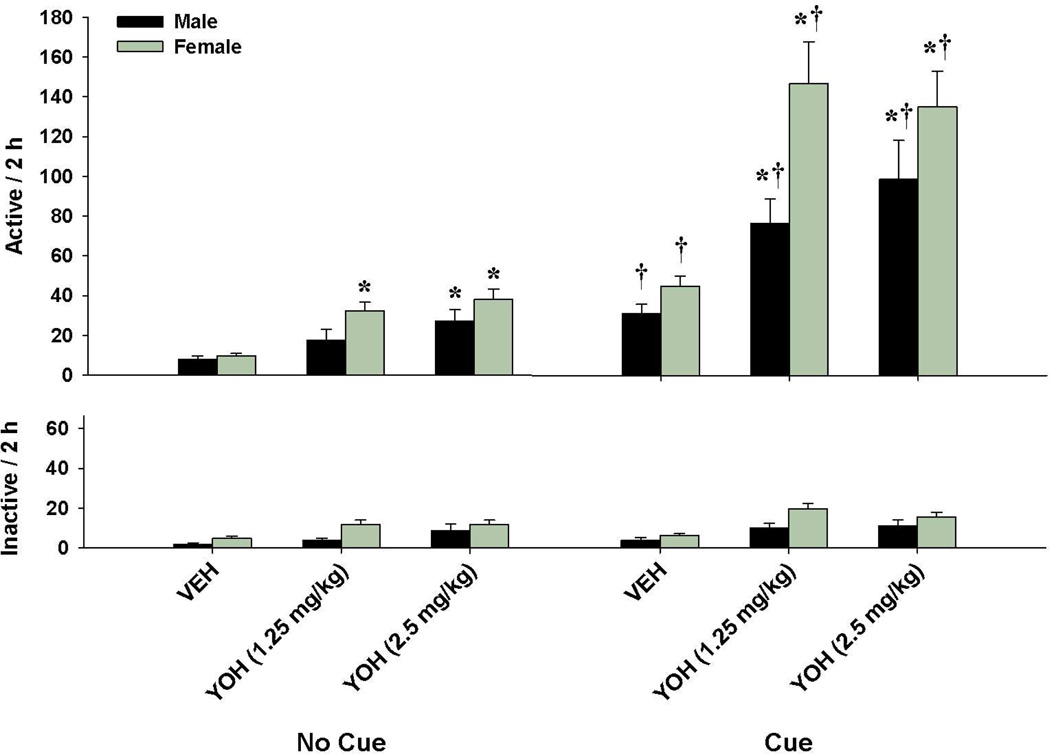
Compared with the “No Cue”-vehicle condition (i.e., extinction responding), yohimbine resulted in 3–5 times higher responding for both males and females under the “No Cue” reinstatement condition (i.e., stress-induced reinstatement; Fig. 2). Similar increases in responding were seen for both groups under the “Cue”-vehicle condition (i.e., cue-induced reinstatement). However, yohimbine + cues robustly enhanced responding in both groups, particularly in females. A 2 × 2 × 3 ANOVA of active lever responding revealed significant main effects for sex (F(1,341)=6.89, p<0.01), cue (F(1,341)=50.27, p<0.001), and dose (F(2,341)=11.96, p<0.001), as well as a significant cue by dose interaction (F(2,341)=4.02, p<0.05). The sex by cue, sex by dose, and sex by cue by dose interactions were not significant.
Fig. 2.
Yohimbine enhancement of cue-induced reinstatement in male (n = 14) and female (n = 47) rats. Active (top) and inactive (bottom) lever responding (mean ± SEM) are shown for the two groups following vehicle or yohimbine injection. Significant differences are indicated for vehicle vs. yohimbine injection (*p<0.05) and for the “No Cue” vs. “Cue” presentation (†p< 0.05).
To further examine the cue by dose interaction, 2 × 3 ANOVAs of active lever responding for the male and female rats revealed significant main effects for cue (Fs(1,78-266)=39.77–68.77, ps<0.001) and dose (Fs(2,78-266)=9.77–16.63, ps<0.001), as well as significant cue by dose interactions (Fs(2,78-266)=3.15–5.86, ps<0.05). One-way ANOVAs for both sexes also revealed significant main effects for dose during the “No Cue” (Fs(2,38-130)=4.63–14.04, ps<0.05) and “Cue” (Fs(2,38-136)=6.42–11.64, ps<0.05) reinstatement tests. Post hoc analyses showed significant increases after 1.25 mg/kg yohimbine during the “Cue” reinstatement test (p<0.05), and after 2.5 mg/kg yohimbine during both reinstatement tests (ps<0.05) in the male rats, and for both yohimbine doses during each reinstatement condition in the female rats (ps<0.05). Cue-induced reinstatement was also evident in both sexes as a significant increase was seen during the “Cue” reinstatement test relative to “No-Cue” (ts(26–87)=4.67–6.66, ps<0.001). Moreover, analyses for each yohimbine treatment condition revealed enhanced responding during the “Cue” reinstatement tests for both sexes (ts(24–90)=3.40–5.20, ps<0.005).
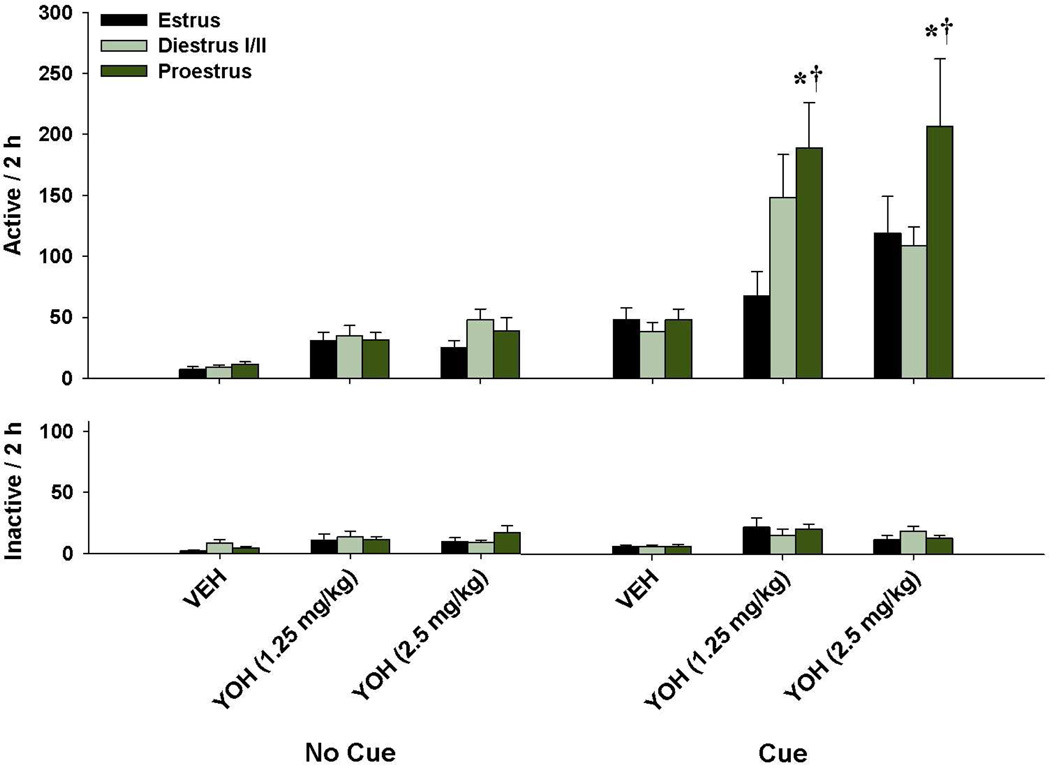
When reinstatement was examined across the different phases of the estrous cycle, an enhancement in active lever responding was noted specifically for rats in proestrus when yohimbine was combined with the previously cocaine-paired cues (Fig. 3). A 2 × 3 × 3 ANOVA of active lever responding revealed significant main effects for cue (F(1,254)=68.95, p<0.001), dose (F(2,254)=16.80, p<0.001), and estrous phase (F(2,254)=4.97, p<0.01), as well as significant cue by estrous phase (F(2,254)=4.10, p<0.05) and cue by dose (F(2,254)=5.58, p<0.005) interactions. The dose by estrous phase and cue by dose by estrous phase interactions were not significant. Post hoc analyses revealed significantly greater reinstatement for female rats in proestrus relative to those in estrus (p<0.01) or diestrus I/II (p<0.05), as well as both doses of yohimbine relative to vehicle (ps<0.001). Based on the significant estrous phase interaction, further analyses for each estrous phase revealed significant main effects for cue (Fs(1,68-94)=18.16–35.87, ps<0.001) and dose (Fs(2,68-94)=4.08–8.71, ps<0.05) for all three estrous cycle phases. However, the cue × dose interaction was only significant for female rats in proestrus (F(2,94)=4.67, p<0.05), with post hoc analyses revealing significant cue and yohimbine-induced reinstatement at the 1.25 and 2.5 mg/kg doses (ps<0.05).
Fig. 3.
Yohimbine enhancement of cue-induced reinstatement in female rats across the estrous cycle. Active (top) and inactive (bottom) lever responding (mean ± SEM) are shown for the three estrous cycle phases (estrus, diestrus I/II, proestrus) following vehicle or yohimbine injection prior to the “No Cue” or “Cue” reinstatement tests (ns=9-22). Significant differences are indicated for vehicle vs. yohimbine injection (*p<0.05) and for the “Cue” vs. “No Cue” presentation (†p< 0.05).
Discussion
Here, we have demonstrated in rats that while both conditioned-cued or yohimbine stress-induced reinstatement showed a similar magnitude in both males and females (i.e., 3–4 times over “No Cue”-vehicle responding), combining these stimuli resulted in a robust enhancement in cocaine-seeking in both groups, but with a greater effect occurring in females (10–12 vs. 14–15 times over No Cue”-vehicle responding for the males and females, respectively). When reinstatement in females was examined as a function of estrous cycle, rats in proestrus displayed the highest levels of responding during yohimbine + cues reinstatement. These results indicate that relative to their male counterparts, female rats show greater sensitivity to stress modulation of cue reactivity.
While a number of studies have utilized intermittent footshock (Brown and Erb 2007; Buffalari and See 2009) and yohimbine (Bongiovanni and See 2008; Feltenstein and See 2006; Kupferschmidt et al. 2009) as a means to study stress-induced reinstatement of drug-seeking in rodents, only one recent study has examined stress-induced reinstatement in females (Anker and Carroll 2010). Relative to males, female rats (cycle phase not determined) showed greater yohimbine-induced reinstatement, an effect consistent with clinical data suggesting that women are more susceptible than men to relapse to cocaine abuse following exposure to a stressor (Hyman et al. 2008; McKay et al. 1996). While we found higher overall levels of reinstatement for female rats in the current study, yohimbine alone (i.e., during the “No Cue” condition) did not appear to significantly increase their level of responding above males. It should be noted that while the male rats were a separate experimental cohort, we have reliably replicated the magnitude of reinstatement in males for cue (current results: 31.2±4.7; Buffalari and See 2009: 26.4±2.5; Feltenstein et al. 2007: 28.4±3.0), yohimbine (current results: 31.1±8.5; Buffalari and See 2011: 23.2±4.3; Bongiovanni and See 2008: 34.8±6.6), and cue + yohimbine (current results: 98.6±19.5; Buffalari and See 2011: 85.3±14.0) reinstatement, suggesting that the observed sex differences are not likely due to experimental procedure, time of year, or seasonal effects. Moreover, the overall enhanced reinstatement noted for the female rats was unlikely due to greater locomotor activity in that a) no significant increases in inactive lever responding occurred, and b) comparable doses of yohimbine (e.g., 2 mg/kg) have actually been shown to decrease locomotor activity (Bowes et al. 1992). While the magnitude of yohimbine-induced reinstatement in the female groups was comparable across the two studies, the significant sex difference noted by Anker and Carroll (2010) was primarily due to levels of responding in males that were lower than those reported previously in response to yohimbine (Banna et al. 2010; Buffalari and See 2011; Shepard et al. 2004). This discrepancy in males may involve procedural differences, including different doses of self-administered cocaine and different yohimbine injection-to-test intervals. Nonetheless, these results overall suggest that female rats may be more sensitive than males to the stress-induced reinstatement effects of yohimbine.
One interesting finding from the current study was the enhancement of stress + cues reinstatement for female rats in proestrus. While the lack of an estrous cycle effect on cue-induced reinstatement is consistent with previous work from our laboratory (Fuchs et al. 2005b), the role of the estrous cycle in mediating stress or stress + cues reinstatement has never been examined. Although there were no cycle differences in stress-induced reinstatement, the enhancement in stress + cues reinstatement for proestrus rats is unique, in that higher levels of cocaine-seeking behavior (e.g., late self-administration, early extinction, cocaine primed-reinstatement, and prolonged withdrawal) have typically been noted for female rats in estrus (Feltenstein et al. 2009; Feltenstein and See 2007; Kerstetter et al. 2008; Kippin et al. 2005). However, basal levels of adrenocorticotrophin hormone (ACTH) and corticosterone have been found to be elevated at the time of proestrus in the rat (Critchlow et al. 1963; Raps et al. 1971). Moreover, when exposed to a stressor, these hormones are highest for females in proestrus (Pollard et al. 1975; Viau and Meaney 1991). In that the stress system has been shown to play a critical role in cue (Goeders and Clampitt 2002) and stress (Erb et al. 1998; Le et al. 2000; Mantsch and Goeders 1999a), but not cocaine-primed (Erb et al. 1998; Mantsch and Goeders 1999b) reinstatement, perhaps enhanced stress hormone levels during proestrus lead to the enhancement in cue + stress reinstatement behavior. Another possible mechanism that may have contributed to this effect could include enhanced striatal DA transport (Thompson and Moss 1997) and uptake sites (Morissette and Di Paolo 1993) during proestrus. Moreover, while relatively selective for NE α2 receptors, the affinity of yohimbine for other receptors (e.g., serotonergic 5-HT1A and dopaminergic D2 receptors) (Millan et al. 2000) may have also contributed to this effect, in addition to an interaction with other systems, including corticotrophin-releasing factor and other neuropeptides (see Shalev et al. 2010, for review).
In that proestrus is characterized by high estradiol levels (see Freeman 1994, for review), estradiol may also have been a contributor to the enhanced reinstatement response. Indeed, women in the luteal phase of their menstrual cycle, when estradiol levels increase, (see Mello and Mendelson 1997, for review) demonstrate enhanced hypothalamic-pituitary-adrenal axis (HPA) mediated responses following exposure to psychological stressors (Kirschbaum et al. 1999; Tersman et al. 1991). In female rats, estradiol replacement has been shown to reverse ovariectomy-induced deficits in ACTH and corticosterone release following an acute stressor (Viau and Meaney 1991), as well as deficits in cocaine-mediated behaviors, including locomotor activity (Sell et al. 2000), self-administration (Grimm and See 1997; Hu et al. 2004; Jackson et al. 2006; Lynch et al. 2001), and reinstatement (Larson et al. 2005). Interestingly, when combined in ovariectomized rats, progesterone reverses these effects of estradiol (Anker et al. 2007; Jackson et al. 2006; Larson et al. 2007; Sell et al. 2000). Furthermore, endogenous plasma levels of progesterone are inversely associated with cocaine-seeking in female rats (Feltenstein and See 2007), while progesterone pretreatment can attenuate cocaine-primed reinstatement (Feltenstein et al. 2009) or cocaine conditioned place preference (CPP) (Russo et al. 2008). Similar clinical results have been reported, in that stress- and cue-induced craving is reduced in women with elevated endogenous levels of progesterone (Sinha et al. 2007), while progesterone pretreatment decreased the subjective effects of cocaine in women (Evans and Foltin 2006; Sofuoglu et al. 2002; Sofuoglu et al. 2004), but not men (Sofuoglu et al. 2004; Sofuoglu et al. 2007). Taken together, these results suggest that estradiol and progesterone may have opposing effects on cocaine-mediated reward and cocaine-seeking behaviors.
The current results support the assertion that women may be more susceptible than men to relapse to cocaine use following exposure to multiple risk factors. While suggestive, our results are limited in that they cannot fully account for the complex interactions of endogenous ovarian hormones as they fluctuate across the cycle in intact animals. It should also be noted that while estradiol may have played a role in mediating reinstatement in the current study, it is also important to note that chronic cocaine exposure has been shown to disrupt estrous cycle activity in primates and rodents (Grimm and See 1997; King et al. 1990; Mello et al. 1997), with previous work in our laboratory demonstrating a decline in the estradiol peak of proestrus during and following chronic cocaine self-administration (Feltenstein and See 2007). Moreover, plasma levels of hormones do not necessarily indicate neuronal alterations that may occur in response to changes in endogenous hormone levels. For example, while data indicates that estradiol and progesterone increase and decrease limbic/striatal DA activity, respectively (Fernandez-Ruiz et al. 1990), these effects only occur 4 h after administration, suggesting a delayed neuronal response to alterations in ovarian hormones. Nonetheless, both preclinical and clinical data indicate that cyclic fluctuations in ovarian hormones may contribute to the motivational effects of cocaine. Future studies should be directed towards understanding how ovarian hormone levels may be used as a means to predict relapse to cocaine-seeking in humans, as well as the efficacy of various drug treatments to attenuate the likelihood of relapse in the animal model and in cocaine-dependent women.
Acknowledgements
This research was supported by NIDA grant DA016511 (RES), NICHHD grant K12 HD055885-01 (MWF), and NIH grant C06 RR015455.
Footnotes
Disclosures/conflict of interest: The authors declare no conflicts of interest.
References
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes MP, Peters RH, Kernan WJ, Jr, Hopper DL. Effects of yohimbine and idazoxan on motor behaviors in male rats. Pharmacol Biochem Behav. 1992;41:707–713. doi: 10.1016/0091-3057(92)90216-3. [DOI] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Erb S. Footshock stress reinstates cocaine seeking in rats after extended post-stress delays. Psychopharmacology (Berl) 2007;195:61–70. doi: 10.1007/s00213-007-0846-4. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D'Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt A, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex differences in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural Brain Research. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Ramos JA. Time-course of the effects of ovarian steroids on the activity of limbic and striatal dopaminergic neurons in female rat brain. Pharmacol Biochem Behav. 1990;36:603–606. doi: 10.1016/0091-3057(90)90262-g. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Freeman M. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill J, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 613–658. [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005a;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005b;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol Clin Exp Res. 2007;31:1441–1445. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- King TS, Schenken RS, Kang IS, Javors MA, Riehl RM. Cocaine disrupts estrous cyclicity and alters the reproductive neuroendocrine axis in the rat. Neuroendocrinology. 1990;51:15–22. doi: 10.1159/000125310. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt DA, Tribe E, Erb S. Effects of repeated yohimbine on the extinction and reinstatement of cocaine seeking. Pharmacol Biochem Behav. 2009;91:473–480. doi: 10.1016/j.pbb.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Li CS, Kemp K, Milivojevic V, Sinha R. Neuroimaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999a;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole does not block cocaine discrimination or the cocaine-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 1999b;64:65–73. doi: 10.1016/s0091-3057(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine's effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J Pharmacol Exp Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Negrete JC, Emil S. Cue-evoked arousal in cocaine users: a study of variance and predictive value. Drug Alcohol Depend. 1992;30:187–192. doi: 10.1016/0376-8716(92)90025-8. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Pollard I, White BM, Bassett JR, Cairncross KD. Plasma glucocorticoid elevation and desynchronization of the estrous cycle following unpredictable stress in the rat. Behav Biol. 1975;14:103–108. doi: 10.1016/s0091-6773(75)90374-0. [DOI] [PubMed] [Google Scholar]
- Raps D, Barthe PL, Desaulles PA. Plasma and adrenal corticosterone levels during the different phases of the sexual cycle in normal female rats. Experientia. 1971;27:339–340. doi: 10.1007/BF02138184. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quinones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Dallman MF, Darlington DN, Cascio CS, Shinsako J. Role of alpha-adrenergic mechanism in effects of morphine on the hypothalamo-pituitary-adrenocortical and cardiovascular systems in the rat. Neuroendocrinology. 1989;49:181–190. doi: 10.1159/000125112. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosom Med. 1991;53:185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229:145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997;44:35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]