Summary
The Escherichia coli dGTP triphosphohydrolase (dGTPase) encoded by the dgt gene catalyzes the hydrolysis of dGTP to deoxyguanosine and triphosphate. The recent discovery of a mutator effect associated with deletion of dgt indicated participation of the triphosphohydrolase in preventing mutagenesis. Here, we have investigated the possible involvement of dgt in facilitating thymine utilization through its ability to provide intracellular deoxyguanosine, which is readily converted by the DeoD phosphorylase, to deoxyribose-1-phosphate, the critical intermediate that enables uptake and utilization of thymine. Indeed, we observed that the minimal amount of thymine required for growth of thymine-requiring (thyA) strains decreased with increased expression level of the dgt gene. As expected, this dgt-mediated effect was dependent on the DeoD purine nucleoside phosphorylase. We also observed that thyA strains experience growth difficulties upon nutritional shift-up and that the dgt gene facilitates adaptation to the new growth conditions. Blockage of the alternative yjjG (dUMP phosphatase) pathway for deoxyribose-1-phosphate generation greatly exacerbated the severity of thymine starvation in enriched media, and under these conditions the dgt pathway becomes crucial in protecting the cells against thymineless death. Overall, our results suggest that the dgt-dependent pathway for deoxyribose-1-phosphate generation may operate under various cell conditions to provide deoxyribosyl donors.
Keywords: dGTPase, E. coli dgt, thyA strains, thymine starvation, nutritional shift, salvage pathways
Introduction
The pathways for biosynthesis of the deoxyribose-5'-triphosphates (dNTPs), the precursors for DNA synthesis, are important for both basic and applied sciences, as the quality and quantities in which these compounds are available for the replication fork directly impact the maintenance of genetic stability and cell viability.
The concentrations of the four canonical dNTPs are carefully regulated, as disturbances of the dNTP pools are mutagenic due to increased replication errors (Kunz et al., 1994). A well-studied case where an extreme imbalance in DNA precursors severely impacts cell physiology is thymineless death, named for the rapid loss of cell viability of thymine-requiring (thyA) strains upon thymine deprivation (Cohen and Barner, 1954; Ahmad et al., 1998). A key factor in the proper balance of dNTPs is the enzyme ribonucleotide reductase (RNR), which reduces ribonucleotides (most often NDPs) to the corresponding deoxynucleotides (Eriksson and Sjöberg, 1989), the so-called de novo pathway for deoxynucleotide synthesis. While dATP, dGTP, and dCTP are generated from the corresponding NDPs by RNR and nucleoside diphosphate kinase, dTTP is synthesized from the non-canonical dNTP, dUTP (Fig. 1). The latter, potentially toxic compound is rapidly converted to dUMP by dUTPase (Kouzminova and Kuzminov, 2004), preventing its incorporation into DNA. dUMP is then converted to dTMP by the enzyme thymidylate synthetase (encoded by thyA), followed by the two-step conversion to dTDP and dTTP.
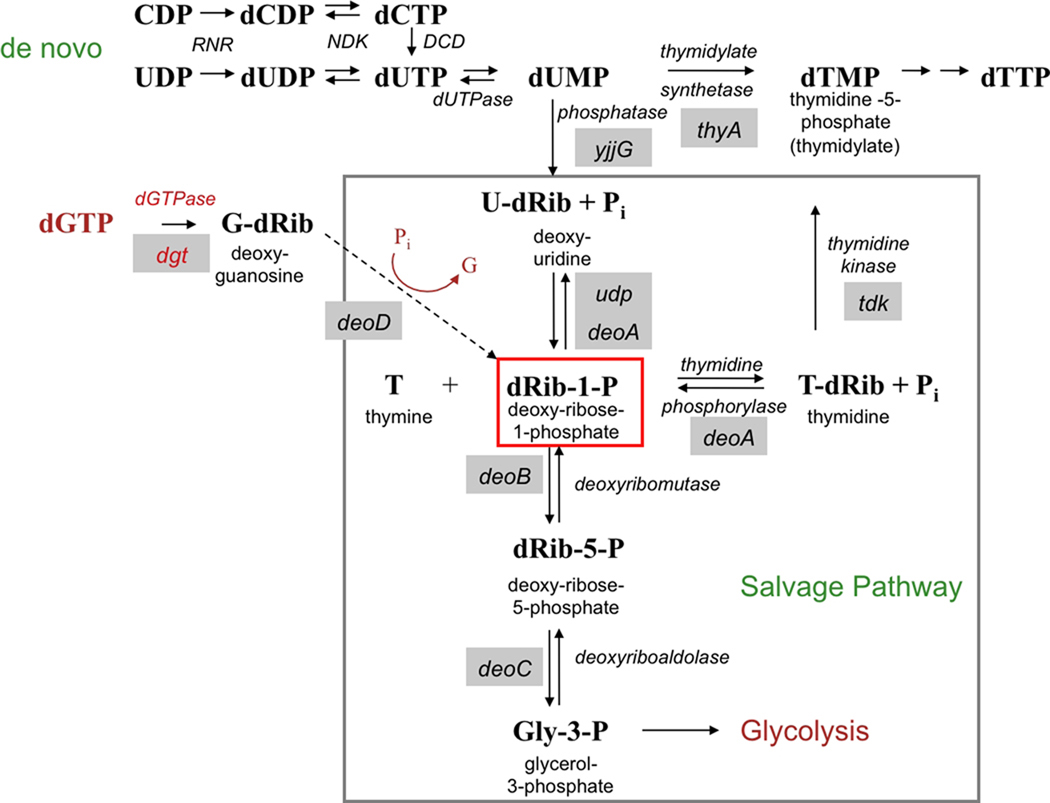
Fig. 1.
de novo and salvage pathways for thymidylate synthesis (adapted from Zaritsky et al., 2006) as present in E. coli. thyA mutants cannot produce thymidylate by the de novo route [mediated by ribonucleotide reductase (RNR), nucleotide diphosphate kinase (NDK) and dCTP deaminase (DCD)] but, in contrast to wild-type cells, they have increased potential for using intra- and extracellular thymine for DNA incorporation. The mechanism involves accumulation of deoxyribose-1-phosphate (dRib-1-P) (central red square) because of increased dUMP hydrolysis due to the thyA block. dUMP phosphatase (encoded by yjjG) converts dUMP to deoxyuridine (U-dRib), which undergoes phosphorolysis to U and dRib-1-P by uridine or thymidine phosphorylase (udp or deoA gene, respectively). dRib-1-P can condense with thymine to generate thymidine in a reaction catalyzed by thymidine phosphorylase (encoded by deoA). Deoxyguanosine (the direct product of the reaction catalyzed by dGTPase (produced by the dgt gene) can further enlarge the dRib-1-P pool through purine nucleoside phosphorylase activity encoded by deoD, thus enhancing thymine incorporation.
Alternatively, deoxynucleotides can be generated via salvage pathways (Fig.1). Such pathways may involve phosphorylation of deoxynucleosides by deoxynucleoside kinases yielding dNMPs. The number of deoxynucleoside kinases varies widely throughout nature. Human cells have a variety of deoxynucleoside kinases with different specificities (Arner and Eriksson, 1995), while insects have only one with broad specificity (Knecht et al., 2000). Much less is known about the diversity of deoxynucleoside kinases in prokaryotic cells (Ives and Ikeda, 1998; Andersen and Neuhard, 2001). Escherichia coli possesses only one type of deoxynucleoside kinase - thymidine kinase. Thus, among the four conventional deoxynucleosides only thymidine can serve as a specific DNA precursor in this organism (Neuhard and Kelln, 1996).
In addition to the activities described above ensuring adequate amounts of DNA precursors, a second issue important for genetic stability is the purity of the dNTP pools. For example, cells possess several dNTP-hydrolyzing enzymes that are essential for sanitizing the dNTP pool from damaged or modified derivatives (Galperin et al., 2006). Incorporation of such alternative dNTPs into the DNA can cause increases in mutation rate due to their ambiguous base-pairing properties (Kamiya, 2003).
One unusual class of dNTP-hydrolyzing enzymes is the dNTP triphosphohydrolases, which hydrolyze dNTPs to the corresponding deoxynucleoside and triphosphate (PPPi). Four members of this group have been investigated in some detail: the E. coli dgt gene product, also referred to as dGTPase (Beauchamp and Richardson 1988; Seto et al., 1988), the Thermus thermophilus TT1383 protein (Kondo et al., 2004), and two triphosphohydrolases from Pseudomonas aeruginosa (P. aeruginosa) PA1124 and PA3043 (Mega et al., 2009).
E. coli dGTPase and P. aeruginosa PA1124 have a preference for hydrolyzing dGTP. The physiological importance of these enzymes is still unclear. However, it has been shown that loss of E. coli dgt leads to a 2-fold increase in the cellular dGTP level (Quirk et al., 1990), while its overexpression (in optA1 strains, which carry a (up) mutation in the dgt promoter) leads to a 30- to 50-fold increased amount of dGTPase (Quirk et al., 1990; Wurgler and Richardson, et al., 1990) and a 5-fold decrease in the dGTP level (Meyers et al., 1987). Recently, our laboratory showed that deletion of the dgt gene results in a mutator phenotype leading, in particular, to an enhancement of G·C→C·G and A·T→G·C base-pair substitutions on an F' episome (Gawel et al., 2008). This observation suggested the likely involvement of the dgt gene in DNA replication fidelity, presumably through its effect on the cellular dNTP pools (Gawel et al., 2008).
In the present study, we have investigated another possibility for the cellular function of the dgt gene, namely, involvement of the dGTPase reaction in thymine salvage. Thymine salvage is the critical pathway for dTTP production in thyA strains, which lack the enzyme thymidylate synthetase of the de novo synthesis pathway. The utilization of external thymine involves its condensation with the internal precursor deoxyribose-1-phosphate by thymidine phosphorylase (deoA gene product) yielding thymidine (see Fig. 1). A major source of the intracellular deoxyribose-1-phosphate in thyA strains has been reported to be the dUMP phosphatase pathway encoded by the yjjG gene (Weiss, 2007a). YjjG hydrolyzes dUMP to deoxyuridine (U-dRib), which is subsequently converted to deoxyribose-1-phosphate by DeoA or uridine phosphorylase (Udp) (Fig. 1). The importance of this pathway in thyA strains reflects, in part, the accumulation of dUMP (Kwon et al. 2010) in these strains resulting from the block of the thyA thymidylate synthetase pathway (Fig. 1).
Previous studies in thyA mutants supplemented with thymine have indicated that it is the size of the deoxyribose-1-phosphate pool that ultimately limits the rate of conversion of thymine to thymidine and, hence, the rate of incorporation into DNA (Kammen, 1967; Beacham et al., 1971; Jensen et al. 1973). Thus, the intracellular deoxyribose-1-phosphate pool determines the external thymine concentration minimally required needed to support growth of thyA mutants. It has also been shown that thymine incorporation can be stimulated by exogenous deoxynucleosides like G-dRib or deoxyadenosine (Kammen, 1967; Jensen et al. 1973), an effect presumably related to the ability of these agents to elevate the deoxyribose-1-phosphate pool (via the DeoD purine nucleoside phosphorylase reaction) (see Fig. 1). Within the context of this model, one may hypothesize that the internal deoxyribose-1-phosphate pool may also be determined, at least in part, by the production of G-dRib through the dgt pathway, and that the minimal external thymine concentration required for thyA mutants would be dependent on the Dgt activity. To test this hypothesis, we generated ΔthyA mutants in strains with different dgt expression levels and investigated their thymine requirements. Our results show that the ability of cells to take up and metabolize extracellular thymine correlates with dgt expression level, most logically due to its ability to generate intracellular deoxyribosyl donors.
Results
Thymine requirements of thyA strains with different dgt expression levels
To test the hypothesis that the dgt gene provides an intracellular G-dRib source that can be used to generate deoxyribose-1-phosphate for thymine incorporation (Fig. 1), we investigated several dgt thyA strains, with different dgt expression levels, for the minimum thymine concentration required for their growth. Specifically, we compared the dgt+ wild-type strain to a dgt null mutant (dgt::mini-Tn10kan) or the dgt overexpressor specified by the optA1 allele. The latter carries a single base change in the dgt promoter creating an enhanced expression allele (Beauchamp, et al., 1988; Quirk et al., 1990). We also tested the interaction between the dgt alleles and the deoD gene encoding the purine nucleoside phosphorylase responsible for the downstream conversion of G-dRib to deoxyribose-1-phosphate and guanine (see Fig. 1).
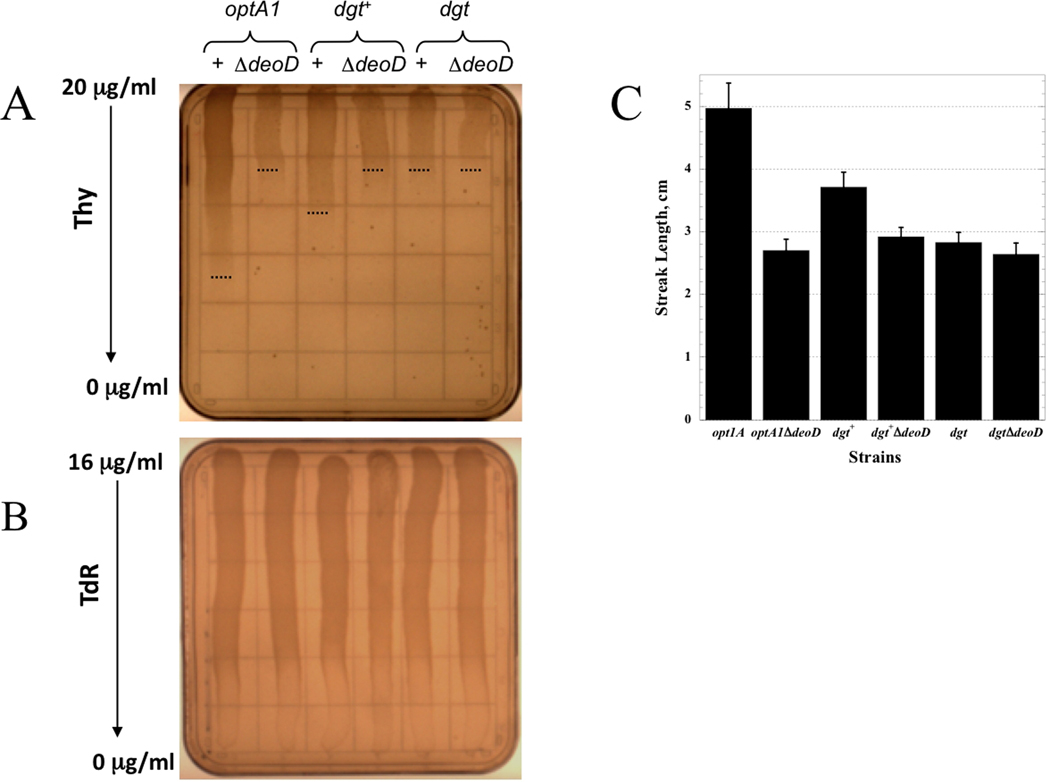
A continuous range of thymine concentrations was tested using thymine gradient plates. As shown in Fig. 2A, these plates clearly showed a positive correlation of growth at lower thymine concentrations with increased dgt expression: the least growth was seen in the dgt strain, while the most growth occurred for the overproducing optA1 strain. In contrast, no such correlation was seen in the corresponding deoD mutants, where the streak lengths do not vary significantly with the dgt level and coincide approximately with that of dgt deoD+ strain. The data are presented quantitatively in the histogram of Fig. 2C. In contrast, no growth differences were observed on plates containing a gradient of thymidine instead of thymine (Fig. 2B).
Fig. 2.
Thymine requirements of thyA and thyA deoD strains with different dgt expression levels as determined on thymine gradient plates. Suspensions of the indicated cultures were streaked on minimal-medium plates containing a gradient from top to bottom of decreasing thymine or thymidine concentrations. A. Thymine gradient from 20 to 0 µg/ml. B. Thymidine gradient from 16 to 0 mg/ml. The thymidine gradient plate also contained CAA. C. Histogram of observed growth (streak lengths) of the corresponding strains on thymine gradient plates. A total of ten plates was used for standard error calculation.
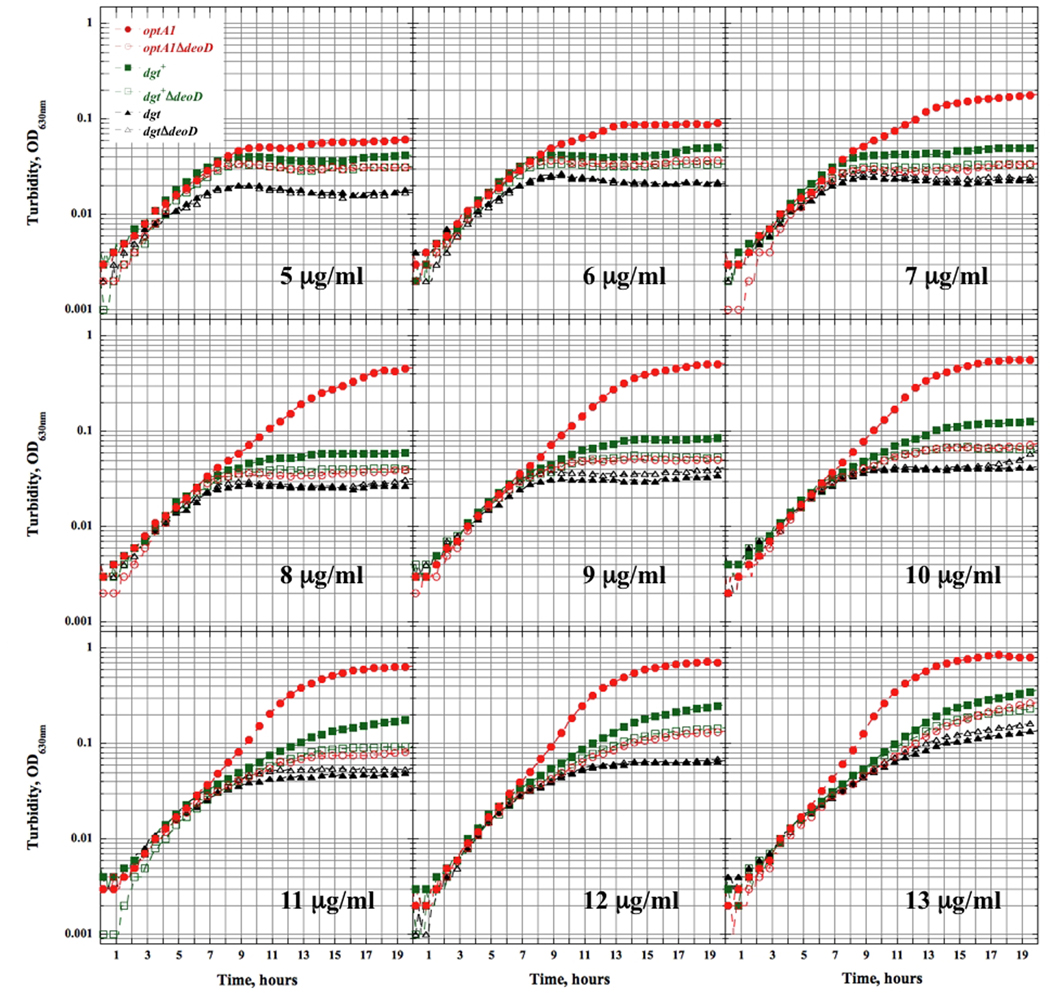
The same trend was seen in a series of experiments using minimal-medium liquid batch cultures supplied with increasing thymine concentrations (Fig. 3). In the deoD+ series, the optA1 strain attained the largest final yield after 19 hours, followed by the dgt+ strain, and the dgt strain showing the lowest yield. In the ΔdeoD series, the optA1 and dgt+ derivatives reached a similar low level, while the dgt derivative attained the lowest yield, which coincides with that of the dgt deoD+ strain.
Fig. 3.
Growth of thyA and thyA deoD strains with different dgt expression levels in liquid minimal medium containing indicated concentrations of thymine.
These combined data clearly suggest that the dGTP triphosphohydrolase encoded by the dgt gene (along with the DeoD purine nucleoside phosphorylase) is an important factor in facilitating thymine incorporation through the production of deoxyribose-1-phosphate from dGTP.
Thymine requirements in the ΔyjjG background
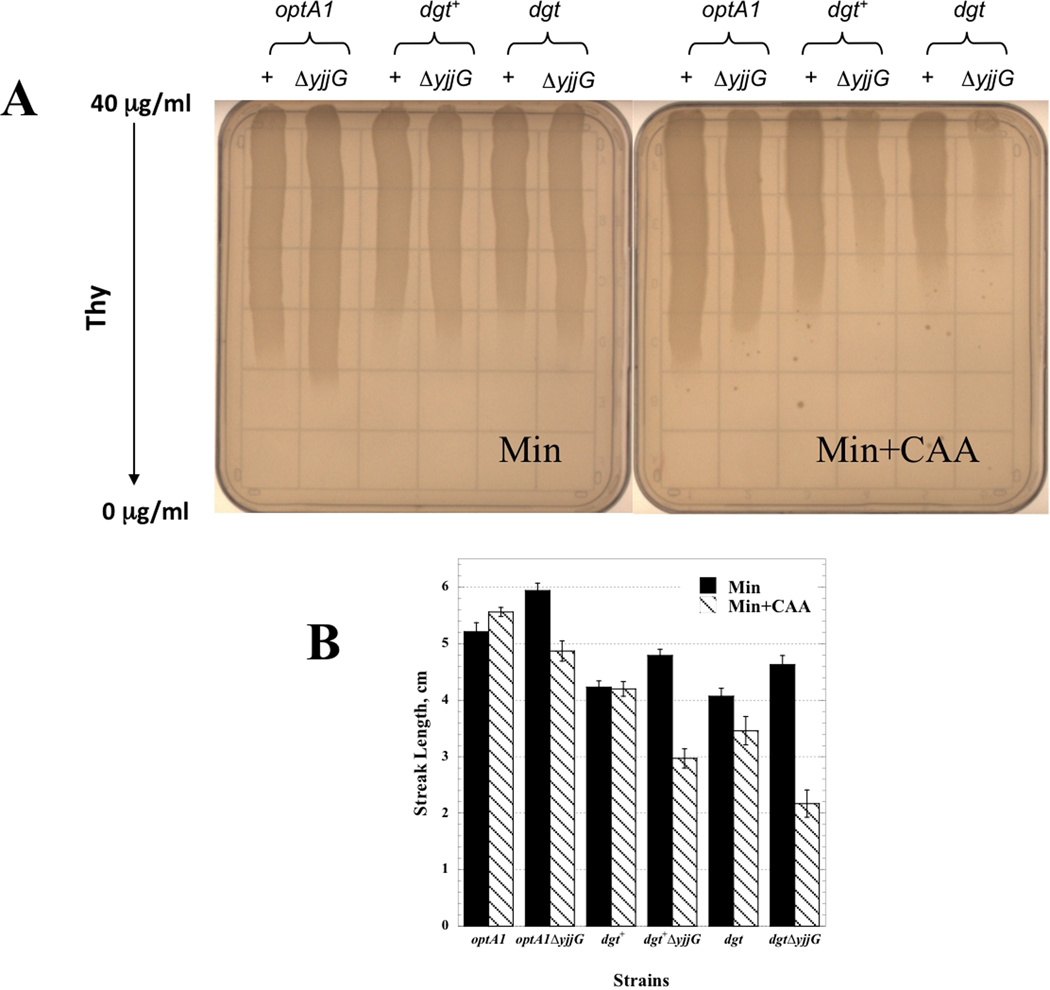
The thymine requirements of thyA strains were also tested under potentially more stringent conditions. For that purpose, we additionally introduced a yjjG deletion into the strains. The yjjG gene encodes a (d)NMP phosphatase (nucleotidase), which has been shown to be responsible, at least in part, for the hydrolysis of the dUMP that accumulates in thyA mutants, yielding deoxyuridine (U-dRib) (Weiss 2007a). The U-dRib is subsequently used to generate deoxyribose-1-phosphate by DeoA (see Fig. 1). In our experiments, we observed little or no effect of the yjjG deletion under the standard conditions of minimal medium (in fact, a slight enhancement of growth may be seen) (Fig. 4A left panel), suggesting that under these conditions the yjjG pathway may, in fact, be minor relative to the dgt pathway. However, when the medium was enriched with casamino acids (CAA) to speed up bacterial growth, the thymine requirements of all yjjG strains were clearly increased, consistent with the previous report (Weiss 2007a) and indicating the importance of this pathway. Importantly, also in this ΔyjjG background, the optA1 derivative shows the most growth, followed by dgt+, with the least growth observed for the dgt strain (Fig. 4A right panel). Thus, also under conditions of rapid growth the dgt pathway makes an important contribution to the intracellular generation of deoxyribose-1-P.
Fig. 4.
Thymine requirements of thyA and thyA yjjG strains with different dgt expression levels determined on thymine gradient plates. A. Suspensions of the indicated cultures were streaked on minimal-medium plates (Min) or minimal-medium plates supplemented with 0.5% casamino acids (Min + CAA). The plates contained a gradient from top to bottom of decreasing thymine concentration from 40 to 0 µg/ml. B. Histogram of streak lengths (extent of growth) for each of the strains: black bars, (Min); dashed bars, (Min+CAA). A total of ten plates was used for standard error calculation. The modest difference between the dgt and dgt+ strains on the Min plate (top left) (0–40 µg/ml gradient) contrasts with the very obvious difference observed between the two strains on the (0–20 µg/ml) gradient plate (Fig. 2A). This phenomenon, observed repeatedly, is likely related to the difference in steepness of the gradients, where a steeper gradient is predicted to diminish the apparent difference in growth (i.e., streak length).
Viability of thymineless dgt strains upon nutritional shift-up
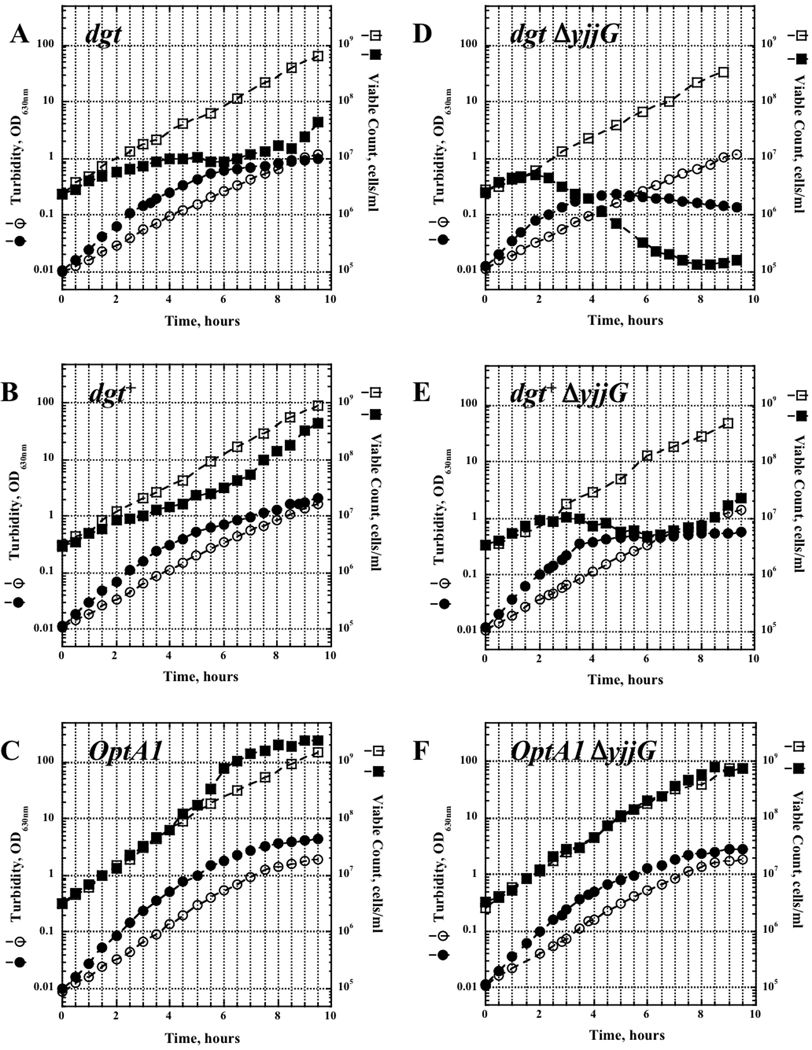
The experiments described above reveal the extra demands that casamino acid-enrichment of the medium places on the thymine requirements of thyA strains. To further investigate this phenomenon, we applied a nutritional shift-up to a series of minimal medium batch cultures growing at steady state (Fishov et al., 1995). A thymine concentration of 20 µg/ml was chosen for this experiment. We followed both the total biomass (OD630) and the total viable cell count. Each of the strains responded to the addition of casamino acids by an immediate 1.5- to 1.9-fold increase in rate of biomass growth (OD630) (Fig. 5 circles). For example, the wild-type (dgt+) strain increased its biomass growth rate from 0.86 doublings/hour to 1.28 doublings/hour upon addition of the casamino acids. However, only the optA1 mutant (panel C) succeeded ultimately to increase its rate of cell division (increase in viable count, closed squares) commensurate with the new nutritional conditions. This acceleration of division rate was observed after a 4-hr period of maintaining the original division rate (rate maintenance, see Kjelgaard et al., 1958, and Discussion). In contrast, no increase in the rate of cell division is seen in the dgt+ and dgt strains (panels A and B). In fact, in the dgt strain cell divisions are completely halted after two hours (panel A), while in the dgt+ strain they slow down and manage to regain the pre-shift rate only after several hours (panel B). Addition of ΔyjjG to the above mutants greatly exacerbates the deleterious consequences of the medium enrichment. The dgt ΔyjjG strain likely undergoes lysis, reflected by the decrease in optical density of the culture approximately two hours after shift-up and a corresponding drop in cell viability (panel D). As thymine starvation is known to lead to SOS induction and prophage induction (Korn and Weissbach, 1962), the strains were checked for the presence of phage under various conditions, including after UV irradiation. No phage was found. Cell divisions of the dgt+ ΔyjjG strain (panel E) stop two hours after the shift-up, and the viable count shows a very slight decrease after four hours, switching to a slow rise after 8 hours possibly due to amplification of preexisting mutants or suppressors. Only the optA1 ΔyjjG strain keeps dividing (panel F), although with the pre-shift rate, and it fails to accelerate the division rate to the level seen in the yjjG+ counterpart. Thus, health and viability of the strains under these conditions is clearly affected by the status of dgt.
Fig. 5.
Biomass growth (OD630) (circles) and viable count (squares) of various indicated thyA strains upon a nutritional shift-up from minimal medium (open) to minimal medium supplemented with 1% casamino acids (closed) at time 0. The thymine concentration was 20 µg/ml in all cases.
Discussion
In this study we describe a second in vivo function of the dgt-encoded dGTPase, in addition to its previously reported role in reducing the frequency of spontaneous mutations (Gawel et al., 2008). This second role is its key participation in a newly described pathway for maintaining the intracellular pool of deoxyribose-1-phosphate (dRib-1-P), the availability of which is the critical parameter for thymine uptake and utilization in thymine-requiring cells.
There is no active transport system for thymine in E. coli (Crawford, 1958; Jensen et al. 1973), and its uptake by diffusion and its utilization is directly coupled to its condensation with intracellular dRib-1-P yielding thymidine. Wild-type cells have a very small pool of dRib-1-P and are, therefore, not able to utilize exogenously provided thymine effectively. In thyA strains, the pool of dRib-1-P is increased due to the accumulation and breakdown of deoxyuridine-5'-phosphate (dUMP) (Fig. 1) (Jensen et al. 1973; Kwon et al. 2010). The minimal thymine concentration required for growth of this kind of strain is about 20 µg/ml. Blockage of the catabolism of dRib-1-P by defects in the deoB or deoC genes (thyA deoB or thyA deoC strains) results in further enlargement of the dRib-1-P pool size (Jensen et al. 1973) and a correspondingly lower minimally required concentration of thymine (as low as 2 µg/ml) (Neuhard and Kelln, 1996). (These strains are called thymine low-requirers) (Ahmad and Pritchard, 1971; Neuhard and Kelln, 1996). The thymine requirement may be further lowered by increasing the expression level of the deo operon (deoCABD). This operon is under double negative control by the DeoR and CytR repressor proteins (Valentin-Hansen et al., 1978). Inactivating these repressors, such as in a triple thyA deoB deoR or thyA deoB cytR mutant, lowers the thymine requirement to only 0.2 µg/ml (Ahmad and Pritchard, 1971; Neuhard and Kelln, 1996) (thymine super-low requirers), as these mutants have both an increased dRib-1-P pool and elevated level of thymidine phosphorylase (DeoA).
The present study demonstrates that the thymine requirement of thyA mutants is controlled additionally by two genes, dgt and deoD, that participate in the two sequential steps converting dGTP into dRib-1-P (Fig. 1). Successive increases in the dgt expression level in the order dgt, dgt+, optA1 lead to a corresponding decline in thymine requirements of a thyA strain, consistent with a progressively increased dRib-1-P pool mediated by the intracellular production of deoxyguanosine (G-dRib). Furthermore, blockage of the downstream conversion of G-dRib into guanine and dRib-1-P in the deoD mutant negates the effects of the dgt expression and returns the thymine requirement to approximately the same, high level (Fig. 2). The slightly higher cell yield obtained in dgt+ΔdeoD and optA1ΔdeoD strains compared to the dgt ΔdeoD strain in the liquid growth experiments (Fig. 3) may be reasonably explained by the activity of some non-specific phosphorylase capable of cleaving accumulated G-dRib, thus contributing to the improvement in bacterial growth on low thymine.
The importance of the dGTP pathway is also readily visible in the nutritional shift-up experiments. When a culture growing in steady state (Fishov et al., 1995) is suddenly enriched by the addition of extra nutrients, the culture undergoes an orderly and highly reproducible series of changes (Neidhardt et al.,1990). The growth rate for total biomass increases immediately to a higher value that is consistent with the new nutritional value of the medium. However, the rate of cell division does not change for a period of C + D min, where C (chromosome time) is the time needed to complete a full round of chromosome replication and D (division time) is the time interval between chromosome termination and cell division. This phenomenon is called “rate maintenance” (Kjeldgaard et al., 1958). It is due to the fact that, while nutritional shift-ups accelerate total DNA synthesis by increasing the frequency of initiations at the chromosomal origin, the corresponding cell divisions are not triggered until these new rounds of replication have completed (C + D) (Helmstetter et al., 1968). In our case of the thyA mutants, only the optA1 derivative demonstrates the expected phenomenon: the pre-shift division rate is maintained for a period of 4–5 hours, after which it accelerates (Fig. 5A). [We also note that this period of rate maintenance is rather long, compared to wild-type strains (Helmstetter et al., 1968; Helmstetter, 1996). We may assume this is due to the very long C time period (slow intrinsic replication rate) that is characteristic of thymineless mutants under conditions of limiting thymine (Pritchard and Zaritsky, 1970)]. In contrast, the dgt and dgt+ derivatives of the thyA strain do not show rate maintenance and actually reduce their division rate 90–120 min after shift-up (Fig. 5A and B, filled squares), with some slow recovery in the dgt+ strain (Fig. 5B) and a complete arrest in the dgt derivative (Fig. 5A). These observations are indicative of the difficulties faced by these strains in maintaining their dTTP pools and DNA synthesis rate when additional replication forks are initiated by the shift up.
A large portion of the pool of dRib-1-P in thyA strains has been reported to be derived from dUMP mediated by the dUMP phosphatase encoded by the yjjG gene (Weiss, 2007a) (see also Fig. 1). Indeed, both our enriched medium experiments (Fig. 4) and nutritional shift-up (Fig. 5) experiments clearly show the negative consequences of loss of the yjjG pathway upon thymine limitation. The progressive loss of viable count that we observe for the dgt ΔyjjG double mutant likely represents a case of thymineless death (Cohen and Barner, 1954; Ahmad et al., 1998). In this case, the extreme lack of thymine leads to persistent damage to the chromosomal origins, along with irreversible SOS-dependent inhibition of cell division (Fonville et al., 2010; Kuong and Kuzminov, 2010; Martin and Guzman, 2010; Sangurdekar et al., 2010). Fewer cells succumb to this mechanism in the dgt+ ΔyjjG derivative, while killing is essentially prevented in the optA1 ΔyjjG derivative, presumably due to the much increased dRib-1-P pool.
The demonstration by Weiss (2007a) of the importance of the yjjG pathway for thymine salvage was based on experiments conducted in casamino acid-enriched medium. Our experiments in such media (Figs. 4 and 5) fully support this role. On the other hand, our experiments in non-enriched medium indicate that the yjjG gene does not play a major role in permitting growth under these conditions. This contrasts to the clear role played by dgt under the same conditions. Possibly, the yjjG expression or activity levels are modulated by the growth conditions compared to other possible dUMP hydrolyzing enzymes, such as YfbR and SurE (Proudfoot et al. 2004, Weiss 2007b). Indeed, it was noted (Weiss, 2007a) that a yjjG mutant still possessed some 50% of its dUMP phosphatase activity. It is also intriguing that the absence of yjjG under minimal conditions slightly improves thymine-limited growth (Fig. 4A, left), regardless of dgt status, suggesting that YjjG can also have a deleterious effect. The specificity of the enzyme is rather broad (Proudfoot et al., 2004; Titz et al., 2007) and includes its ability to hydrolyze dTMP, which might be deleterious under some conditions.
The observed enhancement of thymine starvation in thyA strains by increased protein synthesis (i.e., in rich media) has parallels in other situations. For example, an enhancement of thymine starvation in enriched medium was observed with dcd mutants defective in dCTP deaminase (Weiss and Wang, 1994). As this enzyme supplies most of the dUTP used for thymidylate synthesis (Neuhard and Thomassen, 1971) (see also Fig. 1), dcd mutants are thymidine deficient and, like the dgt ΔthyA mutants, display a greater growth defect when the medium is supplemented with casamino acids (Weiss and Wang, 1994).
The present study clearly demonstrates a role of the dgt-mediated reaction in potentiating thymine incorporation in thymine-requiring strains. A remaining question is the role of dgt in the salvage pathway of wild-type (thy+) strains. Dgt is expected to be equally active in wild-type cells. This is evidenced by the dgt mutator effect caused by its absence (Gawel et al., 2008), as well as a demonstration of dgt-dependent excretion of deoxyguanosine into the medium under conditions when the DeoD purine phosphorylase pathway is blocked (results to be described elsewhere). Thus, also in wild-type cells, Dgt will contribute to the production and maintenance of a dRib-1-P pool. While this pool is reportedly small (Jensen et al., 1973; Munch-Petersen, 1970), it should still be available for salvage, which could provide metabolic flexibility under conditions of change. Although wild-type cells generally use exogenous thymine poorly (Crawford, 1958; Reinhart and Copeland, 1973), which is due to a number of factors (lack of active transport, low dRib-1-P pool, and a limited level of DeoA thymine phosphorylase), nevertheless, detectable thymine usage has been observed upon addition of exogenous deoxynucleosides (Jensen et al., 1973), confirming that indeed the dRib-1-P pool is available for thymine salvage. Maintenance of a modest dRib-1-P pool might also be advantageous for controlling expression of the deoCABD operon, which is under control of the DeoR repressor (Valentin-Hansen et al., 1978). The inducer for the operon is dRib-5-P, which is the next catabolic product down from dRib-1-P (see Fig. 1). Thus, one of the functions of dgt-mediated hydrolysis of dGTP may be aimed at ensuring a sufficient basal expression level of the deo operon, providing metabolic flexibility and adaptability. To our knowledge, thymine utilization has not been investigated under various conditions of stress, including DNA stress, where salvage reactions may be beneficial.
Experimental procedures
Bacterial strains
The Escherichia coli strains used are derivatives of E. coli K-12 (λ−) and are listed in Table 1. All strains used in the experiments testing thymine requirements are derivatives of strain NR16947 [ara, thi, trpE9777, Δprolac, F'prolac (F'CC106)] (Gawel et al., 2008) and also contain the zad-220::Tn10 panD tonA markers, as described in Table 1. Deletions of the chromosomal thyA, deoD, and yjjG genes were created by the method of Datsenko and Wanner using strain BW25113 and the kanamycin resistance cassette specified by plasmid pKD13 or the chloramphenicol resistance specified by plasmid pKD3 (Datsenko and Wanner, 2000). Primers used for generating the required PCR product are described in Table 2. The kanamycin resistance markers of the ΔthyA::kan and ΔdeoD::kan alleles were eliminated using the temperature-sensitive replicon pCP20 (Datsenko and Wanner, 2000). The deletion/insertion constructs were confirmed by PCR reactions. Transfer of genetic markers was accomplished by P1 transduction using P1virA. Due to the lack of a direct selection for optA1 (dgt overexpressor), this allele was co-transduced with the zad-220::Tn10 transposon insertion (Singer et al., 1989) to which it is genetically linked (25–30 %). To check for co-transfer of optA1, tetracycline-resistant transductants were tested for their ability to support growth of the bacteriophage T4 tsL141 mutant (Muzyczka et al., 1972). Clones that restrict growth of this phage at 30°C are optA1, while permissive clones are dgt+ (Gauss et al., 1983). Wild-type phage T4D was used as a positive control. The T4 phages were obtained from Dr. J. W. Drake, NIEHS.
Table 1.
E. coli strains used in this studya
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| BW25113 | lacIq rrnB T14 ΔlacZWJ16 hsdR514 ΔaraBAD AH33 ΔrhaBAD LD78 | Datsenko and Wanner, 2000 |
| CAG12025 | zad-220::Tn10 panD | Singer et al., 1989 |
| JD20450b | dgt::mini-Tn10kan | Nat. Inst. Genet., Japanc |
| NR16947 | ara, thi, Δprolac, trpE9777, F'CC106 | Gawel et al., 2008 |
| NR17063 (HR44 but optA1) | thi-1 argH1 his-1 leu-6 lys-25 thr-1 ara-13 gal-6 lacY1 malA1 mtl xylA1 strR tonA optA1 | J. W. Drake |
| NR17624 | optA1 zad-220::Tn10 tonA panD | NR17063 × P1/CAG12025 |
| NR17642d | BW25113, but ΔthyA::kan | This work |
| NR17647 | optA1 zad-220::Tn10 panD tonA | NR16947 × P1/NR17624 |
| NR17648 | zad-220::Tn10 panD tonA | NR16947 × P1/NR17624 |
| NR17649 | optA1 zad-220::Tn10 panD tonA ΔthyA::kan | NR17647 × P1/NR17642 |
| NR17650 | zad-220::Tn10 panD tonA ΔthyA::kan | NR17648 × P1/NR17642 |
| NR17673 | NR17649, but kans | NR17649 × pCP20 |
| NR17674 | NR17650, but kans | NR17650 × pCP20 |
| NR17685e | BW25113, but ΔdeoD::kan | This work |
| NR17688 | optA1 zad-220::Tn10 panD tonA ΔthyA ΔdeoD::kan | NR17673 × P1/NR17685 |
| NR17689 | zad-220::Tn10 panD tonA ΔthyA ΔdeoD::kan | NR17674 × P1/ NR17685 |
| NR17698 | NR17688, but kans | NR17688 × pCP20 |
| NR17699 | NR17689, but kans | NR17689 × pCP20 |
| NR17726 | dgt::mini-Tn10kan zad-220::Tn10 panD tonA ΔthyA | NR17674 × P1/JD20450 |
| NR17738 | dgt::mini-Tn10kan zad-220::Tn10 panD tonA ΔthyA ΔdeoD | NR17699 × P1/JD20450 |
| NR17755f | BW25113, but ΔyjjG::cam | This work |
| NR17756 | optA1 zad-220::Tn10 panD tonA ΔthyA ΔyjjG::cam | NR17673 × P1/NR17755 |
| NR17757 | zad-220::Tn10 panD tonA ΔthyA ΔyjjG::cam | NR17674 × P1/NR17755 |
| NR17758 | dgt::mini-Tn10kan zad-220::Tn10 panD tonA ΔthyA ΔyjjG::cam | NR17726 × P1/NR17755 |
All strains are E. coli K-12 (λ−)
mini-Tn10kan insertion located at 228 nucleotides from start of dgt gene (insertion site 179,465)
National BioResource Project (NIG, Japan) (http://www.shigen.nig.ac.jp/ecoli/strain/top/top.jsp)
The mutant lacks 87% of thyA gene; kan cassette replaces nucleotides 2,962,433–2,963,127.
The mutant lacks 86% of deoD; kan cassette replaces nucleotides 4,618,906–4,619,625.
The mutant lacks 92% of yjjG; cam cassette replaces nucleotides 4,606,629–4,607,284.
Table 2.
Primers used for chromosomal gene deletions.
| Target gene | Pairs of primers (5'-3') | Templateb |
|---|---|---|
| thyA |
ATGAAACAGTATTTAGAACTGATGCAAAAAGTGCTCGACGAAGGCACACAGTGTAGGCTGGAGCTGCTTC TTAGATAGCCACCGGCGCTTTAATGCCCGGATGCGGATCGTAGCCTTCAAATTCCGGGGATCCGTCGACC |
pKD13 |
| deoD |
ATGGCTACCCCACACATTAATGCAGAAATGGGCGATTTCGCTGACGTAGTGTGTAGGCTGGAGCTGCTTC TTACTCTTTATCGCCCAGCAGAACGGATTCCAGTGCGATTTTGATCATGTATTCCGGGGATCCGTCGACC |
pKD13 |
| yjjG a |
TCATGGCGTTGCCAATCAGTATGTAATACAAGGTGGAATAGTGTAGGCTGGAGCTGCTTC CCAGTTCGTGCAACGAAGAAACGGTCCAGGTGGGCGCGATCATATGAATATCCTCCTTAG |
pKD3 |
Primers were designed according to Weiss, 2007a.
Media
Strains were selected and maintained on LB medium supplemented with thymidine (200 µg/ml), and also containing kanamycin (50 µg/ml) for strains with a kan marker, chloramphenicol (25 µg/ml) for strains with a cam marker, or tetracycline (15 µg/ml) for strains with a Tn10 marker. Minimal Medium (Min) contained Vogel-Bonner (VB) salts (Vogel and Bonner, 1956), glucose (0.4%), proline (50 µg/ml), tryptophan (50 µg/ml), thiamine (5 µg/ml), D-pantothenic acid (5 µM), various concentrations of thymine (5–20 µg/ml), and, where indicated, 1% casamino acids (CAA).
Thymine requirements
Thymine requirements on solid media were determined by measuring the growth of bacterial streaks on thymine gradient plates. The gradient plates were generated as follows: 31 ml of minimal liquid agar containing 20 or 40 µg/ml of thymine (or 16 µg/ml of thymidine) was poured into square Petri dishes, which were immediately tilted on the Petri dish lid. After agar solidification, the Petri dishes were returned to the horizontal position, and 36 ml of MM liquid agar without thymine was poured on top of the skewed surface. Streaks were generated by spreading 10 µl overnight cultures - 10-fold diluted in VB buffer - along the length of the gradient using an inoculating loop (Fisherbrand). The plates were incubated at 37°C for 24hr (minimal medium) or 16hr (minimal medium with CAA) until clearly visible zones of growth were apparent and demarcation lines could be placed. No manipulation of the images in Photoshop or other image-processing program was performed.
Because of the possibility of prophage induction during thymine starvation, the culture supernatants were checked carefully for the presence of phage. None were found. We also checked our strains for phage induction following UV light exposure. Again, none were found.
Thymine requirements in liquid batch culture were tested by measuring biomass growth (optical density at 630 nm) with a Synergy 2 Multi-Detection Microplate Reader (BioTek Instruments, Inc.).
Viable cell counts
Cell viability was determined by colony-forming ability on LB plates (with 200 µg/ml of thymidine) following appropriate dilutions. The number of colonies was counted after 24 h incubation at 37°C.
Acknowledgements
We thank Drs. S. Covo and M. Young of the NIEHS for their critical reading of the manuscript for this paper and helpful comments. We thank Dr. J. Drake (NIEHS) for providing the bacteriophage T4 and E. coli optA1 strains, and the National Institute of Genetics, Japan, for strain JD20450. This research was supported by project number ES101905 of the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Andersen RB, Neuhard J. Deoxynucleoside kinases encoded by the yaaG and yaaF genes of Bacillus subtilis. Substrate specificity and kinetic analysis of deoxyguanosine kinase with UTP as the preferred phosphate donor. J Biol Chem. 2001;276:5518–5524. doi: 10.1074/jbc.M007918200. [DOI] [PubMed] [Google Scholar]
- Ahmad SI, Kirk SH, Eisenstark A. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu Rev Microbiol. 1998;52:591–625. doi: 10.1146/annurev.micro.52.1.591. [DOI] [PubMed] [Google Scholar]
- Ahmad SI, Pritchard RH. A regulatory mutant affecting the synthesis of enzymes involved in the catabolism of nucleosides in Escherichia coli. Mol Gen Genet. 1971;111:77–83. doi: 10.1007/BF00286556. [DOI] [PubMed] [Google Scholar]
- Arner ESJ, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- Beacham IR, Beacham K, Zaritsky A, Pritchard RH. Intracellular thymidine triphosphate concentrations in wild type and in thymine requiring mutants of Escherichia coli 15 and K12. J Mol Biol. 1971;60:75–86. doi: 10.1016/0022-2836(71)90448-7. [DOI] [PubMed] [Google Scholar]
- Beauchamp BB, Richardson CC. A unique deoxyguanosine triphosphatase is responsible for the OptA1 phenotype of Escherichia coli. Proc Natl Acad Sci USA. 1988;85:2563–2567. doi: 10.1073/pnas.85.8.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SS, Barner HD. Studies on unbalanced growth in Escherichia coli. Proc Natl Acad Sci USA. 1954;40:885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LV. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958;30:428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S, Sjöberg BM. Ribonucleotide Reductase. In: Hervé G, editor. Allosteric Enzymes CRC press Inc.; 1989. pp. 189–215. [Google Scholar]
- Fishov I, Zaritsky A, Grover NB. On microbial states of growth. Mol Microbiol. 1995;15:789–794. doi: 10.1111/j.1365-2958.1995.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Fonville NC, Bates D, Hastings PJ, Hanawalt PC, Rosenberg SM. Role of RecA and the SOS response in thymineless death in Escherichia coli. PLoS Genet. 2010;6(3) doi: 10.1371/journal.pgen.1000865. e1000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Moroz OV, Wilson KS, Murzin AG. House cleaning, a part of good housekeeping. Mol Microbiol. 2006;59:5–19. doi: 10.1111/j.1365-2958.2005.04950.x. [DOI] [PubMed] [Google Scholar]
- Gauss P, Doherty DH, Gold L. Bacterial and phage mutations that reveal helix-unwinding activities required for bacteriophage T4 DNA replication. Proc Natl Acad Sci USA. 1983;80:1669–1673. doi: 10.1073/pnas.80.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawel D, Hamilton MD, Schaaper RM. A novel mutator of Escherichia coli carrying a defect in the dgt gene, encoding a dGTP triphosphohydrolase. J Bacteriol. 2008;190:6931–6939. doi: 10.1128/JB.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter CE, Cooper S, Pierucci O, Revelas E. On the bacterial life sequences. Cold Spring Harbor Symp. Quant. Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Helmstetter CE. Timing of Synthetic Activities in the Cell Cycle. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, DC: American Society for Microbiology; 1996. pp. 1627–1639. [Google Scholar]
- Ives DH, Ikeda S. Life on the salvage path: the deoxynucleoside kinase of Lactobacillus acidophilus R-26. Prog Nucleic Acid Res Mol Biol. 1998;59:205–255. doi: 10.1016/s0079-6603(08)61033-8. [DOI] [PubMed] [Google Scholar]
- Jensen KF, Leer JC, Nygaard P. Thymine utilization in Escherichia coli K12 on the role of deoxyribose 1-phosphate and thymidine phosphorylase. Eur J Biochem. 1973;40:345–354. doi: 10.1111/j.1432-1033.1973.tb03203.x. [DOI] [PubMed] [Google Scholar]
- Kammen HO. Thymine metabolism in Escherichia coli. Factors involved in utilisation of exogenous thymine. Biochim Biophys Acta. 1967;134:301–311. [Google Scholar]
- Kamiya H. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Res. 2003;15:517–531. doi: 10.1093/nar/gkg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldgaard NO, Maaloe O, Schaechter M. The transition between different physiological states during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:607–616. doi: 10.1099/00221287-19-3-607. [DOI] [PubMed] [Google Scholar]
- Knecht W, Munch-Petersen B, Piskur J. Identification of residues involved in the specificity and regulation of the highly efficient multisubstrate deoxyribonucleoside kinase from Drosophila melanogaster. J Mol Biol. 2000;301:827–837. doi: 10.1006/jmbi.2000.3990. [DOI] [PubMed] [Google Scholar]
- Kondo N, Kuramitsu S, Masui R. Biochemical characterization of TT1383 from Thermus thermophilus identifies a novel dNTP triphosphohydrolase activity stimulated by dATP and dTTP. J Biochem. 2004;136:221–231. doi: 10.1093/jb/mvh115. [DOI] [PubMed] [Google Scholar]
- Korn D, Weissbach A. Thymineless induction in Escherichia coli K12 (λ) Biochim Biophys Acta. 1962;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Kouzminova EA, Kuzminov A. Chromosomal fragmentation in dUTPase-deficient mutants of Escherichia coli and its recombinational repair. Mol Microbiol. 2004;51:1279–1295. doi: 10.1111/j.1365-2958.2003.03924.x. [DOI] [PubMed] [Google Scholar]
- Kunz BA, Kohalmi SE, Kunkel TA, Mathews CK, McIntosh EM, Reidy JA. Deoxyribonucleoside triphosphate levels: a critical factor in the maintenance of genetic stability. Mutat Res. 1994;318:1–64. doi: 10.1016/0165-1110(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Kuong KJ, Kuzminov A. Stalled replication fork repair and misrepair during thymineless death in Escherichia coli. Genes Cells. 2010;15:619–634. doi: 10.1111/j.1365-2443.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YK, Higgins MB, Rabinowitz JD. Antifolate-induced depletion of intracellular glycine and purines inhibits thymineless death in E. coli. ACS Chem Biol. 2010;5:787–795. doi: 10.1021/cb100096f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín CM, Guzmán EC. DNA replication initiation as a key element in thymineless death. DNA Repair. 2011;10:94–101. doi: 10.1016/j.dnarep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Mega R, Kondo N, Nakagawa N, Kuramitsu S, Masui R. Two dNTP triphosphohydrolases from Pseudomonas aeruginosa possess diverse substrate specificities. FEBS J. 2009;276:3211–3221. doi: 10.1111/j.1742-4658.2009.07035.x. [DOI] [PubMed] [Google Scholar]
- Meyers JA, Beauchamp BB, Richardson CC. Gene 1.2 protein of bacteriophage T7. Effect on deoxyribonucleotide pools. J Biol Chem. 1987;262:5288–5292. [PubMed] [Google Scholar]
- Munch-Petersen A. Deoxyribonucleoside catabolism and thymine incorporation in mutants of Escherichia coli lacking deoxyriboaldolase. Eur J Biochem. 1970;15:191–202. doi: 10.1111/j.1432-1033.1970.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Muzyczka N, Poland RL, Bessman MJ. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972;247:7116–7122. [PubMed] [Google Scholar]
- Neidhardt FC, Ingraham J, Schaechter M. Physiology of the Bacterial Cell: A Molecular Approach. Sunderland, MA: Sinauer Associates; 1990. [Google Scholar]
- Neuhard J, Kelln RA. Biosynthesis and conversion of pyrimidines. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 580–599. [Google Scholar]
- Neuhard J, Thomassen E. Deoxycytidine triphosphate deaminase: identification and function in Salmonella typhimurium. J Bacteriol. 1971;105:657–665. doi: 10.1128/jb.105.2.657-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard RH, Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970;226:126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot M, Kuznetsova E, Brown G, Rao NN, Kitagawa M, Mori H, Savchenko A, Yakunin AF. General enzymatic screens identify three new nucleotidases in Escherichia coli. Biochemical characterization of SurE, YfbR, and YjjG. J Biol Chem. 2004;279:54687–54694. doi: 10.1074/jbc.M411023200. [DOI] [PubMed] [Google Scholar]
- Quirk S, Bhatnagar K, Bessman MJ. Primary structure of the deoxyguanosine triphosphate triphosphohydrolase-encoding gene (dgt) of Escherichia coli. Gene. 1990;89:13–18. doi: 10.1016/0378-1119(90)90200-b. [DOI] [PubMed] [Google Scholar]
- Reinhart KV, Copeland JC. Evidence that thymine is not a normal metabolite in wild-type Bacillus subtilis. Biochim Biophys Acta. 1973;294:1–7. [Google Scholar]
- Sangurdekar DP, Hamann BL, Smirnov D, Srienc F, Hanawalt PC, Khodursky AB. Thymineless death is associated with loss of essential genetic information from the replication origin. Mol Microbiol. 2010;75(6):1455–1467. doi: 10.1111/j.1365-2958.2010.07072.x. [DOI] [PubMed] [Google Scholar]
- Seto D, Bhatnagar SK, Bessman MJ. The purification and properties of deoxyguanosine triphosphate triphosphohydrolase from Escherichia coli. J Biol Chem. 1988;263:1494–1499. [PubMed] [Google Scholar]
- Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, Jaacks KJ, Grossman AD, Erickson JW, Gross CA. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titz D, Häuser R, Engelbrecher A, Uetz P. The Escherichia coli protein YjjG is a house-cleaning nucleotidase in vivo. FEMS Microbiol Lett. 2007;270:49–57. doi: 10.1111/j.1574-6968.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P, Svenningsen BA, Munch-Petersen A, Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978;159:191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Vogel HJ, Bonner DM. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- Weiss B. YjjG, a dUMP phosphatase, is critical for thymine utilization by Escherichia coli K-12. J Bacteriol. 2007a;189:2186–2189. doi: 10.1128/JB.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. The deoxycytidine pathway for thymidylate synthesis in Escherichia coli. J Bacteriol. 2007b;189:7922–7926. doi: 10.1128/JB.00461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B, Wang L. De novo synthesis of thymidylate via deoxycytidine in dcd (dCTP deaminase) mutants of Escherichia coli. J Bacteriol. 1994;176:2194–2199. doi: 10.1128/jb.176.8.2194-2199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler SM, Richardson CC. Structure and regulation of the gene for dGTP triphosphohydrolase from Escherichia coli. Proc Natl Acad Sci USA. 1990;87:2740–2744. doi: 10.1073/pnas.87.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler SM, Richardson CC. DNA binding properties of the deoxyguanosine triphosphate triphosphohydrolase of Escherichia coli. J Biol Chem. 1993;268:20046–20054. [PubMed] [Google Scholar]
- Zaritsky A, Pritchard RH. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973;114:824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A, Woldringh CL, Einav M, Alexeeva S. Use of thymine limitation and thymine starvation to study bacterial physiology and cytology. J Bacteriol. 2006;188:1667–1679. doi: 10.1128/JB.188.5.1667-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]