Abstract
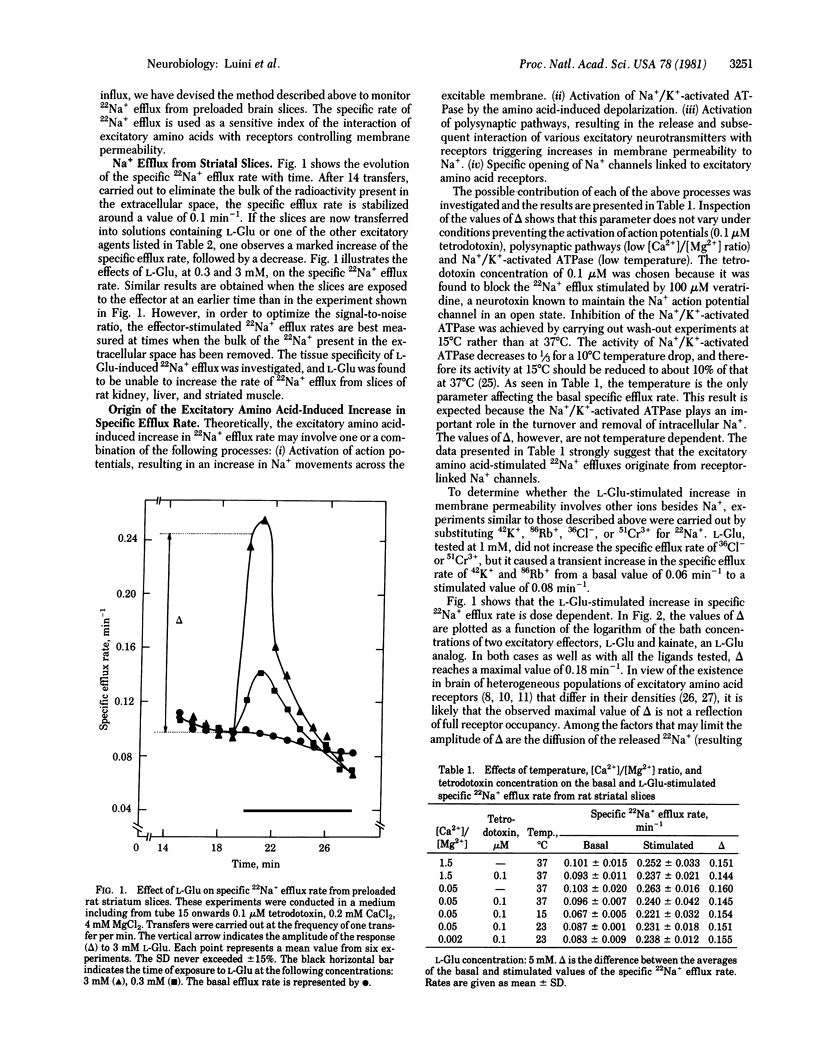
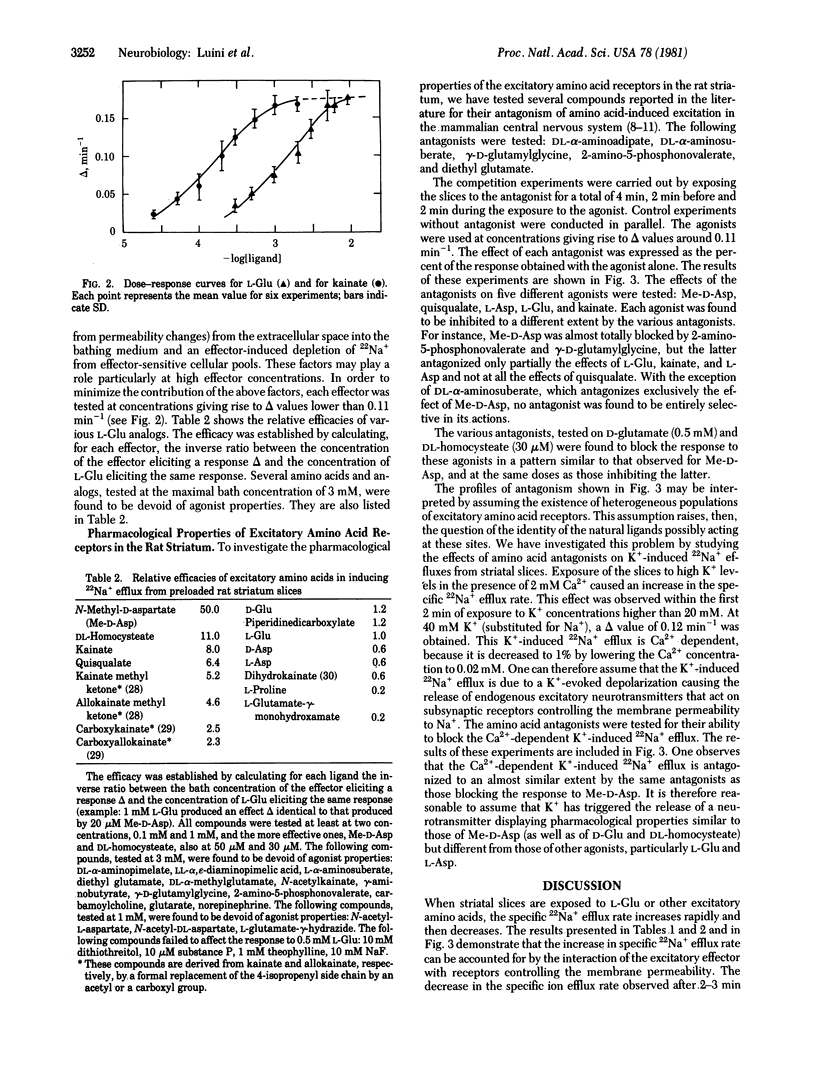
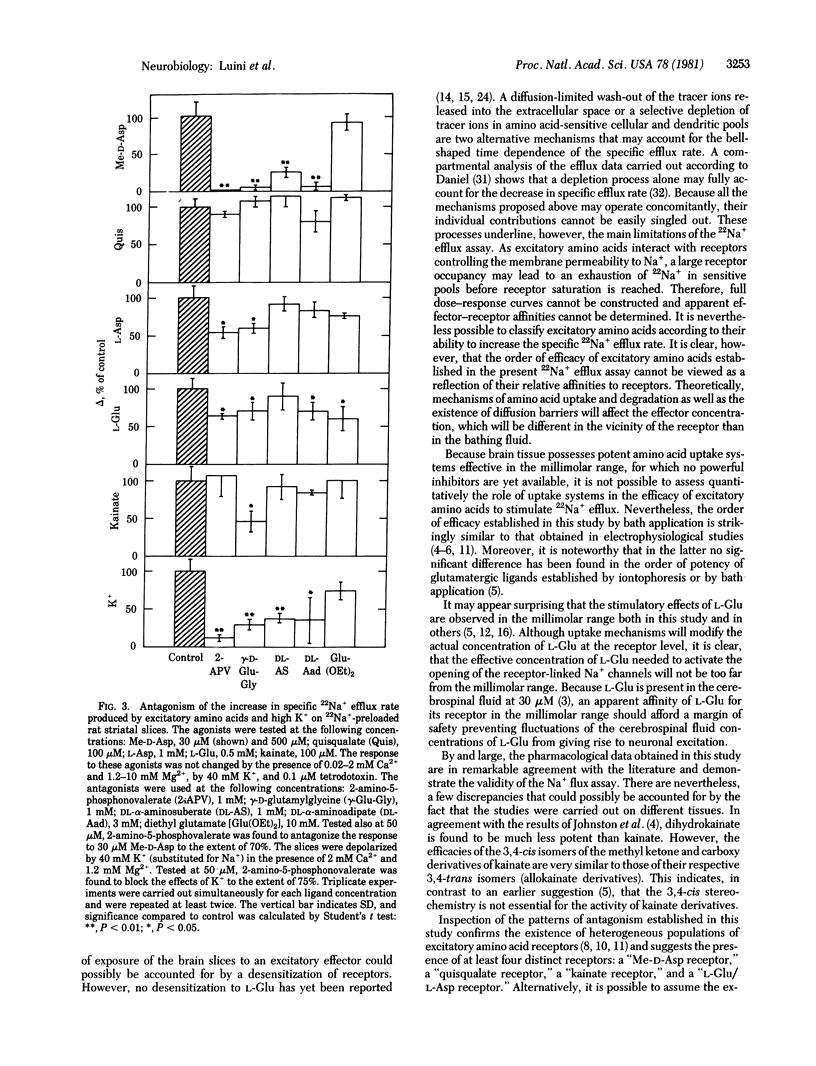
Specific 22Na+ efflux rates from preloaded rat striatal slices are increased in a dose-dependent manner by L-glutamate and other excitatory amino acids displaying the following order of efficiency: N-methyl-D-aspartate greater than DL-homocysteate greater than quisqualate greater than kainate greater than D-glutamate greater than L-glutamate greater than L-aspartate. Amino acid antagonists such as 2-amino-5-phosphonovalerate, gamma-D-glutamylglycine, DL-aminosuberate, DL-aminoadipate, and diethyl glutamate but not nonexcitatory amino acids such as gamma-aminobutyric acid inhibit the amino acid-induced increase in specific 22Na+ efflux rate. Increased K+ concentrations, in the presence of 2 mM Ca2+, increase the specific 22Na+ efflux. The latter and the response to N-methyl-D-aspartate, but not the responses to L-glutamate, L-aspartate, quisqualate, and kainate, are inhibited to similar extents by the same antagonists. These results suggest the release from striatal nerve terminals of a putative neurotransmitter with pharmacological properties different from those of L-glutamate or L-aspartate but similar to those of N-methyl-D-aspartate. The results of this study show that the stimulation of the 22Na+ efflux in brain slices by neuroactive amino acids and K+ ions is a valid and powerful tool for pharmacological investigations of excitatory amino acid receptors and their putative ligands.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biscoe T. J., Davies J., Dray A., Evans R. H., Francis A. A., Martin M. R., Watkins J. C. Depression of synaptic excitation and of amino acid induced excitatory responses of spinal neurones by D-alpha-aminoadipate, alpha,epsilon-diaminopimelic acid and HA-966. Eur J Pharmacol. 1977 Oct 1;45(3):315–316. doi: 10.1016/0014-2999(77)90017-6. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Headley P. M., Martin M. R., Watkins J. C. Structure-activity relations of excitatory amino acids on frog and rat spinal neurones. Br J Pharmacol. 1976 Nov;58(3):373–382. doi: 10.1111/j.1476-5381.1976.tb07714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford H. F., McIlwain H. Ionic basis for the depolarization of cerebral tissues by excitatory acidic amino acids. J Neurochem. 1966 Nov;13(11):1163–1177. doi: 10.1111/j.1471-4159.1966.tb04274.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Charnock J. S., Cook D. A., Casey R. The role of cations and other factors on the apparent energy of activation of (Na + + K + )-ATPase. Arch Biochem Biophys. 1971 Nov;147(1):323–329. doi: 10.1016/0003-9861(71)90340-7. [DOI] [PubMed] [Google Scholar]
- Clarke G., Straughan D. W. Evaluation of the selectivity of antagonists of glutamate and acetylcholine applied microiontophoretically onto cortical neurones. Neuropharmacology. 1977 Jun;16(6):391–398. doi: 10.1016/0028-3908(77)90079-x. [DOI] [PubMed] [Google Scholar]
- Constanti A., Connor J. D., Galvan M., Nistri A. Intracellularly-recorded effects of glutamate and aspartate on neurones in the guinea-pig olfactory cortex slice. Brain Res. 1980 Aug 18;195(2):403–420. doi: 10.1016/0006-8993(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Selective antagonism of amino acid-induced and synaptic excitation in the cat spinal cord. J Physiol. 1979 Dec;297(0):621–635. doi: 10.1113/jphysiol.1979.sp013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Roberts P. J. High affinity l-[3h]glutamate binding to postsynaptic receptor sites on rat cerebellar membranes. J Neurochem. 1978 Dec;31(6):1467–1477. doi: 10.1111/j.1471-4159.1978.tb06574.x. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Harvey J. A., McIlwain H. Excitatory acidic amino acids and the cation content and sodium ion flux of isolated tissues from the brain. Biochem J. 1968 Jun;108(2):269–274. doi: 10.1042/bj1080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hösli L., Hösli E. Action and uptake of neurotransmitters in CNS tissue culture. Rev Physiol Biochem Pharmacol. 1978;81:135–188. doi: 10.1007/BFb0034093. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., Davies J., McCulloch R. M. Spinal interneurone excitation by conformationally restricted analogues of L-glutamic acid. Nature. 1974 Apr 26;248(5451):804–805. doi: 10.1038/248804a0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. D., Coyle J. T. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol Pharmacol. 1979 May;15(3):492–505. [PubMed] [Google Scholar]
- Mauger J. P., Moura A. M., Worcel M. Pharmacology of the adrenoceptors and cholinoceptors of the BC3H1 nonfusing muscle cell line. Br J Pharmacol. 1978 Sep;64(1):29–36. doi: 10.1111/j.1476-5381.1978.tb08637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Scherer U., Singh K. A glutamatergic corticostriatal path? Brain Res. 1977 Jun 10;128(2):369–373. doi: 10.1016/0006-8993(77)91003-4. [DOI] [PubMed] [Google Scholar]
- McLennan H., Lodge D. The antagonism of amino acid-induced excitation of spinal neurones in the cat. Brain Res. 1979 Jun 15;169(1):83–90. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977 Sep;40(5):1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Cuenod M. Glutamate release in vitro from corticostriatal terminals. Brain Res. 1979 Oct 26;176(1):185–188. doi: 10.1016/0006-8993(79)90884-9. [DOI] [PubMed] [Google Scholar]
- Rowlands G. J., Roberts P. J. Specific calcium-dependent release of endogenous glutamate from rat striatum is reduced by destruction of the cortico-striatal tract. Exp Brain Res. 1980;39(2):239–240. doi: 10.1007/BF00237555. [DOI] [PubMed] [Google Scholar]
- Spencer H. J. Antagonism of cortical excitation of striatal neurons by glutamic acid diethyl ester: evidence for glutamic acid as an excitatory transmitter in the rat striatum. Brain Res. 1976 Jan 30;102(1):91–101. doi: 10.1016/0006-8993(76)90577-1. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Olsen R. W. gamma-Aminobutyric acid-stimulated chloride permeability in crayfish muscle. Biochim Biophys Acta. 1977 Feb 4;464(3):519–529. doi: 10.1016/0005-2736(77)90027-x. [DOI] [PubMed] [Google Scholar]
- White W. F., Nadler J. V., Cotman C. W. The effect of acidic amino acid antagonists on synaptic transmission in the hippocampal formation in vitro. Brain Res. 1979 Mar 23;164:177–194. doi: 10.1016/0006-8993(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Puil E. A. Actions of glutamic acid on spinal neurones. Exp Brain Res. 1973 Mar 29;17(1):35–49. doi: 10.1007/BF00234562. [DOI] [PubMed] [Google Scholar]


