Smokers generally gain weight when they quit smoking; this weight gain can lessen some of the health benefits of quitting smoking. We review the effectiveness of behavioral and pharmacological approaches to mitigating weight gain in the context of quitting smoking and consider mechanisms that could potentially account for the effects of smoking and nicotine on body weight. Understanding how nicotine affects body weight may lead to novel pharmacological and behavioral interventions for obesity as well as concurrent obesity and nicotine dependence.
THE IMPACT OF SMOKING AND OF OBESITY ON HEALTH
Approximately 19% of women and 23% of men in the United States are current smokers. Each year, cigarette smoking causes more than 400,000 premature deaths in the United States from cardiovascular and respiratory diseases and cancer.1 The second leading cause of premature morbidity and mortality is excess body weight due to poor diet and insufficient physical activity.1 In the United States, ~30% of women and ~43% of men are overweight, and ~26% of women and ~29% of men are obese.2 Overweight and obesity are associated with an increased risk for cardiovascular disease, hypertension, type 2 diabetes, respiratory problems, musculoskeletal disorders, and certain cancers.2
The idea that cigarette smoking is helpful in controlling body weight has been part of popular culture for many years. Cigarette advertisements from the 1930s suggested that women should “reach for a cigarette instead of a sweet.” For many smokers, the anticipation of weight gain can hinder smoking cessation success.3 Most health-care providers would agree that the decrease in morbidity and mortality associated with smoking cessation far outweighs the health risks associated with post-cessation weight gain. Nevertheless, weight gain can reduce some of the health benefits of quitting smoking. For example, weight gain after smoking cessation contributes to an increased risk of type 2 diabetes4 and hypertension5 and also reduces the improvement in lung function conferred by quitting smoking.6 Overweight and obesity peak at 45–64 years of age. This is also a period when smoking cessation is more likely to occur. Of the population ≥50 years of age, 44% of overweight men and 48% of obese men are former smokers, whereas 27% of overweight women and 27% of obese women are former smokers.7 Optimizing the health benefits of smoking cessation requires greater understanding of the behavioral and biological relationships between smoking and dietary habits in order to prevent weight gain after quitting smoking.
EPIDEMIOLOGY OF SMOKING AND BODY WEIGHT
The link between smoking and body weight is evident as early as adolescence. Adolescents, especially girls, report starting to smoke and continuing with the habit for purposes of weight control and weight loss.8 The more common the dieting habit becomes, the greater the risk of initiating the smoking habit.9 It is not clear whether smoking during adolescence actually influences body weight, but the perception that it does tracks into adulthood. Young adults who are trying to lose weight are 40% more likely to smoke cigarettes.10 Because smoking is often thought of as a way to control appetite and weight, quitting smoking means the absence of this control strategy. As highlighted in Figure 1, adult smokers weigh, on average, 4–5 kg less than nonsmokers, are less likely to be overweight or obese, and tend to gain weight when they quit smoking.2,11
figure 1.
Average changes in body mass index over 10 years by smoking status. Sample size = 9,004 (3,365 men and 5,639 women). The average age of female participants at baseline was 47.1 years, and the average age of male participants at baseline was 43.8 years. Adapted from ref. 11.
There are interindividual differences in the amount of post-cessation weight gain. Individuals who successfully quit smoking typically gain between 7 and 19 pounds within 8 years of quitting, whereas those who continue to smoke gain an average of 4–5 pounds.12,13 As shown in Figure 2, most of the weight gain occurs within the first 6 months of abstinence.14 Approximately 10% of smokers who quit smoking gain close to 30 pounds in weight.11 Smokers who are either underweight (body mass index ~18) or overweight (body mass index >29) tend to have the greatest post-cessation increase in weight.12 Unfortunately, only 25% of former smokers maintain a healthy weight after quitting smoking.12 These data indicate that smoking decelerates aging-related weight gain, creating a greater difference in body weight between smokers and nonsmokers across time; this difference between the groups disappears after smokers quit.11,12
figure 2.
Average weight gain within the first year of attempting to quit smoking. Point prevalence abstinence group includes individuals who were not continuously abstinent but who were abstinent over the 7 days prior to testing. Sample size = 196. The average age of the participants at baseline was 44.5 years. Adapted from ref. 14.
PHYSIOLOGY OF SMOKING EFFECT ON BODY WEIGHT
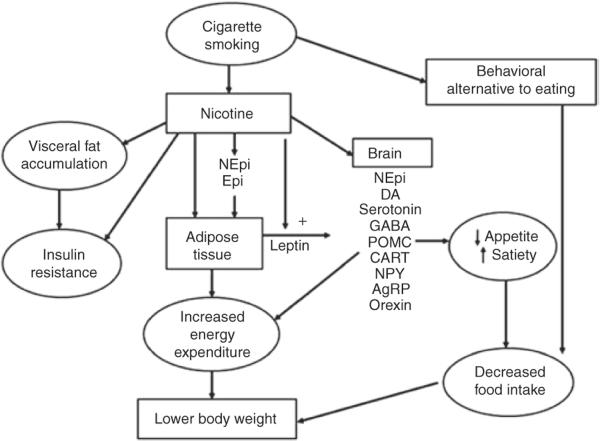
The mechanisms through which smoking decreases body weight are complex and incompletely understood. Most of the effects of cigarette smoking on body weight are mediated by nicotine, although smoking a cigarette may also serve as a behavioral alternative to eating, resulting in decreased food intake (Figure 3). Nicotine acts on nicotinic cholinergic receptors in the brain and autonomic ganglia.15 The binding of nicotine to the nicotinic receptors opens ion channels, allowing entry of sodium and calcium, which, in turn, augment the release of various neurotransmitters. This process includes the systemic release of catecholamines and, in the central nervous system, release of dopamine, norepinephrine, serotonin, acetylcholine, glutamate, γ-aminobutyric acid, and other neurotransmitters.
figure 3.
Mechanisms through which cigarette smoking reduces body weight. Smoking reduces body weight by increasing energy expenditure and inhibiting the expected compensatory increase in caloric intake. Nicotine increases energy expenditure both by direct effects on peripheral tissues, largely mediated by catecholamines, and by effects on central nervous system neuroendocrine circuits. Nicotine's effects on the brain also leads to suppression of appetite, and smoking per se can serve as a behavioral alternative to eating. AgRP, Agouti-related peptide; CART, cocaine amphetamine-regulated transcript; DA, dopamine; Epi, epinephrine; GABA, γ-aminobutyric acid; NEpi, norepinephrine; NPY, neuropeptide Y; POMC, proopiomelanocortin.
Body weight is determined by the balance of caloric intake and daily energy expenditure. Daily energy expenditure is determined by resting metabolic rate, physical activity and the thermic effects of food. Nicotine reduces body weight by raising the resting metabolic rate while blunting the expected increase in food intake in response to the increase in metabolic rate. Like many antiobesity drugs, nicotine is a sympathomimetic agent. Sympathomimetic drugs increase energy expenditure via action on peripheral tissue and through regulation of metabolism in the brain. Nicotine promotes the local release of norepinephrine within body tissues and systemic release of epinephrine from the adrenal glands. Nicotine increases thermogenesis in adipose tissue, partly by increasing lipolysis and subsequent recycling of fatty acids into triglycerides.16,17 Smoking increases 24-h energy expenditure by ~10%18 and increases energy expenditure more during exercise and after eating than while at rest.19 A 10% increase in metabolic rate, corresponding to an expenditure of 200 kcal per 24 h, seems small; however, assuming that there is no change in caloric intake, this increase in energy expenditure caused by nicotine can result in the loss of 10 kg in body weight over 1 year.
Nicotine has many potential effects on central nervous system regulation of eating and energy expenditure. The regulation of eating behavior and metabolic rate by the brain occurs in the hypothalamus, which integrates peripheral signals of satiety and adiposity as well as central motivational and emotional influences. Leptin is released from adipose tissue in proportion to the amount of adipose and acts centrally to suppress food intake and increase metabolic rate. Studies comparing leptin levels in smokers with those in nonsmokers show conflicting results. However, it has been suggested that nicotine may augment the effects of leptin in the brain by enhancing leptin binding or increasing the sensitivity of downstream transduction cascades.20
The release of hormones such as norepinephrine, dopamine, serotonin and γ-aminobutyric acid by the central nervous system influences brain chemicals that suppress eating and increase metabolic rate (such as pro-opiomelanocortin and cocaine-amphetamine-regulated transcript) as well as those that suppress eating and decrease metabolic rate (such as neuropeptide Y, Agouti-related peptide, melanin-concentrating hormone, and orexin).21 Nicotine has complex effects on these hormones; the acute response is consistent with activation of systems that decrease appetite and increase body metabolism, whereas the chronic changes are consistent with activation of systems that increase appetite and decrease metabolic rate.20 Like nicotine, drugs that increase central nervous system levels of norepinephrine, dopamine and/or serotonin (such as phentermine, sibutramine, and bupropion) suppress appetite and facilitate weight loss.22
Nicotine also produces other metabolic effects related to body weight or composition, including insulin resistance, which is most likely related to the release of catecholamines.23 Levels of the adipose-derived protein adiponectin, which modulates insulin sensitivity and has anti-inflammatory effects, are lower in smokers as compared to nonsmokers.24 Insulin resistance is associated with altered body composition, including increased visceral fat. Smokers have a higher percentage of visceral fat compared to total fat and consequently a higher waist-tohip ratio than nonsmokers.25 The link between smoking and increased visceral fat is not well understood, but it may be related to the effects of nicotine, which promotes the release of cortisol and alters the balance of male and female sex hormones. A high waist-to-hip ratio is a risk factor for atherosclerotic cardiovascular disease and appears to be particularly related to the frequency of smoking and the number of cigarettes smoked per day/number of packs smoked per year.
The endocannabinoid system is involved in the rewarding and reinforcing effects of nicotine. Endogenous cannabinoids influence food intake and other appetite-related behaviors.21 Cannabinoids are also involved in brain reward circuits, interacting with opioid and dopamine systems. As discussed below, cannabinoid receptor antagonists have been shown to reduce body weight as well as to promote smoking cessation.
NATURAL HISTORY, PHYSIOLOGICAL CHANGES, AND WEIGHT GAIN AFTER SMOKING CESSATION
As noted previously, smokers weigh, on average, 4–5 kg less than nonsmokers. When smokers quit, they gain, on average, 4.5 kg, within 6–12 months after quitting, and their weight returns to the same weight-age trajectory as that observed in nonsmokers (Figure 1). Some smokers, however, gain much more weight after quitting; 13% of them gain more than 10 kg.11 Risk factors for greater weight gain after smoking cessation include African-American race, age <55 years, being a heavy smoker (more than 25 cigarettes per day), and lower socioeconomic status.26 Genetic factors also influence weight gain after quitting smoking, as demonstrated in twin studies.27
As shown in Figure 2, weight gain is greatest in the first 1–2 months after quitting smoking, although weight continues to increase for 6 or more months. As described below, some pharmacotherapies to aid smoking cessation (nicotine-replacement products and bupropion) blunt weight gain after smoking cessation. However, when these medications are stopped, body weight increases to the level it would have reached without the medication. The weight gain after cessation is primarily accounted for by increased body fat.
The mechanisms of weight gain after smoking cessation include decreased metabolic rate and increased caloric intake, effects opposite to those produced by nicotine. In one study, the average increase in caloric intake in subjects who had quit smoking was 227 calories per day, which could explain 69% of the weight gain observed at 3 months.28 Lower socioeconomic status was found to be a risk factor for weight gain after smoking cessation, probably because it is associated with lower physical activity and the consumption of high-fat and high-calorie diets.
There are several explanations for increased food intake after smoking cessation. One is that the appetite-suppressant effects of nicotine on the brain are reversed, resulting in increased hunger. However, the increased intake of preferred snacks associated with smoking abstinence is not explained by overall changes in hunger, at least acutely.29 Smoking and/or nicotine may have helped control overeating or compulsive eating, and these behaviors are left uninhibited after smoking cessation. Smokers who have a history of binge eating or who indulge in binge eating during smoking-cessation treatment have lower quit rates coupled with greater weight gain.30
Another mechanism for the link between smoking and eating is that the rewarding effects of food substitute for the rewarding effects of smoking (that is, substitutable reinforcers). Research suggests that the absence of nicotine increases the rewarding value of food29 and that individual differences in food reward are explained, in part, by genetic factors.31 Increased intake of foods high in fat and sugar activates reward circuitries in the brain similar to those activated by smoking.32 Nicotine withdrawal produces an elevated reward threshold, meaning that less pleasure is derived from typical reinforcers.33 Therefore greater amounts of highly rewarding foods (“comfort foods,” that is, snacks high in sugar and carbohydrates) may be sought to achieve the pleasure previously derived from smoking. Bupropion has been shown to lower the reward threshold,34 attenuate food reward, and suppress weight gain.35 To maximize the health benefits of smoking cessation and inform behavioral and pharmacologic interventions to mitigate post-cessation weight gain, it is important to identify processes that account for overeating among smokers within the first 6 months after quitting.
IMPLICATIONS OF WEIGHT GAIN FOR SMOKING CESSATION TREATMENT
When smokers quit smoking, the loss of the metabolic boost and appetite suppression conferred by nicotine is often accompanied by increased caloric intake but no increase in physical activity. This positive energy balance leads to weight gain. Behavioral interventions to manage post-cessation weight gain have therefore focused on managing caloric intake, increasing physical activity, or both. Research findings have been inconsistent with respect to whether the subjects were concerned about post-cessation weight gain, although >90% of the participants in these studies were women. A recent meta-analysis evaluated whether weight-related behavioral interventions are an effective way to reduce post-cessation weight gain.36 The results of the systematic review provided evidence of the short-term benefit of weight-control components in smoking cessation interventions, for both smoking abstinence and weight control. However, after 6 months, the beneficial effects of these weight-control interventions were no longer significant. These data confirm the short-term benefits of a combined (smoking cessation and weight control) treatment and, importantly, challenge the long-held notion that concurrently quitting smoking and trying to control dietary intake would be detrimental to smoking cessation success.36 Although not yet investigated, longer-term behavioral interventions may produce more enduring effects on body weight.
An alternative to a weight control/smoking cessation treatment is one in which only the concerns about post-cessation weight gain are addressed, as opposed to the weight gain itself.37 This treatment approach was based on findings that concerns about weight gain are a stronger predictor of relapse than actual weight gain.3 One group of smokers received standard smoking-cessation counseling, a second group received this counseling plus diet advice to prevent weight gain (that is, weight control), and a third group received the standard smoking-cessation program plus counseling to reduce their concerns about gaining weight. At the 1-year follow-up, 21% of the women in the weight-concerns group were abstinent from smoking as compared to 13% in the weight-control group and 9% in the standard smoking-cessation counseling group. Women in the weight-concerns group also gained less weight (5.5 pounds) than women in the weight-control group (11.9 pounds) or the standard smoking-cessation counseling group (16.9 pounds). Therefore, reducing concerns about weight gain, rather than controlling weight gain itself, may be more beneficial for smokers who want to quit smoking but are worried about gaining weight after they quit.
Several pharmacotherapies have been evaluated for preventing post-cessation weight gain. These include bupropion, nicotine-replacement medications, fluoxetine, and varenicline. Overall, these medications appear to delay, rather than prevent, post-cessation weight gain.35 That is, once the drug regimens are completed, the weight increases to a level consistent with what would have been reached if no medication had been used. However, a temporary suppression of weight gain might increase smokers' motivation to quit, allowing time for the smoker who is concerned about weight gain to focus on quitting smoking first and subsequently address dietary intake and physical activity. A recent study found that combining bupropion therapy with a weight-concerns smoking-cessation intervention produced greater levels of smoking abstinence at 6 months as compared to standard smoking-cessation counseling along with either bupropion or placebo (34%, 21%, and 12%, respectively).38 There were no significant post-cessation differences in weight gain among women at 3, 6, or 12 months after the quit date.
TREATMENT OF THE OBESE SMOKER: IMPLICATIONS FOR NEW DRUG DEVELOPMENT
Despite the general weight-reducing effects of cigarette smoking, there are many smokers who, in addition to tobacco dependence, also have metabolic syndrome, including obesity. Smoking and obesity are important cardiovascular risk factors and act synergistically to cause cardiovascular disease. Smoking produces a chronic inflammatory state, causes endothelial dysfunction, enhances thrombogenesis, can cause insulin resistance and diabetes, and is associated with an atherogenic lipid profile.23 Obesity increases blood pressure, causes or aggravates diabetes, produces hyperlipidemia, and also causes a chronic inflammatory state. Although smokers weigh less than they might if they were not smokers, their ratio of visceral fat to subcutaneous fat is high, which is also a cardiovascular risk factor. Estimates suggest that 20% of smokers are obese.10 Somewhat paradoxically, some epidemiological studies have found that those who smoke the most cigarettes per day weigh more and have a higher likelihood of obesity than those who smoke fewer cigarettes per day.25 The higher prevalence of obesity in high-level smokers may reflect a disorder of appetite-related behavior, leading them to overconsume both nicotine and food. Research has shown that obese smokers tend to discriminate less readily between nicotinized and de-nicotinized cigarettes (that is, with reduced reinforcing effects of nicotine) than do their normal-weight counterparts.39
The idea of treating tobacco dependence and obesity together is a provocative one. The first drug to offer such treatment was rimonabant, a cannabinoid receptor antagonist. The cannabinoid 1 receptor appears to be involved in reward pathways for both nicotine and food. In clinical trials, rimonabant was shown to be effective both for smoking cessation and for weight loss and control of metabolic syndrome.22 Unfortunately, because of its adverse psychiatric side effects, the drug was not approved for use in the United States. Bupropion, as mentioned above, is effective for smoking cessation and has also been used for weight loss. Bupropion increases levels of dopamine and norepinephrine in the brain, similar to the effects of nicotine. Indeed, nicotine itself might be considered a potential long-term approach to weight control. The major concern with using nicotine (and other sympathomimetic drugs) as medication is its association with cardiovascular toxicity. Nicotine increases heart rate and cardiac work, potentially contributing to endothelial dysfunction and insulin resistance. However, most of the cardiovascular risk from smoking comes from oxidants and particulates rather than from nicotine. An informative test of the long-term safety of nicotine per se is the use of snus, a form of smokeless tobacco, by men in Sweden. A large percentage of men in Sweden use snus and do not smoke. Nicotine exposure from snus is similar to that from cigarettes, but most of the epidemiological studies in Sweden have found little or no increased risk of cardiovascular events in snus users.40 The efficacy and safety of long-term nicotine medication for weight control remain to be investigated.
In conclusion, across the population, nicotine has undoubtedly been the most effective long-term weight control drug in use over the past century. Unfortunately, nicotine is delivered to most people via cigarette smoke, which is extraordinarily toxic, resulting in the premature death of half of those who are lifelong smokers. Understanding brain mechanisms of how nicotine affects body weight may lead to novel approaches to pharmacotherapy, not only for obesity but also for obesity concomitant with substance abuse.
ACKNOWLEDGMENTS
This review was supported by grants DA02277 from the National Institute on Drug Abuse, P50 CA143187 from the National Cancer Institute, and U01 DA020830 from the National Institute on Drug Abuse.
Footnotes
CONFLICT OF INTEREST N.B. serves as a consultant to several pharmaceutical companies that market or are developing medications to aid smoking cessation. He has served as a paid expert witness in litigation against tobacco companies.
References
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Kruger J, Ham SA, Prohaska TR. Behavioral risk factors associated with overweight and obesity among older adults: the 2005 National Health Interview Survey. Prev. Chronic Dis. 2009;6:A14. [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J. Consult. Clin. Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- 4.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann. Intern. Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janzon E, Hedblad B, Berglund G, Engström G. Changes in blood pressure and body weight following smoking cessation in women. J. Intern. Med. 2004;255:266–272. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 6.Chinn S, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–1635. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- 7.CDC Cigarette smoking among adults and trends in smoking cessation -United States 2008. MMWR Morb. Mortal. Wkly Rep. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 8.Fulkerson JA, French SA. Cigarette smoking for weight loss or control among adolescents: gender and racial/ethnic differences. J. Adolesc. Health. 2003;32:306–313. doi: 10.1016/s1054-139x(02)00566-9. [DOI] [PubMed] [Google Scholar]
- 9.Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. Am. J. Public Health. 2001;91:446–450. doi: 10.2105/ajph.91.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee CC, Rigotti NA, Davis RB, Phillips RS. Relationship between smoking and weight control efforts among adults in the United States. Arch. Intern. Med. 2001;161:546–550. doi: 10.1001/archinte.161.4.546. [DOI] [PubMed] [Google Scholar]
- 11.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 12.Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106:188–196. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am. J. Epidemiol. 1998;148:821–830. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- 14.Klesges RC, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. J. Consult. Clin. Psychol. 1997;65:286–291. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL. Nicotine addiction. N. Engl. J. Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson K, Arner P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int. J. Obes. Relat. Metab. Disord. 2001;25:1225–1232. doi: 10.1038/sj.ijo.0801654. [DOI] [PubMed] [Google Scholar]
- 17.Hellerstein MK, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J. Clin. Invest. 1994;93:265–272. doi: 10.1172/JCI116955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofstetter A, Schutz Y, Jéquier E, Wahren J. Increased 24-hour energy expenditure in cigarette smokers. N. Engl. J. Med. 1986;314:79–82. doi: 10.1056/NEJM198601093140204. [DOI] [PubMed] [Google Scholar]
- 19.Perkins KA. Metabolic effects of cigarette smoking. J. Appl. Physiol. 1992;72:401–409. doi: 10.1152/jappl.1992.72.2.401. [DOI] [PubMed] [Google Scholar]
- 20.Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J. Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008;18:158–168. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Ioannides-Demos LL, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: past, current, and future therapies. J. Obes. 2011;2011:179674. doi: 10.1155/2011/179674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 2003;46:91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki T, Shimada K, Mokuno H, Daida H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart. 2003;89:663. doi: 10.1136/heart.89.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 26.Filozof C, Fernández Pinilla MC, Fernández-Cruz A. Smoking cessation and weight gain. Obes. Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 27.Swan GE, Carmelli D. Characteristics associated with excessive weight gain after smoking cessation in men. Am. J. Public Health. 1995;85:73–77. doi: 10.2105/ajph.85.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamford BA, Matter S, Fell RD, Papanek P. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am. J. Clin. Nutr. 1986;43:486–494. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- 29.Spring B, Pagoto S, McChargue D, Hedeker D, Werth J. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol. Biochem. Behav. 2003;76:351–360. doi: 10.1016/j.pbb.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 30.White MA, Peters EN, Toll BA. Effect of binge eating on treatment outcomes for smoking cessation. Nicotine Tob. Res. 2010;12:1172–1175. doi: 10.1093/ntr/ntq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerman C, et al. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl.) 2004;174:571–577. doi: 10.1007/s00213-004-1823-9. [DOI] [PubMed] [Google Scholar]
- 32.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- 34.Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl.) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- 35.Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 2009:CD006219. doi: 10.1002/14651858.CD006219.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Spring B, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: a systematic review and meta-analysis. Addiction. 2009;104:1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins KA, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J. Consult. Clin. Psychol. 2001;69:604–613. [PubMed] [Google Scholar]
- 38.Levine MD, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch. Intern. Med. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blendy JA, et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology (Berl.) 2005;180:306–315. doi: 10.1007/s00213-005-2167-9. [DOI] [PubMed] [Google Scholar]
- 40.Piano MR, et al. American Heart Association Council on Cardiovascular Nursing Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]