Abstract
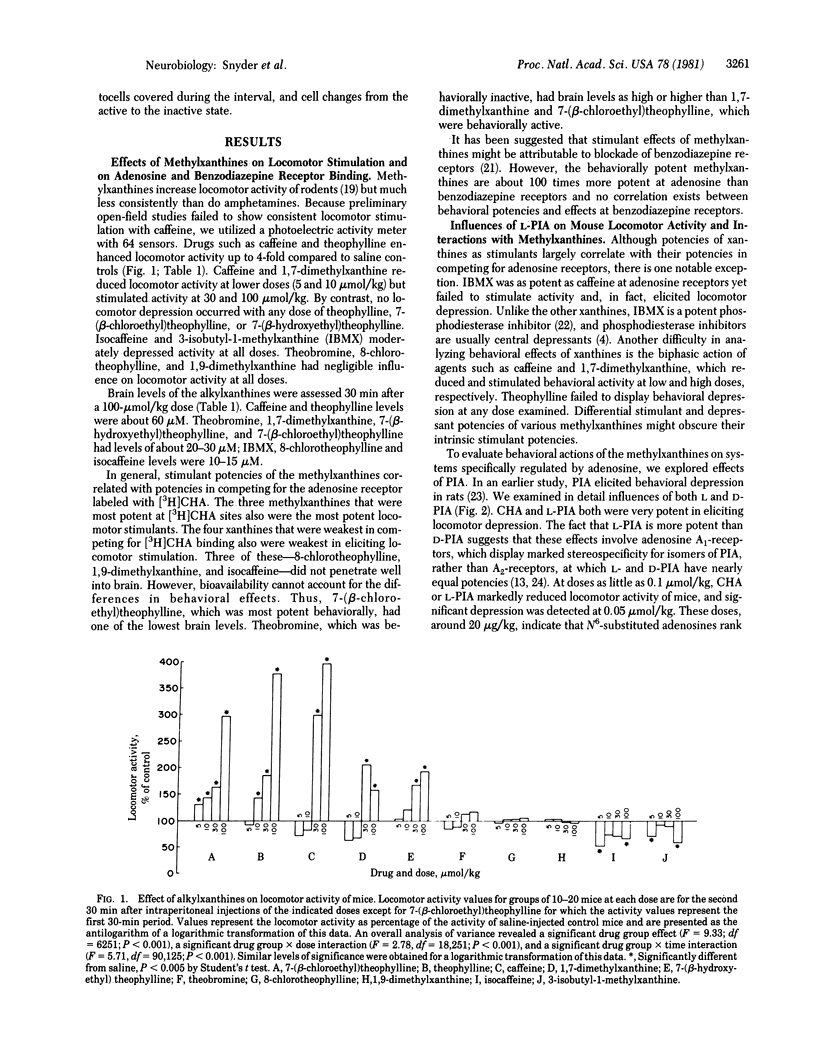
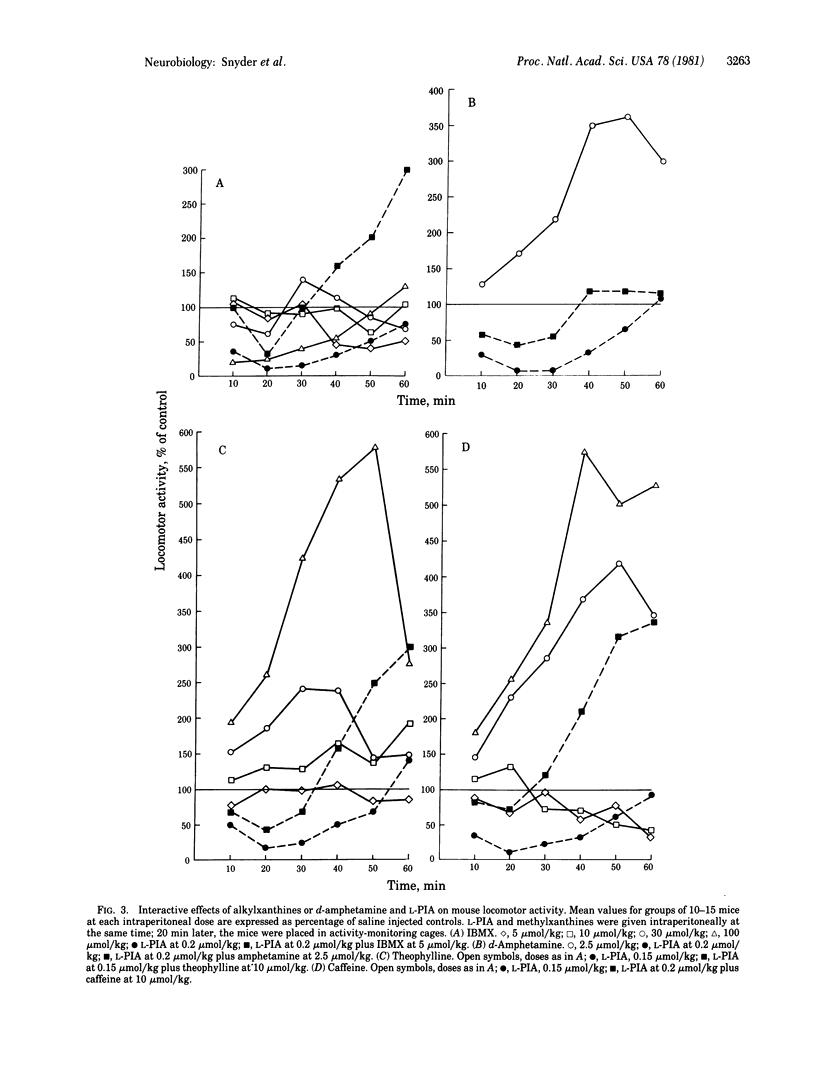
Central stimulant actions of 10 methylxanthines in mice correlate with affinities for adenosine receptors labeled with N6-[3H]cyclohexyladenosine. Affinities of methylxanthines for adenosine receptors are consonant with central levels attained at behaviorally effective doses. The much higher concentrations of methylxanthines required to influence benzodiazepine receptor binding do not correlate with behavioral potency. N6-(L-Phenylisopropyl)adenosine (L-PIA), a metabolically stable analog of adenosine with high affinity for adenosine receptors, is an extremely potent behavioral depressant, reducing locomotor activity of mice at doses as little as 0.05 mumol/kg. The D isomer, which has much less affinity for adenosine receptors, is much less active as a central depressant. Theophylline stimulates locomotor activity and reverses depressant effects of L-PIA. Caffeine or 1,7-dimethylxanthine, when administered alone, elicits biphasic effects, with locomotor depression at lower doses and stimulation at higher doses. When administered with L-PIA, even low doses of caffeine produce marked stimulation. 3-Isobutyl-1-methylxanthine given alone elicits only behavioral depression. However, like theophylline and caffeine, isobutylmethylxanthine reverses the L-PIA-evoked depression, converting it into pronounced locomotor stimulation. The data strongly suggest that the behavioral stimulant effects of methylxanthines involve a blockade of central adenosine receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Beer B., Chasin M., Clody D. E., Vogel J. R. Cyclic adenosine monophosphate phosphodiesterase in brain: effect on anxiety. Science. 1972 Apr 28;176(4033):428–430. doi: 10.1126/science.176.4033.428. [DOI] [PubMed] [Google Scholar]
- Blanchard J., Mohammadi J. D., Conrad K. A. Improved liquid-chromatographic determination of caffeine in plasma. Clin Chem. 1980 Aug;26(9):1351–1354. [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Dutta P., Mustafa S. J. Saturable binding of adenosine to the dog heart microsomal fraction: competitive inhibition by aminophylline. J Pharmacol Exp Ther. 1979 Dec;211(3):496–501. [PubMed] [Google Scholar]
- Fredholm B. B., Fuxe K., Agnati L. Effect of some phosphodiesterase inhibitors on central dopamine mechanisms. Eur J Pharmacol. 1976 Jul;38(1):31–38. doi: 10.1016/0014-2999(76)90198-9. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Hert R. C., Fain J. N. Characterization of [3H]adenosine binding to fat cell membranes. J Biol Chem. 1978 May 10;253(9):3114–3122. [PubMed] [Google Scholar]
- Murphy K. M., Snyder S. H. Adenosine receptors in rat testes: labeling with 3H-cyclohexyladenosine. Life Sci. 1981 Feb 23;28(8):917–920. doi: 10.1016/0024-3205(81)90054-0. [DOI] [PubMed] [Google Scholar]
- Newman M. E., De Lucia R., Patel J., McIlwain H. Adenosine-binding to cerebral preparations in interpretation of adenosine activation of adenosine 3':5'-cyclic monophosphate formation [proceedings]. Biochem Soc Trans. 1980 Feb;8(1):141–142. doi: 10.1042/bst0080141. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Edstrom J. P., Kostopoulos G. K., Kirkpatrick J. R. Effects of adenosine and adenine nucleotides on synaptic transmission in the cerebral cortex. Can J Physiol Pharmacol. 1979 Nov;57(11):1289–1312. doi: 10.1139/y79-194. [DOI] [PubMed] [Google Scholar]
- Sattin A., Rall T. W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3', 5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 1970 Jan;6(1):13–23. [PubMed] [Google Scholar]
- Schwabe U., Kiffe H., Puchstein C., Trost T. Specific binding of 3H-adenosine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):59–67. doi: 10.1007/BF00499875. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Trost T. Characterization of adenosine receptors in rat brain by (-)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Sep;313(3):179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- Skolnick P., Paul S. M., Marangos P. J. Purines as endogenous ligands of the benzodiazepine receptor. Fed Proc. 1980 Oct;39(12):3050–3055. [PubMed] [Google Scholar]
- Smellie F. W., Daly J. W., Dunwiddie T. V., Hoffer B. J. The dextro and levorotatory isomers of N-phenylisopropyladenosine: stereospecific effects on cyclic AMP-formation and evoked synaptic responses in brain slices. Life Sci. 1979 Nov 12;25(20):1739–1748. doi: 10.1016/0024-3205(79)90477-6. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Davis C. W., Daly J. W., Wells J. N. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979 Jun 25;24(26):2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bruns R. F., Daly J. W., Innis R. B. Multiple neurotransmitter receptors in the brain: amines, adenosine, and cholecystokinin. Fed Proc. 1981 Feb;40(2):142–146. [PubMed] [Google Scholar]
- Thithapandha A., Maling H. M., Gillette J. R. Effects of caffeine and theophylline on activity of rats in relation to brain xanthine concentrations. Proc Soc Exp Biol Med. 1972 Feb;139(2):582–586. doi: 10.3181/00379727-139-36191. [DOI] [PubMed] [Google Scholar]
- Vapaatalo H., Onken D., Neuvonen P. J., Westermann E. Stereospecificity in some central and circulatory effects of phenylisopropyl-adenosine (PIA). Arzneimittelforschung. 1975 Mar;25(3):407–410. [PubMed] [Google Scholar]
- Williams M., Risley E. A. Biochemical characterization of putative central purinergic receptors by using 2-chloro[3H]adenosine, a stable analog of adenosine. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6892–6896. doi: 10.1073/pnas.77.11.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Balls K., Rinaldi B. Specific binding of 2-[3H]chloroadenosine to rat brain cortical membranes. Can J Physiol Pharmacol. 1980 May;58(5):576–579. doi: 10.1139/y80-096. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


