Abstract
Thymic Stromal Lymphopoietin (TSLP) is crucial for the development of atopic diseases in humans and mice. Mice that express a lung-specific TSLP transgene (SPC-TSLP) develop a spontaneous and progressive asthma-like disease, suggesting that TSLP expression alone was sufficient for disease development. Here we show that, in fact, TSLP alone only causes a weak innate response that is insufficient for development of full airway inflammatory disease. Complete disease development requires both TSLP and antigenic stimulation. These data suggest that the spontaneous lung inflammation observed in SPC-TSLP mice reflects a TSLP-driven predisposition towards the development of aberrant responses against innocuous environmental antigens. This provides evidence that TSLP may act directly to induce susceptibility to the inappropriate allergic responses that characterize atopy and asthma. We additionally show that disease development requires CD4 T cells but not B cells. Further, we reveal a TSLP-driven innate response involving mucus overproduction and goblet cell metaplasia. Taken together, these data suggest a multi-faceted model of TSLP-mediated airway inflammation, with an initial activation of resident innate immune cells, followed by activation of the adaptive immune system and full disease development. This study provides new insight into the unique features of the asthma pathology contributed by the innate and adaptive immune responses in response to TSLP stimulation.
Keywords: Cytokines, Allergy, Inflammation, Lung
Introduction
Asthma is a chronic airway inflammatory disease characterized by infiltration of the bronchial mucosa by leukocytes, subepithelial fibrosis, mucus hyperproduction, goblet cell metaplasia, airway hyper-responsiveness (AHR), and elevated serum IgE(1). Disease development follows sensitization of susceptible individuals with aero-allergen, followed by challenge with the same antigen(2, 3). The challenge phases of asthma, which are characterized by rapid reoccurrence of disease symptoms, are relatively well-defined, involving rapid degranulation of lung-resident mast cells in response to cross-linking of FcεRI by IgE bound to allergen(4). This results in the release of large amounts of pro-inflammatory mediators into the lung tissue and airways followed by rapid onset of bronchoconstriction, mucous production, and airway remodeling. In contrast, the initial stages of disease development are much less-well understood, in particular the events leading to allergen sensitization and further progression to asthma. The state of sensitization to environmental allergens, commonly referred to as atopy, is defined by the presence of allergen-specific IgE in sera or bronchoalveolar lavage (BAL) and/or a positive inflammatory reaction in response to a classic skin-prick test with allergen, indicative of T-cell sensitization. The finding that fewer than half of all atopic individuals develop asthma symptoms(5, 6) indicates that factors beyond sensitization to allergen are necessary for the development of asthma.
Thymic Stromal Lymphopoietin (TSLP) is an IL-7 related cytokine shown to be critical to the pathogenesis of airway inflammation(7, 8). Though originally discovered due to its potent effects on B and T cell growth and differentiation (9, 10), (11), TSLP has also been shown to be associated with atopic diseases. For instance, epidermal TSLP expression in lesional skin of individuals with atopic dermatitis is markedly elevated(12), and, in human asthmatics, there is a high degree of correlation between TSLP expression in lung tissue and severity of disease (13). Murine models have extended these findings, showing that TSLP is both necessary and sufficient for the development of airway inflammation(7, 8). Wild type mice subjected to an antigen-induced model of asthma showed a dramatic upregulation of TSLP expression in the lungs(7) and mice deficient in the TSLP receptor (TSLPR−/−) were resistant to development of disease in this model(7, 8). Furthermore, mice that express a lung-specific TSLP transgene, driven by the Surfactant Protein C promoter (SPC-TSLP), developed severe airway inflammation characterized by leukocyte infiltration of the bronchial mucosa, AHR, excess mucous secretion, goblet cell metaplasia, and elevated serum IgE(7), all hallmark features of asthma(1). These data demonstrate a critical role for TSLP in the development of airway inflammation. A larger question remains as to the role of TSLP in disease progression, and to the extent that TSLP regulates innate and adaptive immune responses in the lung. In particular, the phenotype observed in SPC-TSLP mice develops spontaneously over a 2–3 month period without the overt sensitization and challenge necessary in other models of asthma. These data suggest that TSLP may either induce the development of asthma-like disease in the absence of allergen-specific adaptive immunity or establish an immunologic environment where sensitization to otherwise innocuous environmental allergens can occur, as is believed to take place in human asthma. To investigate these possibilities we developed an acute model of TSLP-induced airway inflammation enabling us to systematically test the role of antigen and the adaptive response in this disease. Interestingly, development of disease in this acute system is entirely dependent on the presence of foreign antigen and CD4 T cells, demonstrating for the first time that an adaptive immune response is a crucial aspect of TSLP-driven airway inflammation. These studies, when paired with the SPC-TSLP data, suggest that dysregulated expression of TSLP may represent an important susceptibility factor in the generation of aberrant Th2-responses against otherwise innocuous environmental allergens.
Materials and Methods
Mice
Balb/c mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) or Taconic. Rag2−/− and C3H/Hej-Balb/c (TLR4mut) mice were also purchased from The Jackson Laboratory. TSLPR-deficient mice were described previously(14). JHD−/− mice were purchased from Taconic. SPC-TSLP mice were described previously(7). All mice were greater than 10 generation backcrossed onto Balb/c. All animals were housed in specific pathogen free conditions in the Benaroya Research Institute animal facility and all experiments were performed as approved by the Benaroya Research Institute Institutional Animal Care Committee (Seattle, Wa).
Intranasal treament
Anesthetized animals were injected i.n. with 500 ng TSLP (R&D systems, generous gift of Amgen Inc.), 25 µg MSA (Sigma), 25 µg OVA (Sigma) or a combination of TSLP + MSA or OVA in a total volume of 40 µl PBS. Following i.n. administration mice were suspended in an upright position for 10 minutes to ensure complete uptake of the treatment solution.
Bronchial alveolar lavage, tissue fixation and staining
Mice were euthanized by lethal injection with 1 ml of 2.5% Avertin in PBS, administered i.p. . The lungs were subjected to BAL four times, each with 1 ml of PBS through a tracheal cannula. The BAL was centrifuged at 1400 × g for 5 min in order to collect the cell pellet. BAL cells were resuspended in PBS plus 1% BSA and counted using a hemocytometer. Differential cell counts were performed on cytospin cell preparations stained with a modified Wright-Geimsa stain on a Hematek 2000 slide stainer (Bayer Corp, Diagnostics Division, Elkhart, Ind).
After BAL, lungs were excised completely from the chest cavity, inflated with 10% neutral buffered formalin (Fisher BioTech) and fixed at room temperature, overnight, in the same solution. Tissues were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) and periodic acid schiff (PAS) stains.
Evaluation of AHR
Enhanced pause (Penh) measurements of AHR were made one day prior to sacrifice in response to increasing doses of aerosolized methacholine (Sigma) in PBS using unrestrained whole body plethysmography (Buxco Electronics, Troy, NY) as previously described with slight modification (7). Each methacholine dose was given over a 3-minute period and the average Penh value was measured during a subsequent 5-minute interval.
Adoptive Transfers
For transfers into rag2−/− mice CD4+ T cells were isolated by negative selection using Dynal CD4 No Touch kits from spleen and lymph nodes of naïve mice. 5 × 105 cells were transferred via retro-orbital injection into rag2−/− recipients. Beginning at day 1 post-transfer recipient mice were treated with MSA in 40 µl PBS or TSLP + OVA in 40 µl PBS every other day for 14 days. Following the treatment regimen mice were euthanized and analyzed as per the above protocols.
Acute CD4 depletion
Animals were i.p. injected on Day −7, 0, and 7 with 250 µg of anti-CD4 antibody (clone: GK1.5, UCSF Monoclonal Antibody Core) or 250 µg rIgG (Sigma) in a total volume of 100 µl PBS. Beginning on Day 0 animals were treated i.n. with PBS or TSLP + OVA in 40 µl PBS every other day and sacrificed on Day 14 as described above.
Statistics
All statistical analysis was performed using GraphPad Prism 5. Unless otherwise indicated all statistical tests are one-way ANOVA with a Tukey post-hoc test with significance between groups represented as * for p≤0.05, ** for p≤0.01, and *** for p≤0.001.
Results
The development of TSLP-mediated airway inflammatory disease is antigen dependent
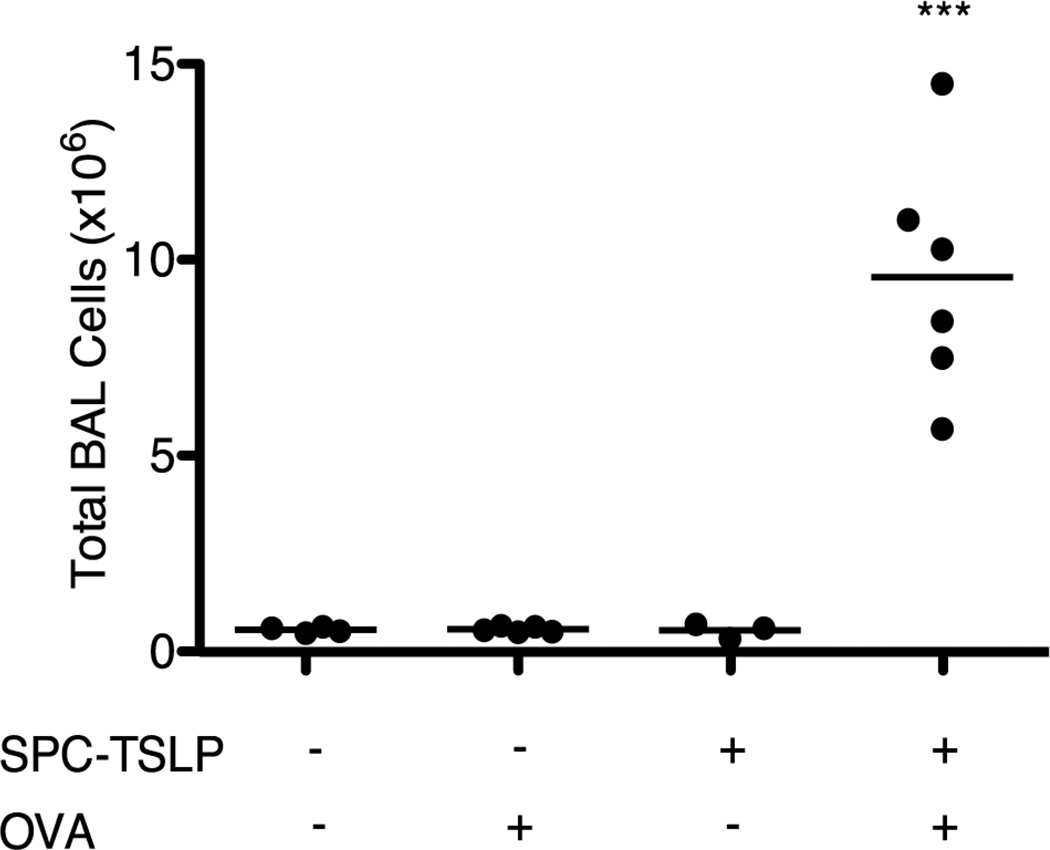
Previous studies have shown that mice expressing a lung-localized TSLP transgene exhibit a robust Th2-type inflammatory response that closely mirrors human asthma(7). In contrast to human allergic asthma, which is recognized as an antigen-driven process(1), the disease that develops in SPC-TSLP mice occurs spontaneously in the absence of overt antigen administration. This observation initially suggested that the disease observed in these animals was directly due to pathologic changes induced by TSLP without a requirement for antigenic stimulation. To investigate whether antigen does in fact play a role in TSLP-driven airway inflammation, SPC-TSLP mice were challenged intranasally (i.n.) with ovalbumin (25 µg every other day for two weeks), beginning at ~4–6 weeks of age (approximately 1–2 months prior to the onset of spontaneous symptoms.) OVA-challenged SPC-TSLP animals rapidly developed disease while neither PBS-treated SPC-TSLP nor OVA-treated WT mice displayed inflammation at that time. This is evidenced by increased infiltration of inflammatory cells in BAL (Figure 1), as well as the development of airway eosinophilia and AHR. These data show that the disease that develops in SPC-TSLP mice can be accelerated by overt airway antigen challenge.
Figure 1.
SPC-TSLP mice develop airway inflammation with earlier onset in response to OVA challenge relative to non-OVA treated SPC-TSLP mice. Total BAL cell counts from SPC-TSLP mice treated i.n. with OVA or PBS every other day for 14 days beginning at 4–6 weeks of age. Mice were analyzed between 6 and 8 weeks of age. Statistic are one-way ANOVA with Tukey post-test, ***=p≤0.001 when compared to all other groups.
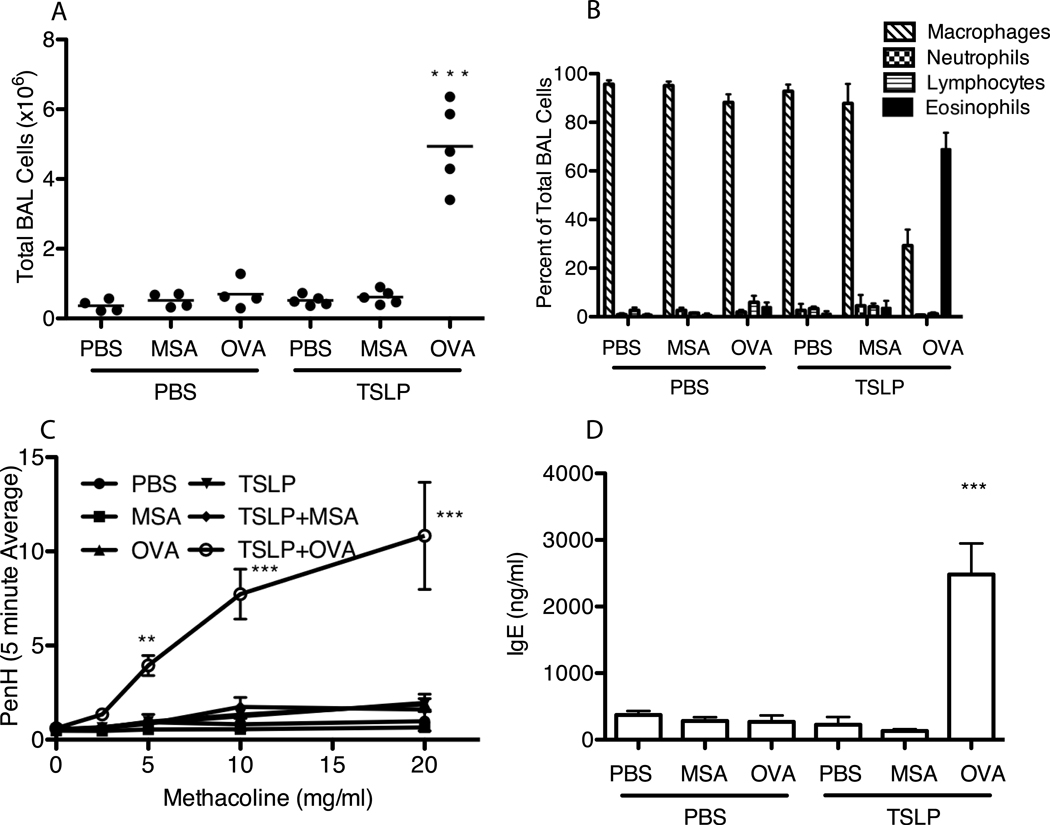
To systematically evaluate the role of antigen and the adaptive immune response in TSLP-mediated airway inflammation we developed an acute model of TSLP-induced asthma. Balb/c mice were subjected to i.n. TSLP, administered in the presence or absence of OVA (mouse serum albumin, MSA, was used as a control), every other day for two weeks. Only those mice that received both TSLP and OVA displayed significant increases across all disease parameters. Total BAL cell counts performed on TSLP + OVA treated animals produced an average of 4.94 × 106 cells, a nearly ten-fold increase over animals in all other treatment groups, all of which displayed approximately equivalent cell counts (Figure 2A). Furthermore, only TSLP + OVA treated mice displayed airway eosinophilia (Figure 2B), AHR (Figure 2C), and elevated serum IgE (Figure 2D), consistent with asthma development in this treatment group.
Figure 2.
Acute intranasal TSLP induces airway inflammation only in the presence of foreign antigen. WT Balb/c mice were treated i.n. every other day for 14 days with PBS or 500ng TSLP with or without 25 mg MSA or OVA. (A) Total number of cells isolated from BAL. (B) Cells isolated from Bal were cytospun onto glass specimen slides and stained with modified wright-giemsa stain to different between macrophages, neutrophils, lymphocytes, and eosinophils. Values shown are mean ± SEM with n≥4 for each group. (C) PenH as a measure of AHR on Day 14 in response to increasing concentrations of methacoline. (D) Serum from whole blood collected from animals at sacrifice was assayed for total IgE by ELISA. Values shown represent mean ± SEM with n=3 per group, *** indicates that labeled group is significant against all other groups with p≤0.001.
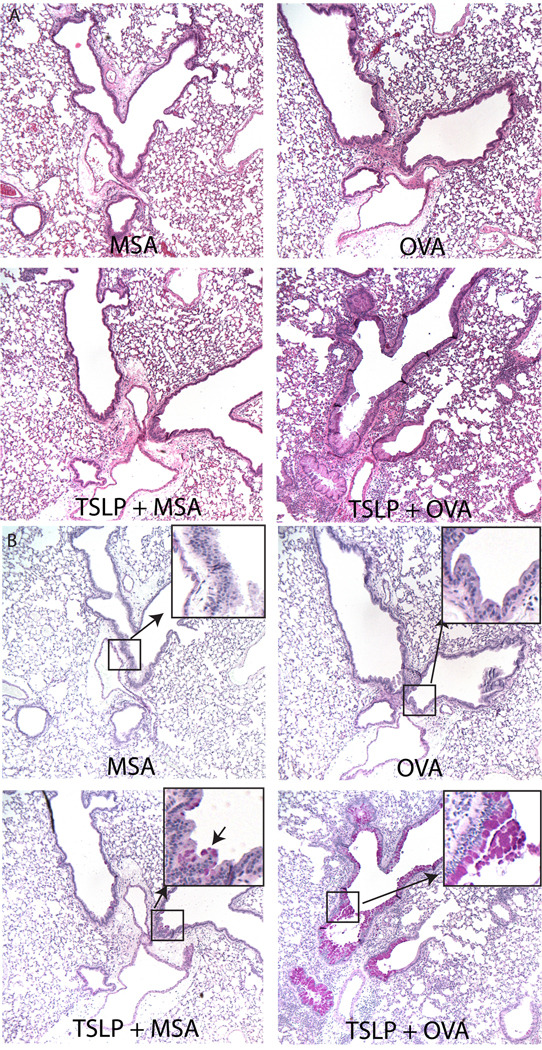
Histological examination of the lungs from these animals yielded similar results. Control groups (PBS, MSA, OVA, TSLP, or TSLP + MSA treated animals) showed only rare, if any, infiltrating cells in the lung tissue (Figure 3A and data not shown). In contrast, TSLP + OVA treated animals had dramatic infiltrates around blood vessels, large proximal airways, and less frequently, small distal airways. The cellular infiltrates consisted primarily of eosinophils (Figure 3A). PAS-stained lung sections showed extensive goblet cell metaplasia and mucous production (pink staining cells, Figure 3B) in TSLP + OVA treated mice compared to all other groups. Interestingly, all mice that received TSLP displayed some goblet cell metaplasia in spite of the lack of infiltrating cells in TSLP and TSLP+MSA-treated animals. (Figure 3B and data not shown). In contrast, PAS-staining cells were undetectable in mice that did not receive TSLP.
Figure 3.
Disease phenotype in the lung of intranasal TSLP-treated mice varies depending on the presence of antigen. Paraffin sections of lungs excised from mice treated with MSA, OVA, TSLP+MSA, or TSLP+OVA every other day for 14 days were stained with (A) H&E or (B) PAS. Pink staining cells under PAS represent mucous producing goblet cells. Magnified inset highlights rare pink-staining cells in airway of TSLP+MSA treated animals not present in MSA or OVA alone treated animals.
In order to ensure that the disease we observed using this model system was TSLP-dependent, and not due to the influence of potential contaminating factors such as LPS in either the TSLP or OVA stocks, TSLPR-deficient and LPS-nonresponsive, TLR4-mutant, mice were treated with i.n. TSLP + OVA. Consistent with this being a TSLP-dependent process, TSLPR-deficient mice failed to show any significant increase in lung inflammation (Supplemental Figure 1B), while the TLR4mut mice developed disease indistinguishable from that seen in WT mice (Supplemental Figure 1A).
An intact lymphocyte compartment is required for the acute development of TSLP-induced allergic airway inflammation
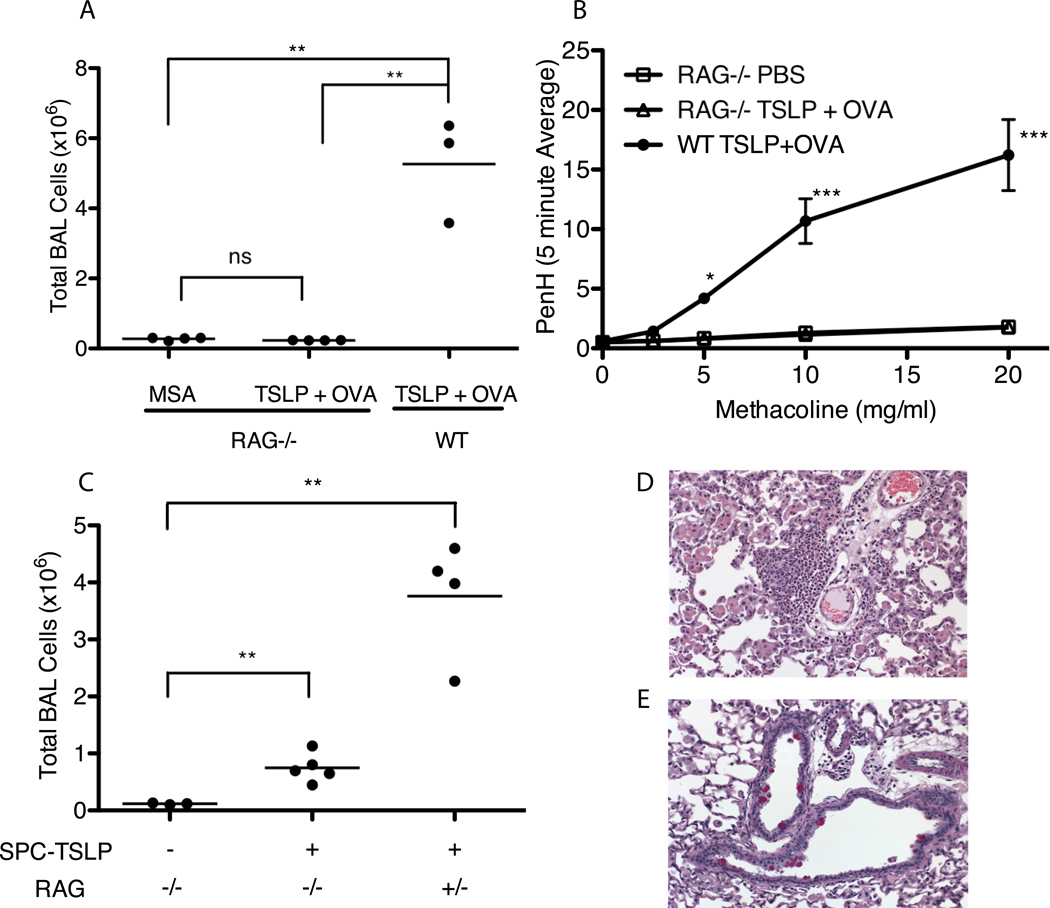
The finding that TSLP-mediated airway inflammation is antigen-dependent predicted that the adaptive arm of the immune response is likely a crucial factor in the ability of TSLP to generate severe airway inflammation. To test this directly, rag2−/− mice, which lack T and B cells, were treated with MSA alone (as control) or TSLP + OVA (Figure 4). Consistent with a role for the adaptive immune system in mediating the pathogenic features of this disease, Rag2-deficient mice were protected from the development of allergic airway inflammation. Rag2−/− mice that received TSLP + OVA exhibited no significant increase in the number of cells in BAL(Figure 4A), airway eosinophilia, lung tissue infiltration, goblet cell metaplasia, and failed to develop AHR in contrast to the WT TSLP+OVA-treated mice (Figure 4B and data not shown).
Figure 4.
TSLP-mediated airway inflammatory disease requires an intact lymphocyte compartment for full disease development. (A) Total number of cells isolated from BAL taken from Rag2−/− or WT Balb/c mice that were treated every other day for 14 days with MSA or TSLP+OVA. Statistics are from one-way ANOVA with a Tukey post-test, ** = significant with p≤0.01 (B) AHR, represented as the PenH value measured over increasing doses of methacoline. Points are mean±SEM. Statistics are two-way repeated measures ANOVA with Bon Ferroni post-tests, *** = p≤0.001 and *=p≤0.05 with n≥3 for each group. (C) Total BAL Cell counts from SPC-TSLP X Rag−/− or SPC-TSLP X Rag +/− (heterozygous control) mice sacrificed between 4 – 6 months of age. Statistics are from two-tailed student T-tests comparing groups, *** = p≤0.001 and **=p≤0.01 (D&E) Representative paraffin sections of lung excised from SPC-TSLP X Rag−/− mice and stained with H&E (D) or PAS (E) showing presence of rare pockets of lung infiltrating cells and pink staining goblet cells.
Our findings were somewhat different with the more chronic SPC-TSLP transgenic system. SPC-TSLP/rag2−/− mice showed a dramatic decrease in inflammation relative to Rag2-sufficient SPC-TSLP animals, consistent with the results from mice given TSLP intranasally. However, SPC-TSLP/rag2−/− did develop mild but significant BAL infiltration at 5–6 months of age (Fig 4C), showing an approximately 6-fold increase in BAL cells over the transgene negative controls (Rag2-sufficient SPC-TSLP animals display an approximately 30-fold increase over transgene negative controls.) Additionally, lung histology revealed very rare sites of eosinophilic infiltration (Figure 4D) and metaplastic goblet cells (Figure 4E), similar to what we observed in mice that received only TSLP in our acute system (Figure 3B). We hypothesize that the late-developing phenotype observed in SPC-TSLP/rag2−/− mice is indicative of the effect of TSLP on lung-resident innate cells.
The absence of disease development seen in rag2−/− mice is largely due to the absence of CD4 T cells not B cells
There is considerable evidence for important roles for CD4 T cells and B cells (largely through the production of IgE) in the recurrent episodes that characterize human asthma(1). However, the contribution of these two cell populations to the initiation of airway inflammation is not as well characterized and more over hasn’t been evaluated in the context of TSLP-driven disease. To determine the contribution of CD4 T cells and B cells in TSLP-induced airway inflammation, we subjected animals genetically deficient in B cells (JHD−/−) or acutely depleted of CD4 T cells to i.n. TSLP administration.
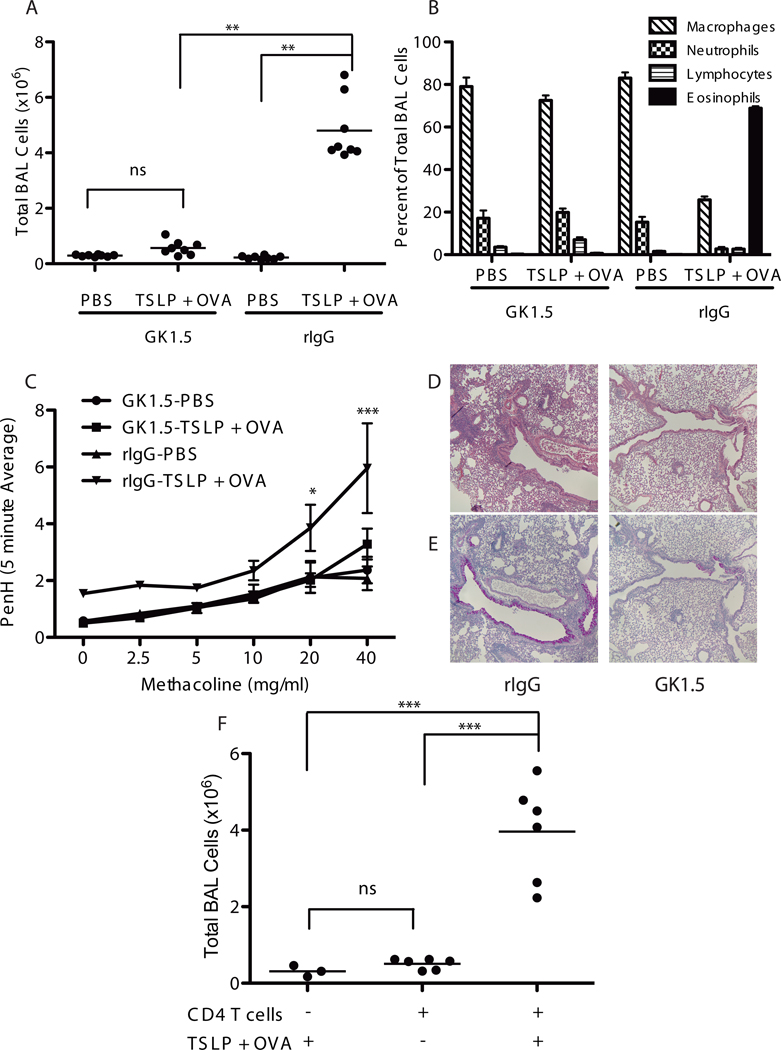
Two experimental protocols were used to assess the role of CD4 T cells in TSLP-mediated airway inflammation. First, mice were treated with either the CD4-depleting antibody GK1.5 or isotype control antibody once per week for 3 weeks. Beginning at the second administration, mice were given i.n. TSLP + OVA every other day for 2 weeks. This protocol led to a nearly complete loss of CD4+ T cells in the GK1.5-treated animals (data not shown). Acute depletion of CD4 T cells led to a dramatic reduction in the development of disease including BAL infiltrates, airway eosinophilia, AHR, and mucus production (Figure 5).
Figure 5.
CD4 T cells are required for TSLP-mediated airway inflammation. (A) Total BAL cell counts from WT Balb/c mice treated i.p. with rIgG or anti-CD4 antibody (GK1.5), to acutely deplete CD4 T cells, over the time course of i.n. treatment with PBS or TSLP+OVA.(B)Differential cell counts performed on wright-giemsa stained BAL cells. (C) Assessment of AHR, points are mean±SEM. Statistics are two-way repeated measures ANOVA with Bon Ferroni post-tests, *** = p≤0.001 and *=p≤0.05 with n=4 for each group. (D,E) Paraffin sections from lungs excised from TSLP+OVA treated mice that received either rIgG or GK1.5 and stained with H&E (D) or PAS (E). (F) Total BAL cell counts from rag2−/− mice that were adoptively transferred with 5 × 106 CD4 T Cells one day prior to initiating PBS or TSLP+OVA treatment every other day for 14 days. (A&F) Statistics are one-way ANOVA with Tukey post-test ***=p≤0.001 and **=p≤0.01.
The second approach involved adoptive transfer of CD4 T cells into Rag2-deficient mice, followed by TSLP + OVA treatment. As shown in Figure 5F, reconstituting Rag-2-deficient mice with CD4 T cells completely rescued the airway inflammatory phenotype in response to i.n. TSLP + OVA. Taken together, these data demonstrate a critical role of CD4+ T cells in the inflammation seen in these mice following TSLP + OVA treatment.
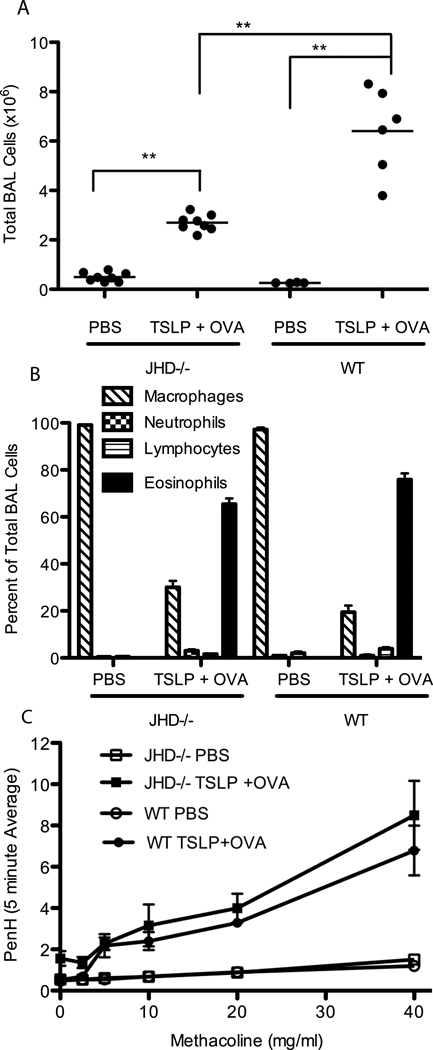
In contrast, TSLP + OVA treated JHD−/− mice, genetically deficient in B cells, developed significant disease symptoms, although the level of BAL cellularity was decreased relative to WT mice treated with TSLP + OVA (Figure 6A). Importantly, the characteristic parameters of disease were unchanged relative to WT mice, including airway eosinophilia, AHR, and goblet cell metaplasia (Figure 6B, C and data not shown), indicating that B cells were not required for the development of airway inflammation in response to TSLP. However, the decrease in overall cellularity was greater than what would be expected from simply a loss of B cells. Suggesting that, while not required, B cells do contribute to the overall inflammatory phenotype in an amplifying capacity.
Figure 6.
B cells aren’t required for TSLP induced airway inflammation but serve to amplify the response. JHD−/− (B cell deficient) or WT Balb/c mice were treated i.n. with PBS or TSLP+OVA every other day for 14 days. (A) Total BAL cell counts, (B) differential cell counts performed on BAL cells (C) assessment of AHR as measured by PenH. (A) Statistics are one-way ANOVA with Tukey post-test ***=p≤0.001 and **=p≤0.01. (C) Points are mean±SEM, both JHD−/− and WT treated with TSLP+OVA are significantly different (p≤0.001) than either the JHD−/− or WT PBS treated groups but not from each other by two-way repeated measures ANOVA with Bon Ferroni post tests.
Discussion
It has been clearly shown that the cytokine TSLP is critical for the development of asthma-like disease in mice, as well as playing a role in humans(7, 13). However, the sequence of events and the factors involved in that response have not fully been elucidated. Previous studies have led to a proposed model in which TSLP, expressed by cells of epithelial origin, acts on dendritic cells to facilitate their activation and the subsequent priming of T cells to a Th2 phenotype in the lymph node(15). Integral to this model is the antigen-specific nature of the interaction between the DCs and T cells, however the role of antigen-specificity in the pathogenesis of TSLP induced asthma has not been previously addressed. Several lines of evidence have in fact suggested that T cells, and consequently antigen responsiveness, may not be involved in TSLP-induced inflammatory diseases. In particular, Th2-associated disease develops spontaneously in lung-specific (SPC-TSLP) and skin-specific (K5-TSLP) TSLP transgenic mouse models (asthma and atopic dermatitis respectively) without an overt antigenic component(7, 16). Furthermore, T cell-deficient K5-TSLP mice developed disease with the same kinetics and pathology as T cell-sufficient K5-TSLP mice, suggesting that innate not adaptive immune responses were critical for disease in this model (16). These data taken together suggested that TSLP might function in a fashion similar to the effector cytokine IL-13, which has been shown to directly induce pathogenic changes in the lung irrespective of antigen involvement(17–19) However, the data presented in this study demonstrates for the first time that the pathogenic phenotype that develops in the lung in response to TSLP is governed almost exclusively by a TSLP-facilitated adaptive response directed against foreign antigen.
One unanswered question in human asthma concerns the fact that only about 50% of individuals who display atopy (antigen sensitization) go on to develop an allergic or asthmatic phenotype(6). Importantly, our finding that acute treatment with TSLP is insufficient to generate a complete and robust asthma-like phenotype in the absence of antigenic stimulus (Figures 1 and 2) suggests that TSLP may function to establish the proper immunologic context to enable sensitization to otherwise innocuous antigens. Support for this hypothesis comes from the spontaneous disease observed in SPC-TSLP mice, which likely represents a TSLP-dependent response against otherwise harmless environmental allergens, such as what could be found in food and bedding. This type of response is highly reminiscent of the various presumptive etiologies of human asthma. Additionally, recent studies have identified allergy-associated SNPs present in either the TSLP-gene itself(20), or in the gene encoding the IL-7R α-chain(21), which pairs with the TSLPR to form the functional TSLP receptor complex. Collectively, these data suggest an important role for TSLP as a factor predisposing individuals towards the generation of inappropriate responses against environmental antigens. Further, highlighting the impact of relatively minor changes in TSLP expression is the fact that SPC-TSLP mice display only a 1.5–2 fold increase in TSLP expression in the lung, as compared to non-transgenic control animals(7). In spite of this relatively small change in expression, 100% of these mice develop severe airway disease that is rarely, if ever, seen spontaneously in WT mice. These data indicate that minor increases in TSLP expression have the potential to result in dramatic consequences for the host, and illustrate the importance of tight regulation of TSLP expression.
While the majority of the asthma-like phenotypic changes induced by TSLP are dependent on the presence of antigen, we also found in two separate settings that TSLP is able to directly induce minor pathogenic changes in the lung. This is evidenced both by the presence of mucin-producing goblet cells seen in animals treated with only intranasal TSLP (Figure 3), as well as the diminished, but present, inflammatory phenotype displayed by Rag2-deficient SPC-TSLP animals (Figure 4). Interestingly, in the SPC-TSLP/rag2−/− mice, even in the absence of an adaptive response, the Th2-character of the disease that develops remains intact. These data suggest that the classic Th2 features, such as eosinophilia and mucous production, are not necessarily a byproduct of a skewed adaptive response but rather, may be an intrinsic characteristic of TSLP exposed adaptive and innate immune cells. Recent studies have established that mast cells(22) and NKT cells(23) represent potential lung-resident populations capable of producing of various cytokines, including IL-5 and IL-13, in direct response to TSLP. Furthermore, IL-13 has been shown to be an important mediator of goblet cell metaplasia and mucous production(19). These data suggest that prolonged exposure to TSLP alone is capable of activating a mild innate response, possibly through induction of IL-13 production from lung-resident cells, but that adaptive responses are required for complete inflammatory disease development. The extent of the contribution of this TSLP driven innate response to the overall asthma phenotype remains to be fully examined.
Seshasayee et al., using a similar system to what is described here, have recently shown that blockade of OX40L abrogated the development of airway inflammation mediated by TSLP(24). This report suggested that TSLP treatment alone was sufficient to drive a minimal inflammatory response; however, we do not see a similar response in mice in our facility (Fig. 2 and data not shown). . One explanation for the discrepancy is that the response seen in Seshasayee et al. represents the early stage of an adaptive response to an environmental antigen present in their animal colony, and that our colony either lacks this antigen, or has antigens with different immunogenicities. This possibility is consistent with the nearly 100-fold difference in magnitude (as assessed by Total BAL infiltration) between TSLP + OVA treated mice in our system versus TSLP-treated animals in Seshayee et al. Additional support for this interpretation is the complete lack of inflammatory cell infiltration in RAG−/− animals treated acutely with TSLP (Figure 4.) Taken together these data lend further support to our hypothesis that TSLP is capable of driving the development of immunopathologic responses against environmental antigens that would otherwise be unnoticed by the immune system.
In this study we additionally evaluated the contribution of various components of the adaptive response downstream of TSLP in generating the overall asthma phenotype. By acutely depleting CD4 T cells prior to intranasal treatment with TSLP and OVA we found CD4 T cells to be essential to TSLP-induced asthma (Figure 5). This is in contrast to the previously published finding that there isn’t a requirement for T cells downstream of TSLP activity in the development of atopic dermatitis (using the K5-TSLP mouse line) (16). Importantly, these data suggest that the cellular mediators of TSLP-induced responses can vary considerably, dependent on the tissue involved. However, as both atopic dermatitis and asthma share a very similar Th2 phenotype, this lends further credence to hypothesis that the Th2 character of TSLP-driven responses is intrinsic to the biology of TSLP itself rather than being a product of the exact cells that act as downstream players in the response.
Additional support for this model comes from work using helminth-driven Th2-type lung inflammation, a system sharing a very similar disease phenotype to what is observed in asthma. In these studies, CD4 T cells, as well as the classic Th2 cytokines IL-4 and IL-13, are required for the generation of a Th2 response to clear the helminth Nippostrongilus brasiliensis in the lung. However, the source of the cytokines appears to be an as yet undetermined myeloid cell population rather than the CD4 T cells(25). Although the source of IL-4 and IL-13 in both Nippostrongilus infection and the TSLP-mediated disease remains to be determined, it is clear that there is significant interplay between the innate and adaptive immune systems in generating a Th2-type inflammatory response in the lung.
Taken together, the above data demonstrate a critical role for an antigen-driven adaptive response in the development of TSLP-induced airway inflammation. However, in the absence of an adaptive immune response prolonged exposure to TSLP has mild effects on cells of the innate immune system, suggesting that TSLP is capable of coordinating innate and adaptive responses in the lung. In the normal setting, TSLP has been shown to be involved in conditioning tissue-resident DCs for Th2 homeostasis(26, 27). These data, with in vitro data demonstrating that TSLP-activated DCs can drive the differentiation of inflammatory Th2 cells(12, 28), suggest the following 2-stage model for TSLP-mediated airway inflammation. TSLP, produced by airway epithelial cells(12), acts on DCs to drive the differentiation of allergen-specific Th2 cells and also activates lung resident innate immune cells to begin airway remodeling, possibly through production of IL-13. The allergen-specific Th2 cells are largely responsible for organizing the subsequent pathogenic changes in the lung. The implications of this have wide-reaching importance both for the basic understanding of the biology of TSLP, as well as for potential roles for this cytokine in facilitating/predisposing the development of immunopathologic responses against non-pathogenic environmental antigens leading to asthma development.
Supplementary Material
Acknowledgements
We thank Mary Beauchamp, Xiaocui Sun, and Sterling Eckard for technical assistance, Drs. Jessica Hamerman and Daniel Campbell for critical discussion of manuscript, and members of the Ziegler laboratory for helpful discussions. We thank Matt Warren for his administrative support.
Footnotes
This work was partially supported by NIH grants AI44259, AI68731, AI71130; DOD grant USAMRAA W81XWH-07-0246 to S.F.Z and NCI training grant Basic Immunology T32, CA009537 to M.B.H.
References
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annual review of immunology. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 2.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Bradding P. The re-emergence of the mast cell as a pivotal cell in asthma pathogenesis. Current allergy and asthma reports. 2005;5:130–135. doi: 10.1007/s11882-005-0086-9. [DOI] [PubMed] [Google Scholar]
- 5.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nature reviews. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 6.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, Hudkins K, Dooley J, Farr A, Alpers CE, Ziegler SF, Rawlings DJ. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. 2007;8:522–531. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 10.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 11.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 12.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 13.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 14.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, Ihle JN. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 16.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. The Journal of allergy and clinical immunology. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 18.Venkayya R, Lam M, Willkom M, Grunig G, Corry DB, Erle DJ. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26:202–208. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, Avila L, Liang C, Lake SL, Hudson TJ, Spesny M, Fournier E, Sylvia JS, Freimer NB, Klanderman BJ, Raby BA, Celedon JC. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177:830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamim Z, Muller K, Svejgaard A, Poulsen LK, Bodtger U, Ryder LP. Association between genetic polymorphisms in the human interleukin-7 receptor alpha-chain and inhalation allergy. International journal of immunogenetics. 2007;34:149–151. doi: 10.1111/j.1744-313X.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 22.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. International archives of allergy and immunology. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 24.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YM, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 27.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.