Abstract
Naïve CD4 T cells can differentiate into a number of functional subsets in response to antigen, including Foxp3+ induced regulatory T cells (iTreg). The in vivo development and function of iTreg has been primarily demonstrated in systems involving antigen encountered systemically or delivered via the intestinal mucosa. In this study, we demonstrate that de novo Foxp3 expression in naïve CD4 T cells is a critical mechanism for establishing tolerance for a tissue-restricted neo-self antigen. Naïve CD4 T cells lacking a functional Foxp3 gene cannot achieve tolerance, but can be suppressed in vivo in the presence of wild-type naïve CD4 T cells. Exposure to non-specific inflammation during priming undermines tolerance through impaired Foxp3 induction, suggesting that the microenvironment also plays a role. Together, these data show that de novo Foxp3 expression is an integral component of establishment and maintenance of tolerance among naïve peripheral CD4 T cells.
INTRODUCTION
Naïve CD4 T cells can develop into a variety of specialized effector or regulatory subsets1. Some priming mechanisms result in tolerance, particularly to self antigen. Deletion and anergy can be achieved intrinsically by signals that lead to programmed death or inactivation in which cells are refractory to further stimulation2. Additionally, effective trans-suppression is mediated by regulatory T cells (Treg) that express the transcription factor Foxp33. The immune suppressive functions of persistent pathogens can work partly through induction of Foxp3 in microbe-specific CD4 T cells4. Expression of Foxp3 is known to dampen CD4 T cell effector responses, suggesting that de novo Foxp3 expression may be both an intrinsic and extrinsic mechanism of antigen-specific tolerance.
It is well appreciated that TGFβ and retinoic acid are key mediators of peripheral Foxp3 induction, and these signals are enhanced in a subpopulation of antigen-presenting cells such as dendritic cells expressing CD103 and/or DEC-2055. Antigen exposure in the context of chronic microbial infection, low-level peptide infusion, systemic soluble antigen, or oral administration can all induce Foxp3+ Treg from naïve CD4 T cell precursors6. Inflammatory cytokines such as IL6, IL12, IL27 and IFNγ can undermine TGFβ-mediated induction of Foxp3, although IL27 and IFNγ may also enhance Foxp3 induction7–10.
The significance of de novo Foxp3 expression in the maintenance of tissue-specific tolerance is unclear. The estimated frequency of peripherally generated Foxp3+ Treg ranges from 30%11 to less than 10% of total Treg12. Normal peripheral Treg conversion is thought to occur at mucosal surfaces in response to antigens derived from food, commensal bacteria or inhaled particles. Peripheral de novo Treg conversion in response to tissue antigen is not well defined. Here we demonstrate a model of islet tolerance in which de novo expression of Foxp3 is critical for prevention of autoimmunity. Peripheral Treg are shown to be effective for trans-suppression of autoreactive effector T cells. These results provide compelling evidence of a role for de novo Foxp3 expression in the maintenance of tissue-specific CD4 T cell self tolerance.
MATERIALS AND METHODS
Mice
DO11.10 mice and CD45.1 congenic B6.SJL-Ptprca Pepcb/BoyJ mice were purchased from the Jackson Laboratory. CD45.1+ mice were backcrossed to Balb/c mice for at least 10 generations. RO/RAG° mice on the Balb/c background were provided by A. Abbas (UCSF, San Francisco, CA). Foxp3-GFP mice were provided by A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY) and backcrossed to Balb/c mice for at least 10 generations. Influenza HA-specific HNT transgenic mice were provided by Dr. S. Swain (University of Massachusetts, Worcester, MA). All mice were maintained in a specific pathogen-free facility. All protocols were conducted as approved by the Animal Care and Use Committee of the Benaroya Research Institute.
Diabetes monitoring and induction
Blood glucose levels (BGL) in chimeric mice were monitored twice a week using an Ascensia Contour blood glucose monitoring device. Mice were considered diabetic after two consecutive BGL readings above 250 mg/dL. As indicated, mice were treated with 100µg of Ovalbumin peptide 323–349 (New England Peptide) emulsified in Incomplete Freund’s Adjuvant (IFA) injected subcutaneously. Mice treated with polyIC (Invivogen) were given intraperitoneal injections of 100µg of polyIC in PBS at the indicated time points.
IL-2 complex treatment
Recombinant murine IL-2 (eBioscience) was incubated with anti-IL-2 monoclonal antibody clone JES6-1 (UCSF Monoclonal Antibody Core) in sterile PBS at 25C for 30–60 minutes. Following incubation, the complex was injected into mice (1.5ug IL-2, 50ug IL-2 mAb per mouse) intraperitoneally every other day for a total of three treatments. Five days after the first treatment, spleens were harvested and CD4 T lymphocytes were analyzed for intracellular Foxp3 expression.
Flow cytometry and cell sorting
Fluorochrome-conjugated mAbs for murine CD4 (RM4-5), DO11.10 TCR (KJ1-26), Foxp3 (FJK16s), CD45.1 (A20), IFNg (XMG1.2), and IL17A (TC11-18H10.1) were purchased from eBioscience or Biolegend. Intracellular detection of Foxp3 was achieved using fixation and permablization protocol and buffers purchased from eBioscience. To purify CD4 T cells, no-touch magnetic bead purification was conducted using the Miltenyi Biotec CD4 T cell isolation kit.
Histology
Pancreata were snap-frozen on dry ice in O.C.T. (Tissue-Tek) and frozen blocks were sectioned into 5µm slices. Slides were fixed in acetone, dried and stained with Hematoxylin and Eosin. Analysis used Leica DM2500 light microscope and images were acquired with attached SPOT Insight 4 digital camera with SPOT 4 Advanced imaging software. No post-acquisition image modifications were made, other than incorporation of scale bars.
Intracellular cytokine production
Single-cell splenocyte suspensions were incubated at 37C in complete RPMI media (cRPMI) alone or in cRPMI with 1µg/mL Ovalbumin 323–349 peptide. For CD3/CD28 stimulation, tissue-culture plates were coated with 3 µg/ml anti-CD3 (145.2C11) and 1µg/ml anti-CD28 (PV-1) in plain PBS overnight, antibodies from UCSF Monoclonal Antibody Core. After one hour of incubation, Golgi Stop (BD Bioscience) was added and cells were incubated for an additional four hours.
RESULTS AND DISCUSSION
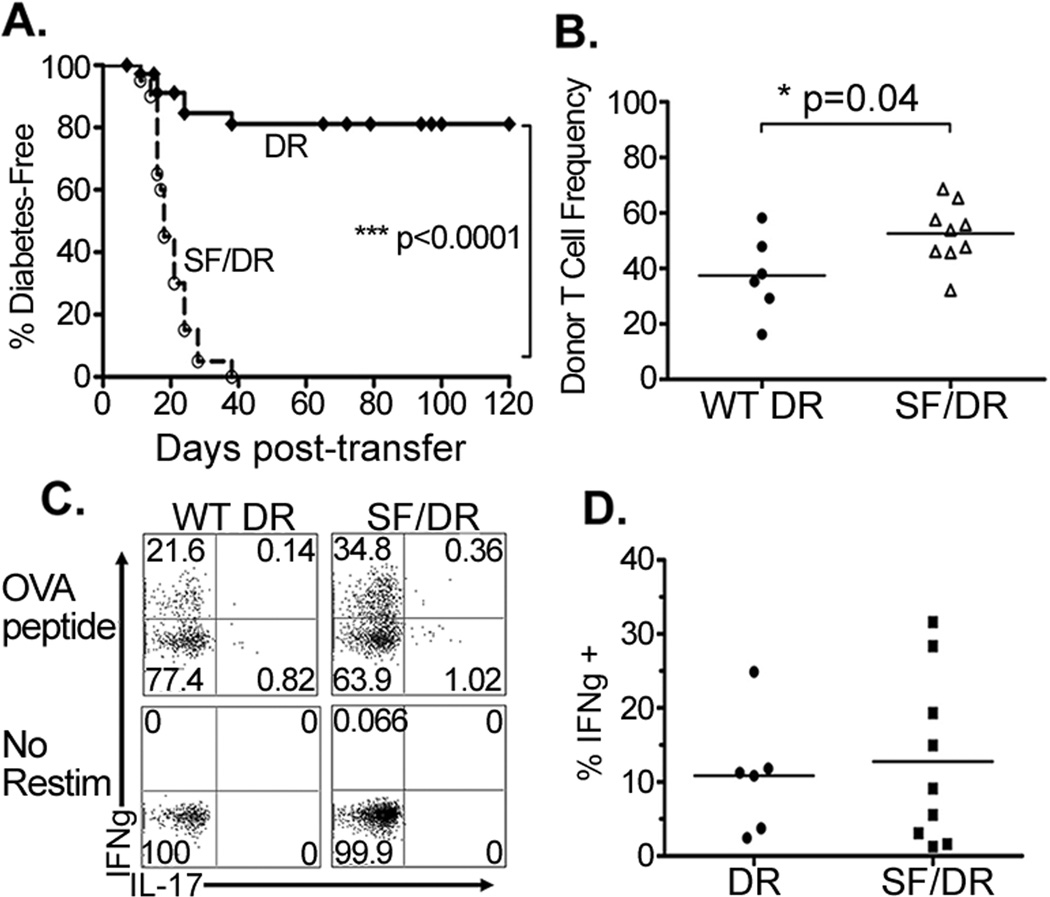
To generate antigen-specific CD4 T cells that lack endogenous Foxp3+ regulatory T cells, DO11.10 mice were crossed onto a Recombination Activating Gene deficient (RAG°)-background (referred to as DR mice or cells)13,14. These mice contained a monoclonal naïve CD4 T cells with specificity restricted to an epitope from ovalbumin (OVA323–339). To determine the ability of these cells to promote autoimmune disease in the absence of endogenous Treg, naïve DR CD4 T cells were transferred into RAG° mice expressing a transgene encoding membrane-bound OVA under control of the Rat Insulin Promoter (RO/RAG°). As a control, DR T cells were transferred into RAG° mice lacking the RO transgene. By day 80 post-transfer, none of the control mice had become diabetic, and >80% of the RO/RAG° recipients also remained diabetes-free (Figure 1a and data not shown). The low incidence of autoimmunity in the RO/RAG° recipients indicates that even in the absence of endogenous regulatory T cells, naïve tissue-specific Foxp3-negative CD4 T cells have an intrinsic mechanism for long-lasting tolerance. To determine the role of Foxp3 in tolerance generation we crossed DR mice with Scurfy mutant mice (SF/DR), which harbor a loss-of-function mutation of the foxp3 gene15. Because the DR cognate antigen is not expressed in these mice, they remain healthy and viable (13,16 and our unpublished data). When SF/DR donor CD4 T cells were transferred into RO/RAG° mice, 100% of the chimeric mice became diabetic within 40 days of the adoptive transfer (Figure 1a). Consistent with a robust effector T cell response, SF/DR T cells accumulated more in draining pancreatic lymph nodes than the WT DR T cells (Figure 1b). Together these data clearly demonstrate that naïve CD4 T cell tolerance to a self tissue antigen is dependent upon the ability to express functional Foxp3.
Figure 1. Naïve CD4 T cells establish long-term Foxp3-dependent tolerance in response to cognate tissue antigen, despite tissue infiltration and Th1 development.
1–2×105 naïve Foxp3-negative DR or Foxp3-deficient SF/DR CD4 T cells were adoptively transferred into RO/RAG° hosts. (A) Diabetes incidence was evaluated by bi-weekly blood-glucose monitoring, graph shows diabetes-free survival over time, and is composite data from at least four independent experiments (DR n=39, SF/DR n=20). (B) Frequencies of donor CD4+ DO11.10 TCR+ T cells among lymphocytes from the pancreatic lymph nodes of chimeric mice, compiled data from two independent experiments shown. Cells were harvested upon determination of diabetes in the SF/DR recipient cohort. (C) Splenocytes were incubated in media alone (No Restim) or with ovalbumin peptide (OVA peptide) and assessed for intracellular IFNγ, IL17 and IL4 (not shown). Representative FACS plots depicted. (D) Composite data from three independent experiments showing frequencies of IFNγ+ donor CD4 T cells.
An examination of the donor CD4 T cells showed that a subset of both WT DR and SF/DR CD4 T cells had differentiated into T-helper type I (Th1) effector cells, as shown by intracellular expression of IFNγ upon restimulation with OVA peptide in vitro (Figure 1c), with a trend toward greater frequencies of IFNγ+ cells in mice with SF/DR cells. In addition, donor CD4 T cells from healthy mice expressed no detectable IL-10 and TGFβ expression was unchanged (not shown). Upon histological examination of pancreata from chimeric mice, antigen-dependent islet infiltration was seen in both healthy and diabetic mice (Supplemental Figure 1). Together these data show that in this system, Th1 effector populations develop and are capable of infiltrating the islets, but the presence of iTreg prevents autoimmune disease.
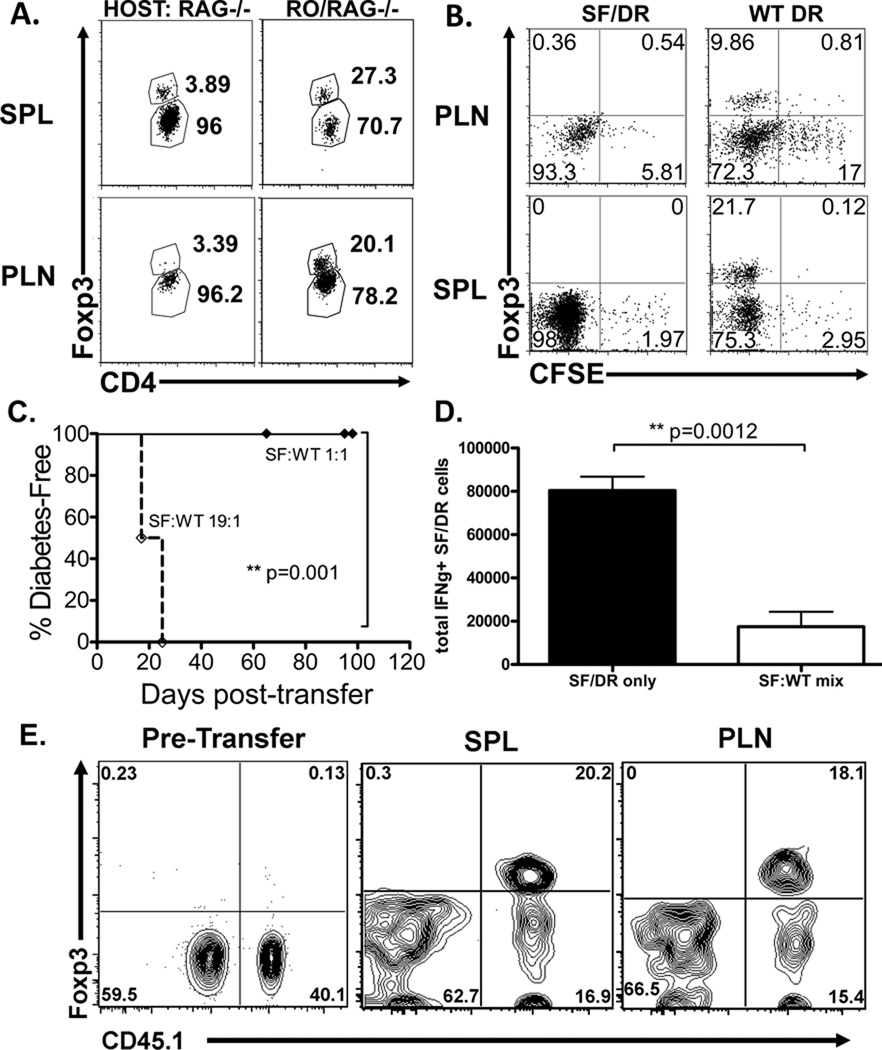
Upon ex vivo examination of donor DR cells, RO/RAG° hosts had substantial populations of Foxp3+ cells in spleen and pancreatic lymph nodes, with only a nominal Foxp3+ population in RAG° hosts (Figure 2a). As expected, both WT DR and SF/DR cells proliferated after adoptive transfer, with the WT DR exhibiting a somewhat diminished proliferation compared to SF/DR (Figure 2b). The enhanced proliferation and expansion in the absence of a functional Foxp3 protein is consistent with the notion that effector cell responses may be curtailed by a suppression of cell division by Foxp3+ Treg. These data demonstrate that an expanded Foxp3+ Treg population responding to cognate antigen expressed in self tissues is an important factor in the establishment of self tolerance.
Figure 2. Foxp3+ donor cells arise from naïve CD4 T cells and can provide dominant suppression against CD4 T cell-mediated autoimmunity.
Donor DR CD4 T cells were transferred into RO/RAG° hosts as described in Figure 1. Cellular analysis was conducted when a cohort of experimental mice was determined to be diabetic. (A) Spleens (SPL) and pancreatic lymph nodes (PLN) from RAG° or RO/RAG° hosts were harvested and donor DR cells were analyzed by FACS by gating on CD4+ DO11.10 TCR+ lymphocytes. Numbers indicate percentages of Foxp3 positive (upper) and Foxp3 negative (lower) donor DR as determined by intracellular staining. Representative FACS plots depicted, n=3 for RAG° recipients and n=4 for RO/RAG° recipients. (B) Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled WT DR or SF/DR CD4 T cells were adoptively transferred into RO/RAG° hosts and donor DO11.10 cells were analyzed by FACS for CFSE dilution and intracellular Foxp3 expression. (C) DR and SF/DR CD4 T cells were mixed at either a 1:1 or 1:19 ratio and 2×105 total CD4 T cells were transferred into RO/RAG° mice. Diabetes induction was monitored as previously described. Data shows diabetes-free survival of indicated chimeric mice, 1:1 mix n=9, 1:19 mix n=7, 2 independent experiments. (D–E) SF/DR CD4 T cells were transferred alone or at equal ratios with CD45.1+ WT DR CD4 T cells and transferred into RO/RAG° mice. (D) Total numbers of intracellular IFNγ+ SF/DR cells from splenocytes stimulated with anti-CD3/CD28. CD45.1-negative cells were from chimeric mice that received only SF/DR cells (SF/DR only) or the 1:1 mixed SF/DR and WT DR donors (SF:WT mix). (E) Analysis of mixed donor WT DR and SF/DR CD4 T cells. Left panel shows mixed donor populations prior to adoptive transfer (Pre-transfer), middle and right panels show ex vivo mixed donor populations from spleen (SPL) and pancreatic lymph node (PLN) after 20 days post-transfer, representative FACS plot depicted, n=3 to 4 mice per group.
Transfer of purified Foxp3+ DO11 CD4 T cells was sufficient to inhibit autoimmunity when mixed at a 1:1 ratio with SF/DR cells (data not shown), demonstrating that Treg can control this autoreactive T cell population. To directly determine the capacity of de novo Foxp3+ iTreg population to control autoimmunity, naïve DR CD4 T cells were mixed with SF/DR CD4 T cells and transferred into RO/RAG° mice. When transferred in equal numbers the DR CD4 T cells established tolerance and mice did not develop disease, but this could be overcome with higher frequencies of SF/DR donor cells (Figure 2c). To determine whether protection by naïve WT DR CD4 T cells was simply the ability of those cells to out-compete the SF/DR CD4 T cells, SF/DR cells were co-transferred with monoclonal CD4 T cells from mice expressing an HA-specific TCR (HNT)17 into RO/RAG° hosts, and these were not protected from diabetes (not shown). A non-significant trend was seen toward increased total expansion of SF/DR cells in the absence of WT DR cells (not shown). When total numbers of IFNγ+ SF/DR cells was calculated, a significant difference emerged, indicating that the de novo Treg in the mixed populations may suppress disease by inhibiting overall expansion of Th1 effector cells (Figure 2d). Ex vivo analysis confirmed that a substantial fraction of DR cells had converted into Foxp3+ Treg (Figure 2e); notably, the pre-transfer ratio of WT to SF donor cells was similar at day 20 post-transfer. These results establish that Treg that arise from the donor WT DR population are capable of exerting a dominant state of tolerance in a dose-dependent manner.
The expanded Foxp3+ donor CD4 T cell population could have originated either from a small undetectable precursor population of pre-existing Treg, or de novo from a fraction of the naïve CD4 T cells. Recent studies using BDC2.5 TCR+ T cells have concluded that the islet-specific Treg population arises from expansion of thymically-generated “natural Treg” precursors18,19. However, in the DR model the cognate ligand is not expressed in the donor mouse, whereas the natural ligand for the BDC2.5 TCR, chromogranin, is present in the RAG°BDC2.5 mice as a potential Treg-selecting antigen20. To address these possibilities, two approaches were taken.
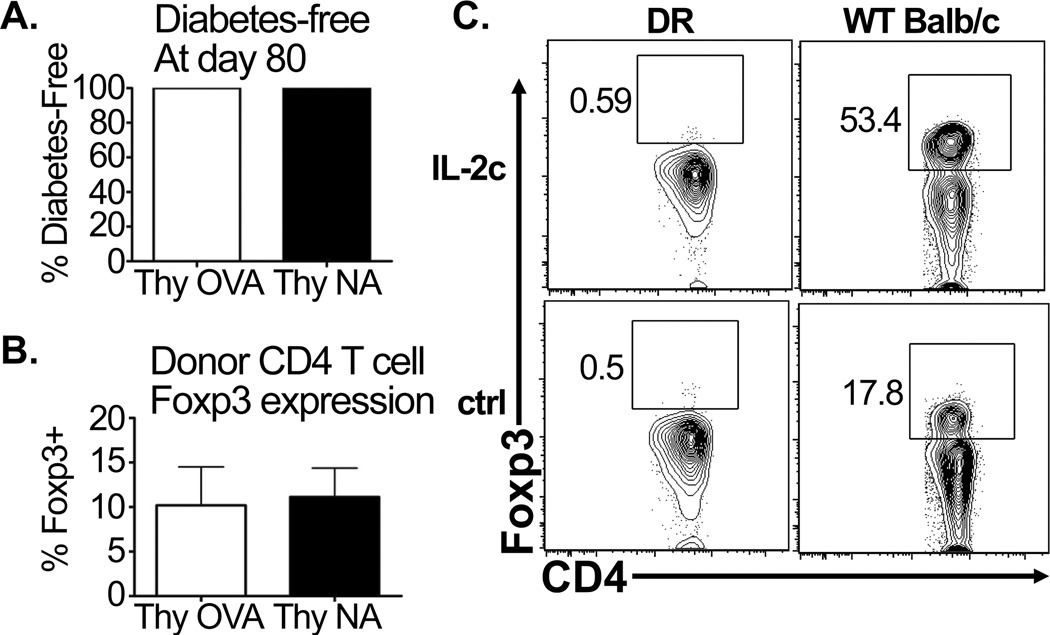
First, DR mice were crossed to mice containing a Foxp3-GFP functional reporter gene16. Bone-marrow chimeric mice were generated using Foxp3-GFP DR donor bone marrow engrafted into lethally irradiated RAG° or RO/RAG° recipients, to generate monoclonal Treg-deficient and sufficient DR donors, respectively. FACS-sorted GFP-negative cells were then transferred into RO/RAG° hosts (Supplemental Figure 2). In this way, donor cells could be obtained from either an environment in which the Treg selecting ligand is absent (RAG°) or present (RO/RAG°). Transfer of sorted GFP− DR CD4 T cells (Foxp3−) into RO/RAG° hosts resulted in long-term tolerance and appearance of similar levels of Foxp3 donor cells (Figure 3a,b). These data demonstrate that tissue specific tolerance can be achieved and Treg can arise from a population that has been rigorously selected to exclude precursor Foxp3+ cells. Additionally, tolerant CD4 T cells exhibit similar levels of Foxp3+ populations, indicating that even when the cognate ligand is present to select Treg precursors, the naïve Foxp3-negative donor population retains the potential to give rise to Foxp3+ cells upon encountering antigen in the RO/RAG° host.
Figure 3. Foxp3+ iTreg arise de novo from naïve CD4 T cell donor precursors.
Sorted Foxp3GFP-negative DR donor cells were transferred into RO/RAG° recipients as described in Supplemental Figure 1. (A) Frequency of diabetes-free RO/RAG° mice that had received GFP-negative donor CD4 T cells derived from the RAG° (Thy NA) and RO/RAG° (Thy OVA) bone marrow chimeras (n≥3 mice per group). (B) Foxp3+ frequencies of donor CD4 T cells from the mice described above. (C) Wild-type Balb/c and DR mice were treated with IL-2/IL-2mAb complex on days 0 and 2 and 4 (IL-2). Control mice received no treatment (ctrl). At day 5 spleens were harvested and CD4 T cells were analyzed by FACS for intracellular Foxp3. N=3 mice per group.
Second, DR mice were treated with an IL-2/IL-2mAb complex which selectively expands Treg21. Following treatment, Foxp3+ cells were not detectable in the DR mice, while wild-type Balb/c mice displayed robust Treg expansion (Figure 3c). This result contrasts with recent studies concluding that monoclonal TCR transgenic mice harbor a normally undetectable population of Foxp3+ cells, again using the BDC2.5 model26. Taken together, these data demonstrate that transfer of naive, Foxp3-negative DR T cells into RO/RAG° hosts results in differentiation of these T cells into iTregs through de novo expression of Foxp3.
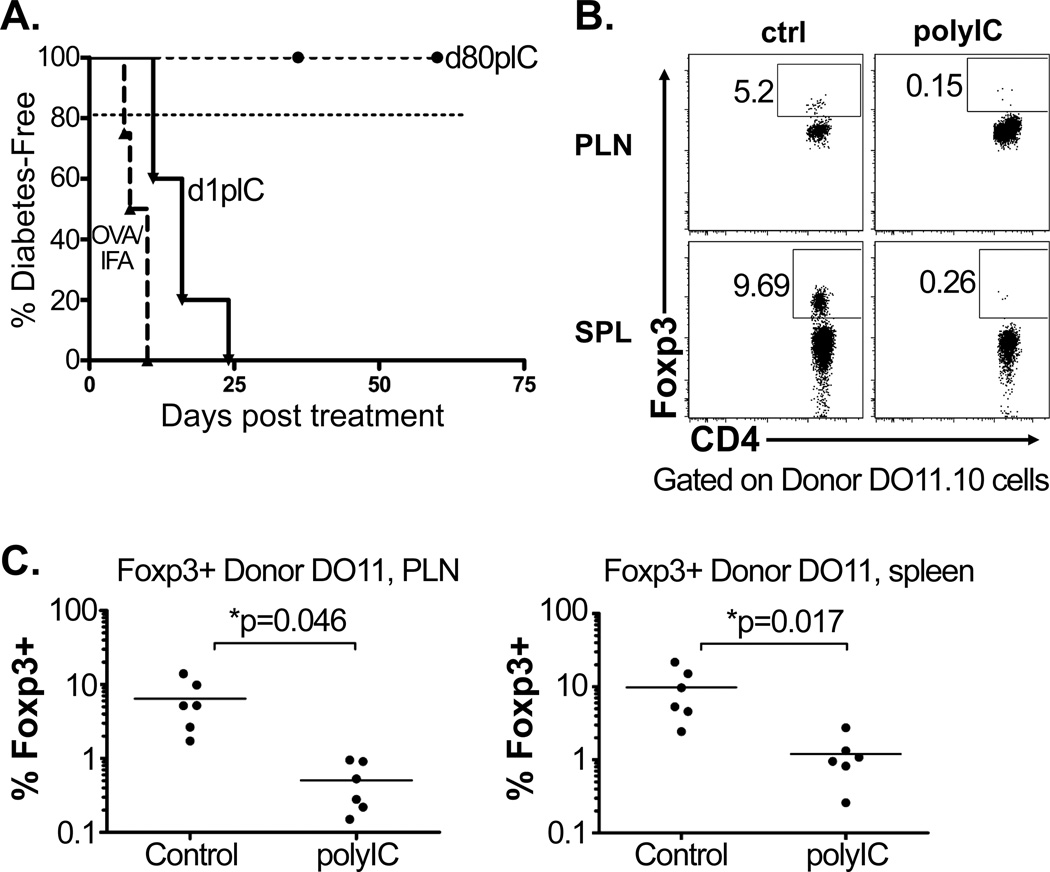
Transfer of naïve DR CD4 T cells, followed by immunization with OVA peptide in IFA, causes diabetes in the RO/RAG° host (Figure 4a and22). Therefore, with antigen-mediated activation, naïve monoclonal CD4 T cells develop effector function and drive autoimmunity. To determine whether non-specific inflammatory stimulation in the absence immunization could trigger autoimmunity, chimeric mice were treated with the viral mimetic polyIC to induce a systemic inflammatory response. When polyIC was given at day 1 following transfer of the DR cells, tolerance was undermined and 100% of the mice became diabetic within 30 days (Figure 4a). However, if polyIC was delivered once tolerance was established at day 80 after DR transfer, tolerance was maintained and the mice remained diabetes-free (Figure 4a). Upon ex-vivo analysis at day 18 post-transfer, the fraction of Foxp3+ donor cells was drastically reduced in mice that received day 1 polyIC treatment compared to control mice (Figure 4b). Furthermore, donor DR cells from day 1 polyIC-treated chimeras developed into Th1 effectors (Supplemental Figure 3). This would suggest that when naïve CD4 T cells encounter a normally tolerizing self-antigen in the context of non-specific systemic inflammation, signals that lead to tolerance and de novo Foxp3 expression are subverted and effector differentiation proceeds. Furthermore, the lack of tolerance breakdown when systemic inflammation is encountered at a late timepoint would suggest that once the initial tolerizing “program” has been established it is durable.
Figure 4. CD4 T cell tissue antigen tolerance and de novo Foxp3 expression are undermined by inflammatory stimuli during priming.
Adoptive transfer of DR donor cells into RO/RAG° hosts as described in Figure 1. As indicated, chimeric mice were given 100µg of ovalbumin peptide with IFA s.c. at day 1 post-transfer (n=4), or 100µg of polyIC at days 1 and 3 (n=5) or days 80 and 82 post-transfer (n=7). (A) Diabetes incidence evaluated by blood-glucose monitoring as described in Figure 1, compiled data from two independent experiments. For comparison, dashed line depicts data from Figure 1 showing diabetes incidence of untreated chimeras. (B) SPL and PLN donor DR CD4 T cells were analyzed for Foxp3 expression at day 18 post-transfer, showing cells from untreated controls (ctrl) vs. cells from diabetic chimeras that had been treated with polyIC (polyIC) at days 1 and 3 post transfer, representative FACS plots shown, n=3 for each. (C) Frequency of Foxp3 expression among control and polyIC treated donor DR cells, compiled data from two independent experiments.
These concepts provide an important insight into the mechanisms of CD4 T cells tolerance. The results shown here suggest a model in which de novo expression of Foxp3 is a critical determinant of the tolerogenic response to tissue antigen. Importantly, if tissue-specific naïve CD4 T cells cannot express Foxp3, they will not establish tolerance. The heterogeneity of outcomes achieved by the naïve antigen-specific CD4 T cell precursors shown here demonstrates that the outcome of tolerance or effector differentiation is likely achieved by a balance of the signals that act on the naïve T cells as they undergo priming. The key to this balance is the relative proportion of CD4 T cells that can upregulate Foxp3, and the nature of the environment in which they encounter antigen. In the presence of inflammatory signals, Foxp3 fails to be induced and effector CD4 T cell differentiation dominates, resulting in autoimmunity. However, under normal homeostatic condition, de novo induction of Treg limits the damage that effector T cells can inflict upon self tissues. These findings lend an important insight into an important cell-intrinsic mechanism for tolerogenic CD4 T cell responses.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank S. Ma and M. Beauchamps for technical assistiance, and D. Campbell and J. Hamerman for helpful discussion. We also thank Matt Warren for his administrative assistance.
This work was supported by NIAID grant T32 AI007411.
ABBREVIATIONS
- DR
DO11.10/RAG°
- RAG°
Recomination Activating Gene-Deficient
- RO
Rat Insulin Promoter membrane-bound OVA transgenic
- SF
Scurfy
- Treg
Regulatory T Cell
REFERENCE LIST
- 1.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Lohr J, Knoechel B, Nagabhushanam V. T cell tolerance and autoimmunity. Autoimmun.Rev. 2004;3:471–475. doi: 10.1016/j.autrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally Arising CD4+ Regulatory T Cells for Immunologic Self-Tolerance and Negative Control of Immune Responses. Annual Review of Immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid Y, Tarbell. K. Regulatory T cells in the control of host-microorganism interactions (*) Annu.Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 8.Wei JO, Duramad O, Perng A, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc.Natl.Acad.Sci.U.S.A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, Schmidt-Weber CB. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros Transcription Factor Family Member, Differentiates Thymic-Derived from Peripherally Induced Foxp3+ T Regulatory Cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J.Exp.Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin.Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Scott B, Liblau R, Degermann S, Marconi LA, Ogata L, Caton AJ, McDevitt HO, Lo D. A role for non-MHC genetic polymorphism in susceptibility to spontaneous autoimmunity. Immunity. 1994;1:73–82. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 18.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp.Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor Çôspecified peripheral niche constraints. J.Exp.Med. 2010;207:1879–1889. doi: 10.1084/jem.20100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2 ÇômAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J.Exp.Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.