Abstract
Objective
To compare quantitative ultrasound (QUS) measurements in adolescents with anorexia nervosa (AN) with that in healthy control subjects and to determine the utility of QUS as a tool to evaluate skeletal status in these patients.
Study design
Female adolescents with AN (n = 41) and healthy control subjects (n = 105) were recruited. Speed of sound (SOS) was measured at the radius and tibia. Participants with AN also had hip and spinal areal bone mineral density measurements by dual-energy x-ray absorptiometry (DXA); bone mineral apparent density (BMAD) was calculated.
Results
Subjects with AN had higher mean radial SOS (4044 ± 99 m/s) than did control subjects (3947 ± 116 m/s; P < .0001). These results were replicated at the tibia (AN, 3918 ± 85 m/s vs control subjects, 3827 ± 106 m/s; P < .0001). Neither DXA measures of areal bone mineral density nor BMAD were correlated with SOS. Weight and body mass index were negative predictors of tibial but not radial SOS. AN status remained a significant predictor of SOS after controlling for body mass index, age, and race.
Conclusions
Subjects with AN had higher mean tibial and radial SOS than did control subjects. QUS variables did not correlate with DXA measures, calculated BMAD, or anthropometric variables. QUS measurements of SOS do not appear to be appropriate for bone density screening in patients with AN.
Bone loss is a well-established complication of anorexia nervosa (AN).1–4 Given that adolescence is the crucial time for establishment of peak bone mass, this loss is clinically significant and may place these young women at higher risk for fracture.5 Dual-energy x-ray absorptiometry (DXA) has been the most widely used tool for assessment of bone mass in this patient population. DXA measures bone in two dimensions and allows for calculation of areal bone mineral density (aBMD, g/cm2). The greatest challenge in the interpretation of aBMD in the adolescent age group is that it is highly influenced by bone and body size.6–8 Additionally, although DXA measures are highly correlated with bone strength, strength depends on skeletal properties such as geometry, elasticity, and internal architecture, which are not reflected directly in DXA measurements.9,10
Quantitative ultrasound (QUS) is an attractive alternative method for the evaluation of skeletal status. QUS assesses peripheral bone by measuring the speed of sound (SOS) of an ultrasound wave as it is propagated along the bone. The speed of propagation along bone is influenced by bone density, elasticity modulus, and the microarchitecture of bone.7 Studies have shown that QUS can predict fracture risk in older women, independent of aBMD, and monitor skeletal responses to exercise with good sensitivity.11–14 The use of QUS is appealing because of its portability, speed, low cost, and lack of ionizing radiation. QUS could also be used as a screening tool for low bone mass or provide information beyond that obtained by current bone density measurement techniques. However, to our knowledge, this modality has not been investigated in young women with AN at peripheral sites other than the calcaneus.15–17
The aim of the current study was to compare QUS measurements in patients with AN with that in healthy control subjects and to determine the utility of QUS as a tool to evaluate skeletal status in this patient population.
METHODS
Subjects
Female adolescents and young women ages 13 to 26 years with a diagnosis of AN (n = 41) were recruited from the Eating Disorders Program at an adolescent medicine clinic for participation in a randomized, controlled trial. Entry criteria included having a diagnosis of AN by Diagnostic and Statistical Manual IV (DSM-IV) criteria and amenorrhea for at least 3 months. All patients with AN were at least 2 years postmenarche. Patients were excluded who had a chronic disease in addition to the eating disorder or if they were receiving medications known to have skeletal effects.
Healthy female adolescents (n = 105) within the same age range were recruited as control subjects from the same adolescent clinic. All control subjects had a body mass index (BMI, kg/m2) between the 5th and 95th percentiles for age, based on Centers for Disease Control growth charts published in 2000.18 Subjects were excluded if they were taking medications known to affect bone health, such as contraceptive hormones (oral contraceptive pills, depot medroxyprogesterone), glucocorticoids, or anticonvulsants during the 3 months before study enrollment. We also excluded potential participants who were premenarchal, pregnant, lactating, or had other medical conditions known to affect aBMD.
All study procedures were reviewed and approved by the local institutional review board. Informed consent was obtained from all subjects or their parents. Minor subjects provided assent for participation.
Data Collection
All participants completed a semistructured interview for demographic information and health history, including information about medication use, menstrual history, smoking, and family history of osteoporosis. Height (cm) was measured with the use of a wall-mounted stadiometer. Weight (kg) was measured after voiding, with subjects wearing a hospital gown. The same stadiometer and calibrated scale were used for all measurements. BMI was calculated, and BMI percentile was determined with the use of standard percentile tables.18
Bone Density Evaluation
The SOS through bone is a ratio of the distance traveled to transit time and is expressed as meters per second. The SOS measurement records the shortest time between pulse transmission and the first reception of a signal. The axial technique was used, with one transducer both transmitting and receiving the signal. SOS was measured on the nondominant limb at the distal one third of the radius and the mid-shaft of the tibia, using the Omnisense 7000P QUS (Sunlight Medical Ltd, Tel-Aviv, Israel). The dominant limb was determined by asking patients to report their hand preference. All measurements were performed by two highly trained researchers. The percent coefficient of variation at the tibia was 0.55% between technicians and 0.82% at the radius. No inter-rater differences were noted at either site.
AN subjects also had aBMD (g/cm2) measured by DXA, using the QDR-4500 with Delphi upgrade (Hologic, Inc, Waltham, Mass). Measurements were performed at the left total proximal hip and lumbar spine (L1–L4). Hip, spine, and total body measurements were compared with age- and sex-matched control subjects.19,20 For participants younger than 20 years of age, pediatric normative data were used.21
To correct for the influence of body size on the measured areal BMD, we performed a volumetric estimation of BMD by using DXA data. Using the method published by Carter et al,22 we calculated bone mineral apparent density (BMAD, g/cm3), using the equation BMAD = BMC/Ap1.5, where Ap is the projected area (cm2) of interest.
Statistical Analysis
Student t tests were used to compare anthropometric characteristics, age, and SOS between groups. Differences in categoric demographic variables (race, family history of osteoporosis) were evaluated through the use of χ2 tests. Pearson correlation analyses were used to evaluate relations among the DXA variables and SOS. Multiple regression was used to determine predictors of SOS while controlling for potential confounding variables. The analysis was performed separately for the radius and tibia. Statistical analyses were performed with SAS (SAS Institute, Inc, Cary, NC). The level of significance was set at a value of P < .05.
RESULTS
We studied 41 adolescents and young women with AN and 105 healthy control subjects (Table I). Mean age of all participants was 17.5 ± 2.8 years; subjects with AN were slightly older than the control group (P = .01). As expected, based on the underlying disease of interest, the two groups differed significantly with respect to weight, height, BMI, and race. There were also group differences noted with respect to family history, with control subjects less likely to report a positive family history of osteoporosis (Table I).
Table I.
Anthropometric measures and demographic characteristics of healthy female adolescents and adolescents with anorexia nervosa
| Control subjects (n = 105) |
AN subjects (n = 41) |
P value | |
|---|---|---|---|
| Age (y) | 17.1 ± 2.7 | 18.4 ± 2.9 | .01 |
| Height (cm) | 161.3 ± 6.3 | 164.9 ± 7.0 | .01 |
| Weight (kg) | 59.2 ± 9.3 | 49.0 ± 6.1 | <.0001 |
| BMI (kg/m2) | 22.8 ± 3.3 | 17.9 ± 1.7 | <.0001 |
| Duration of AN (mo) | 24.9 ± 29.9 | ||
| Duration of amenorrhea (mo) | 12.3 ± 13.6 | ||
| White (%) | 19.1 | 97.6 | <.0001 |
| Positive family history of osteoporosis (%) | 14.9 | 34.3 | .01 |
AN, Anorexia nervosa; BMI, body mass index.
Data are presented as mean ± SD.
We also completed a subgroup analysis, limiting the analysis to only control subjects who reported white race (n = 20). When white control subjects and subjects with AN were compared, the two groups were significantly different only in terms of weight and BMI (Table II).
Table II.
Demographic characteristics and anthropometric measures of white healthy female adolescents and adolescents with anorexia nervosa
| Control subjects (n = 20) |
AN subjects (n = 40) |
P value | |
|---|---|---|---|
| Age (y) | 17.4 ± 3.1 | 18.4 ± 2.9 | .22 |
| Height (cm) | 165.2 ± 6.4 | 164.7 ± 7.0 | .82 |
| Weight (kg) | 62.5 ± 7.9 | 49.2 ± 6.1 | <.0001 |
| BMI (kg/m2) | 22.9 ± 2.7 | 18.1 ± 1.6 | <.0001 |
| Positive family history of osteoporosis (%) | 30.0 | 35.3 | .69 |
BMI, Body mass index.
Data are presented as mean ± SD.
QUS measurements were significantly different between the two groups (Figure 1). Subjects with AN had a higher SOS at the radius than control subjects (P < .0001). These results were replicated at the tibia (P < .0001). The group differences remained significant at both sites when the control group was restricted to white subjects and were even more pronounced at the tibia (P < .0001). There was no difference in SOS at the radius or the tibia between white and nonwhite control subjects.
Figure 1.
Speed of sound at the radius and tibia in subjects with anorexia nervosa compared with healthy control subjects. *Boxes represent the median speed of sound (SOS, m/s) and quartile limits. Normal nonwhite indicates healthy, nonwhite control subjects; normal white indicates healthy, white control subjects; AN, subjects with anorexia nervosa. *Difference between subjects with AN and control subjects was significant, P < .0001 at both anatomic sites.
Bone density as measured by DXA was low (aBMD Z-score ≤ −1.0 SD) in the subjects with AN. The mean hip aBMD by DXA in the patients with AN was 0.874 ± 0.109 g/cm2. The mean aBMD Z-score was −0.45 ± 0.92, with a range of −2.1 to 1.6. Total hip aBMD Z-scores were between −1.0 and −2.0 SD in 34% of patients and ≤ −2.0 SD in 3% of patients. The mean hip BMAD was 0.153 ± 0.019 g/cm3. At the lumbar spine, mean aBMD in the subjects with AN was 0.872 ± 0.089 g/cm2. The mean Z-score was −1.12 ± 0.90, with a range of −3.1 to 0.5. Spinal BMD Z-scores were between −1.0 and −2.0 SD in 59% of patients, and ≤ −2.0 SD in 18% of patients. The mean spine BMAD was 0.115 ± 0.011 g/cm3.
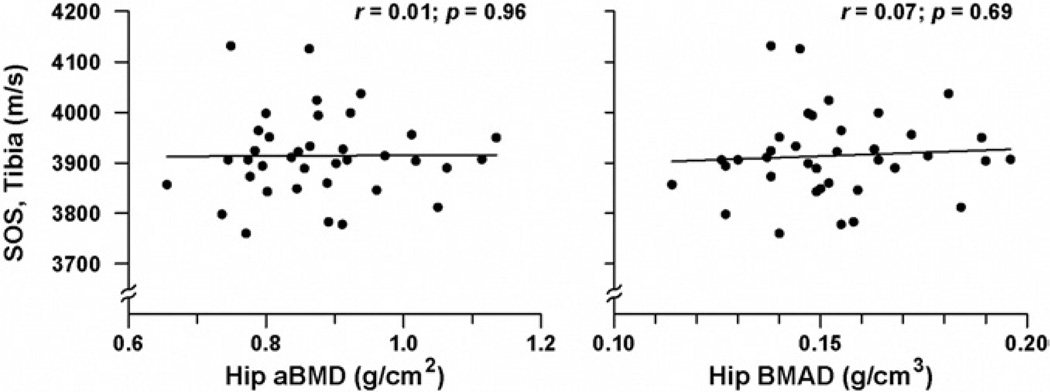
In participants with AN, neither hip nor spinal aBMD by DXA was significantly correlated with QUS measurements of SOS at either the radius or tibia (Figure 2). The relationship between BMAD (at the hip and spine) and SOS measures (at the radius and tibia) followed an almost identical trend (Figure 2); no significant associations were found between BMAD and SOS (P = .30 to 0.68). No association was found between severity of AN (expressed as duration of illness or duration of amenorrhea) and SOS measures. Moderate correlations were observed between subject age and SOS results at both the radius and tibia (r = 0.36 and r = 0.37, respectively; P = 0.02). In patients with AN, anthropometric measures did not correlate with SOS at either anatomic site.
Figure 2.
Relationship between hip aBMD or hip BMAD by DXA and speed of sound at the tibia in subjects with anorexia nervosa. SOS, Speed of sound; aBMD, areal bone mineral density; BMAD, bone mineral apparent density.
Regression analyses of SOS at the radius and tibia were carried out (Table III; available at www.jpeds.com). In univariate analyses of the entire sample, weight and BMI were significant negative predictors of SOS at the tibia but not at the radius. Age and height positively predicted SOS at both sites. Neither race nor family history of osteoporosis was a significant predictor of SOS. In the final multivariate regression model, AN status remained a significant predictor of SOS at the radius even after controlling for baseline group differences in BMI, race, and age. Neither BMI nor race remained important predictors of SOS in the final model. At the tibia, age and BMI remained significant predictors of SOS, although AN status was borderline-significant (P = .056) after controlling for these variables and race.
Table III.
Regression analyses of speed of sound at the radius and tibia
| Models/predictors | Δ SOS radius (m/s) AN vs control |
P value | Δ SOS tibia (m/s) AN vs control |
P value |
|---|---|---|---|---|
| AN | 97.5 (20.7) | <.0001 | 91.2 (9.6) | <.0001 |
| AN, adjusted for age | 69.3 (18.5) | .0003 | 72.3 (17.9) | <.0001 |
| AN, adjusted for race | 85.7 (29.7) | .005 | 109.7 (26.7) | <.0001 |
| AN, white subjects only | 94.7 (28.7) | .002 | 119.4 (25.2) | <.0001 |
| AN, adjusted for BMI, race, age | 63.9 (29.9) | .03 | 53.1 (28) | .059 |
| Univariate analyses | ||||
| Age (years) | 22.7 (2.9) | <.0001 | 16.1 (2.8) | <.0001 |
| Weight (kg) | −0.8 (1) | .41 | −3.0 (0.9) | .001 |
| Height (cm) | 3.4 (1.5) | .02 | 2.8 (1.3) | .04 |
| BMI (kg/m2) | −5.79 (2.7) | .06 | −10.6 (2.3) | <.0001 |
| White | 70.8 (19.2) | .004 | 47.7 (17.7) | .01 |
| Family history of osteoporosis | 43.7 (24.4) | .08 | 15.5 (22.8) | .51 |
SOS, Speed of sound; AN, anorexia nervosa status (yes/no); BMI, body mass index.
Data are presented as parameter estimate (β), standard error in parentheses, and indicate the change in SOS (m/s) per unit change of the covariate.
DISCUSSION
In this study, we have evaluated quantitative ultrasound as a tool to measure bone density in adolescents and young women with AN. Although measurements of aBMD by DXA were low, replicating results from our and others’ previous studies in this patient population,2,3,23 we found a paradoxically higher SOS at both the radius and the tibia in young women with AN compared with healthy control subjects. This unexpected result was contrary to our hypothesis that SOS would be reduced in AN patients.
In the group with AN, 37% of subjects had a total hip aBMD Z-score more than 1 SD below the mean, and 77% had a lumbar spine aBMD Z-score more than 1 SD below the mean of age- and sex-matched reference data. Unexpectedly, there were no significant correlations between ultrasound measurements of SOS and either aBMD or BMAD. In other patient populations, including children with rheumatologic disease or celiac disease, QUS end points have been highly correlated with site-matched aBMD values.24,25 As DXA is a well-established methodology for the evaluation of skeletal status in young women with AN, and our results confirm the low bone mass frequently demonstrated in this population, it appears that SOS measures are problematic in this group.
Previous studies have used QUS to evaluate bone mass at the calcaneus in patients with AN.15,16 Milos et al16 measured SOS at the calcaneus in 36 adult women with AN and 30 healthy adult female control subjects. Their group found no significant difference in SOS between the two subject cohorts. Similarly, Kutilek et al15 measured SOS at the calcaneus in 26 adolescents with AN. They found that SOS values were significantly higher than reference data obtained from healthy girls of similar age. In contrast, broadband ultrasound attenuation (BUA) measurements by QUS in patients with AN were lower than in the healthy subjects. The BUA results at the calcaneus were replicated by Resch et al,17 who additionally demonstrated significant correlations between BUA and BMD of the femoral neck and lumbar spine. No significant relations were found between BUA and peripheral quantitative computed tomography values of the distal radius.17 That study did not compare SOS measurements between groups. The Omnisense 7000P QUS used in the current study does not have the capability to measure BUA; thus, this information could not be obtained in the current participants.
This study used QUS to evaluate the tibia and radius in subjects with AN. As the tibia is a weight-bearing site and a common site for stress fracture in patients with AN who frequently participate in excessive exercise, bone ultrasound measurements at this site would have provided a convenient screening tool for clinicians in the outpatient setting. However, our data suggest that the accuracy of the SOS measurements at the tibia and radius are compromised. This is potentially due to the minimal soft tissue in these malnourished young women that may violate underlying operating assumptions of this methodology. In our analysis, BMI was a significant predictor of SOS at the tibia, but in a negative direction. The direction of the association indicates that SOS decreased as BMI increased, the opposite of what has been demonstrated with aBMD measurements by DXA.26 Although unexpected, our results replicate findings from other patient populations. Littner et al27 studied bone SOS at the tibia in small for gestational age infants, a group that also exhibits minimal body fat. The small for gestational age infants studied had higher bone SOS than the predicted SOS of appropriate for gestational age infants. These results corroborate with literature that has shown an inverse correlation between tibial SOS and measures of adiposity (percentage of body fat and sum of skinfold measurements).14 At the opposite extreme, Eliakim et al28 found low QUS values in obese children. Future studies are needed in which body composition data are obtained as well as bone assessments by ultrasound to answer more definitively why SOS measurements are higher in malnourished young women with AN.
Another explanation for the higher SOS measures in AN patients may be related to an internal lack of fat. With axial measurement of SOS by QUS, the “fastest” signal will be detected by the receiver. The signal pathway traverses cortical bone and also penetrates the trabecular portion or bone marrow cavity. Thus, if the marrow cavity decreases (like AN patients who may have either a hypoplastic bone marrow or possess decreased marrow fat29), the inner course will also decrease, leading to an increased transmission speed.8 These hypotheses are speculative and merit future research.
It is also possible that the higher SOS measures seen at this weight-bearing site in patients with AN were related to the high levels of physical activity often reported by these young women. A significant association between level of physical activity and bone SOS has been reported in healthy young female athletes.14 However, aBMD by DXA was low in our subjects. If the patients with AN were engaging in protective physical activity, it would have been anticipated that aBMD would be normal or high.
The role of bone size in measurements of SOS has been disputed. For axial measurements by QUS, the ultrasound wave is passing within a small layer underneath the surface of the cortical bone. In one previous study, it was reported that body height and therefore bone size influenced SOS measured at the thumb and patella in healthy children, teenagers, and adults.30 The authors concluded that as a result of an increase in bone size, cortical thickness increases. Sound waves thus propagate at a longer distance through the cortical bone in larger skeletal elements, resulting in a dependence of SOS on growth measures. However, this hypothesis has been refuted by larger studies that have found that QUS variables were not affected by body size in healthy adolescents and athletes.14,31 In our patient sample, however, AN subjects were significantly taller than control subjects. If the results were influenced by bone size, the measured SOS in AN subjects should have been lower than that observed in the control subjects. We also demonstrated that there was no association between SOS measures and both aBMD and BMAD, the latter calculated in an attempt to account for differences in bone size.
Matching patients with control subjects on the basis of stage of maturation is critical in the evaluation of bone density. Because all study subjects were postmenarchal, pubertal stage was not a contributing factor to the differences noted between groups. We elected to include all control participants despite the racial diversity of the control group and recognize this as a study limitation. However, although the two study populations differed in terms of race, the differences between groups were only more pronounced when white control subjects alone were included in the analysis. The family history differences align with the racial make-up of the two groups (as white individuals are more likely to have a low bone mass and are at higher risk for osteoporosis). However, family history of osteoporosis was not a significant predictor of SOS at the tibia or the radius. Although BMI, race, and age were important contributing factors to the SOS results, AN status remained a significant predictor, even when these variables were included in the multivariate analyses.
In conclusion, we found that subjects with AN had a higher SOS as measured by QUS at the radius and tibia than healthy control subjects. SOS values did not correlate with aBMD measures by DXA or calculated BMAD or anthropometric variables in adolescents and young women with AN. Based on these results, QUS measurements of SOS at the radius or tibia do not appear to be appropriate for carrying out bone density evaluations in patients with AN.
Acknowledgments
Supported by grant RO1 HD043869 from NICHD; NIH grant MO1-RR-2172 to the Children’s Hospital Boston General Clinical Research Center; the Department of Defense, US Army Bone Health and Military Readiness Program; and Project 5-T71-MC-00009-14 from the Maternal and Child Health Bureau.
Glossary
- aBMD
Areal bone mineral density
- AN
Anorexia nervosa
- BMI
Body mass index
- BUA
Broadband ultrasound attenuation
- DXA
Dual-energy x-ray absorptiometry
- QUS
Quantitative ultrasound
- SOS
Speed of sound
REFERENCES
- 1.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa: a longitudinal study of cortical bone mass. JAMA. 1991;265:1133–1138. [PubMed] [Google Scholar]
- 2.Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440–447. [PubMed] [Google Scholar]
- 3.Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141:64–70. doi: 10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- 4.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–4496. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 5.Lucas AR, Melton LJ, III, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74:972–977. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 6.Bachrach LK. Dual energy X-ray absorptiometry (DEXA) measurements of bone density and body composition: promise and pitfalls. J Pediatr Endocrinol Metab. 2000;13 suppl 2:983–988. [PubMed] [Google Scholar]
- 7.Specker BL, Schoenau E. Quantitative bone analysis in children: current methods and recommendations. J Pediatr. 2005;146:726–731. doi: 10.1016/j.jpeds.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Schonau E. Problems of bone analysis in childhood and adolescence. Pediatr Nephrol. 1998;12:420–429. doi: 10.1007/s004670050479. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–782. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 10.Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study. Bone. 2004;35:750–759. doi: 10.1016/j.bone.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Fielding KT, Nix DA, Bachrach LK. Comparison of calcaneus ultrasound and dual X-ray absorptiometry in children at risk of osteopenia. J Clin Densitom. 2003;6:7–15. doi: 10.1385/jcd:6:1:7. [DOI] [PubMed] [Google Scholar]
- 12.Hartl F, Tyndall A, Kraenzlin M, Bachmeier C, Guckel C, Senn U, et al. Discriminatory ability of quantitative ultrasound parameters and bone mineral density in a population-based sample of postmenopausal women with vertebral fractures: results of the Basel Osteoporosis Study. J Bone Miner Res. 2002;17:321–330. doi: 10.1359/jbmr.2002.17.2.321. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen TV, Center JR, Eisman JA. Bone mineral density-independent association of quantitative ultrasound measurements and fracture risk in women. Osteoporos Int. 2004;15:942–947. doi: 10.1007/s00198-004-1717-z. [DOI] [PubMed] [Google Scholar]
- 14.Falk B, Bronshtein Z, Zigel L, Constantini NW, Eliakim A. Quantitative ultrasound of the tibia and radius in prepubertal and early-pubertal female athletes. Arch Pediatr Adolesc Med. 2003;157:139–143. doi: 10.1001/archpedi.157.2.139. [DOI] [PubMed] [Google Scholar]
- 15.Kutilek S, Bayer M. Ultrasound parameters of calcaneal bone density in girls with anorexia nervosa. Eat Weight Disord. 2001;6:220–224. doi: 10.1007/BF03339746. [DOI] [PubMed] [Google Scholar]
- 16.Milos G, Spindler A, Ruegsegger P, Seifert B, Muhlebach S, Uebelhart D, et al. Cortical and trabecular bone density and structure in anorexia nervosa. Osteoporos Int. 2005;16:783–790. doi: 10.1007/s00198-004-1759-2. [DOI] [PubMed] [Google Scholar]
- 17.Resch H, Newrkla S, Grampp S, Resch A, Zapf S, Piringer S, et al. Ultrasound and X-ray-based bone densitometry in patients with anorexia nervosa. Calcif Tissue Int. 2000;66:338–341. doi: 10.1007/s002230010070. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics, in collaboration with Center for Disease Control. 2000 http://www.cdc.gov/growthcharts.
- 19.Kelly TL. Bone mineral density reference databases for American men and women. J Bone Miner Res. 1990;5:S249. [Google Scholar]
- 20.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5:389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 21.Zemel BS, Leonard MB, Kalkwarf HJ, Specker BL, Moyer-Mileur LJ, Shepherd JA, et al. Reference data for the whole body, lumbar spine, and proximal femur for American children relative to age, gender, and body size. J Bone Miner Res. 2004;S231:1. [Google Scholar]
- 22.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 23.Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114:1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 24.Hartman C, Hino B, Lerner A, Eshach-Adiv O, Berkowitz D, Shaoul R, et al. Bone quantitative ultrasound and bone mineral density in children with celiac disease. J Pediatr Gastroenterol Nutr. 2004;39:504–510. doi: 10.1097/00005176-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hartman C, Shamir R, Eshach-Adiv O, Iosilevsky G, Brik R. Assessment of osteoporosis by quantitative ultrasound versus dual energy X-ray absorptiometry in children with chronic rheumatic diseases. J Rheumatol. 2004;31:981–985. [PubMed] [Google Scholar]
- 26.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 27.Littner Y, Mandel D, Mimouni FB, Dollberg S. Bone ultrasound velocity of infants born small for gestational age. J Pediatr Endocrinol Metab. 2005;18:793–797. doi: 10.1515/jpem.2005.18.8.793. [DOI] [PubMed] [Google Scholar]
- 28.Eliakim A, Nemet D, Wolach B. Quantitative ultrasound measurements of bone strength in obese children and adolescents. J Pediatr Endocrinol Metab. 2001;14:159–164. doi: 10.1515/jpem.2001.14.2.159. [DOI] [PubMed] [Google Scholar]
- 29.Geiser F, Murtz P, Lutterbey G, Traber F, Block W, Imbierowicz K, et al. Magnetic resonance spectroscopic and relaxometric determination of bone marrow changes in anorexia nervosa. Psychosom Med. 2001;63:631–637. doi: 10.1097/00006842-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Fricke O, Tutlewski B, Schwahn B, Schoenau E. Speed of sound: relation to geometric characteristics of bone in children, adolescents, and adults. J Pediatr. 2005;146:764–768. doi: 10.1016/j.jpeds.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 31.Zadik Z, Price D, Diamond G. Pediatric reference curves for multi-site quantitative ultrasound and its modulators. Osteoporos Int. 2003;14:857–862. doi: 10.1007/s00198-003-1456-6. [DOI] [PubMed] [Google Scholar]