Abstract
Background
A polymorphism in the promoter region of the monoamine oxidase A gene (MAOA) has been shown to alter the effect of persistent drinking and childhood maltreatment on the risk for violent and antisocial behaviors. These findings indicate that MAOA could contribute to inter-individual differences in stress resiliency.
Methods
Recidivism in severe violent crimes was assessed after 8 years of nonincarcerated follow-up in a male sample of 174 impulsive Finnish alcoholic violent offenders, the majority of whom exhibited antisocial (ASPD) or borderline personality disorder (BPD) or both. We examined whether MAOA genotype alters the effects of heavy drinking and childhood physical abuse (CPA) on the risk for committing impulsive recidivistic violent crimes.
Results
Logistic regression analyses showed that both heavy drinking and CPA were significant independent predictors of recidivism in violent behavior (OR 5.2, p = 0.004 and OR 5.3, p = 0.003) among offenders having the high MAOA activity genotype (MAOA-H), but these predictors showed no effect among offenders carrying the low MAOA activity genotype (MAOA-L).
Conclusion
Carriers of the MAOA-H allele have a high risk to commit severe recidivistic impulsive violent crimes after exposure to heavy drinking and CPA.
Keywords: Violence, Alcoholism, Alcohol Consumption, Antisocial Behavior, Personality Disorder, Impulsivity, Childhood Adversity, Physical Abuse, Monoamine Oxidase A, Crime
The polymorphism of the enzyme monoamine oxidase genotype A (MAOA), alcoholism, childhood adversities, impulsivity, and violence forms an association complex that is recognized but not well clarified. On one hand, strong evidence suggest that alcoholism (Bye, 2007; Zhang et al., 1997), some childhood adversities (Caspi et al., 2002; Reif et al., 2007), and impulsivity (Zhang et al., 1997) are linked to violence. Alcoholism (Grucza et al., 2006; Linnoila et al., 1989; Zhang et al., 1997) and childhood adversities (Johnson et al., 1999; Zanarini et al., 1997), on the other hand, are also connected with impulsivity and cluster B personality disorders. Moreover, some childhood adversities have been associated with adulthood alcoholism (Brown and Anderson, 1991). Prevention of childhood adversities by early supportive family interventions has resulted in lower rates of adolescent antisocial behavior and substance abuse in a long-term follow-up (Olds et al., 1998).
Brunner and colleagues identified a rare stop-coding variant in the MAOA in a Dutch family that was prone to aggressive behaviors (Brunner et al., 1993). MAOA is an outer membrane mitochondrial enzyme that inactivates monoamines such as serotonin, noradrenalin, and dopamine (Shih et al., 1999). The MAOA gene is located on the X chromosome (Xp 11.23–11.4) (Levy et al., 1989), and a common polymorphism in the MAOA gene’s transcriptional control region [“MAOA linked polymorphism region” (MAOA-LPR)] has been shown to affect transcriptional activity that results in either high- or low-MAOA activity (MAOA-H and MAOA-L) (Denney et al., 1999; Sabol et al., 1998). Alleles differ in the number of copies (2, 3, 3.5, 4, 5, or 6) of a 30-bp repeat sequence, and the most common alleles are those that contain 3 or 4 copies of the 30-bp repeat sequence. The 4-repeat and 3.5-repeat alleles (MAOA-H) correspond to a greater amount and higher activity of MAOA when compared with the 3- repeat allele (MAOA-L) (Denney et al., 1999; Sabol et al., 1998).
The role of MAOA in human aggression is not well understood. A subdivision of human aggression into impulsive and premeditated could in part explain the mixed results that have been reported in this field of research. A discernable trend in the literature suggests that the MAOA-H genotype connects with impulsive aggression whereas the MAOA-L genotype links to more premeditated aggression. The MAOA-H genotype has been associated with reconvictions of impulsive alcohol-related severe violent crimes (Tikkanen et al., 2009), persistent childhood aggression (Beitchman et al., 2004), and dispositional aggression with impulsive features (Manuck et al., 2000). The MAOA-L genotype, on the other hand, has shown a main effect on aggression defined as predisposition toward intentional physical aggression against another person (Reif et al., 2007). Likewise, after exposure to certain childhood adversities, MAOA-H individuals have presented impulsivity/aggression in terms of problematic alcohol-related behavior (Nilsson et al., 2008), whereas maltreated MAOA-L individuals have shown attitudes that accept usage of inter-personal violence (Caspi et al., 2002). Weder and colleagues (2009) showed that the level of childhood adversities may determine which MAOA genotype presents more aggression, rule-breaking, and inattention.
Reports on which MAOA alleles are associated with alcoholism have also presented divergent results. Some studies of Caucasian samples suggest that the MAOA-L allele is more frequent among alcoholics (Samochowiec et al., 1999; Schmidt et al., 2000) while the MAOA-H allele has also been associated with alcohol and drug dependence with related antisocial lifestyle and reconvictions of impulsive alcohol-related severe violent crimes (Gade et al., 1998; Tikkanen et al., 2009). Nilsson and colleagues (2007, 2008) reported on the outcome of adolescent alcohol-related destructive behavior after exposure to adverse psychosocial environments. They concluded that the MAOA-L genotype increased risk among boys, whereas the MAOA-H genotype increased the risk among girls. Ducci and colleagues (2008) observed that only after exposure to childhood sexual abuse were MAOA-L genotyped adult women at an increased risk for antisocial alcoholism.
One aim of the current study was to test the hypotheses that childhood physical abuse (CPA) leads to both persistent excessive drinking and impulsive acts of violence in adulthood, and that the putative effect of CPA on the risk of recidivistic impulsive violent crimes is mediated via heavy drinking in a sample comprising impulsive-aggressive alcoholics that had been convicted of severe violent crimes. Another focus of the study was to examine the role of MAOA conjunct to heavy drinking and CPA as predictors of new acts of impulsive violence. We expected that heavy drinking and CPA would show a larger effect among the MAOA-H genotype, as we recently observed that this group was prone to commit impulsive violent crimes under the influence of alcohol after a history of persistent excessive drinking (Tikkanen et al., 2009).
MATERIALS AND METHODS
The Ethics Board of the Helsinki University Central Hospital approved the study, and all subjects provided their informed consent before participating in the study.
Subjects
The study sample comprised 174 Finnish MAOA-LPR-genotyped male violent alcoholic offenders recruited between 1990 and 1998. Because of the violent nature of their crimes, the subjects were remanded to a mental status examination and spent 2 months within the in-patient care unit at the Department of Psychiatry, Helsinki University Central Hospital. This sample included many subjects who participated in several genetic studies that concerned violent alcoholic offenders, mainly with ASPD (Belfer et al., 2006, 2007; Ducci et al., 2006, 2009; Enoch et al., 2006; Xu et al., 2007). Psychosis and an IQ of <70 served as exclusion criteria. Mean age at the time of evaluation was 32.5 (SD ± 9.7), and mean IQ (WAIS) was 97.2 (SD ± 14.5). The majority of the offenders belonged to lower socio-economic groups. Their occupational status mainly included semi-skilled workers, and many were unemployed at the time of recruitment for this study.
Psychiatric Assessment
Each subject was interviewed with the Structured Clinical Interview for DSM-III-R (Spitzer et al., 1990) to detect lifetime mental disorders (APA, 1987). Interviewers were experienced licensed psychiatrists, and diagnoses were double-checked by psychiatrists at the National Institute of Alcohol Abuse and Alcoholism in Bethesda, Maryland. Axis II diagnoses were ASPD (61; 36%), borderline personality disorder (BPD) (21; 12%), ASPD and BPD comorbidity (46; 26%), narcissistic personality disorder (8; 5%), paranoid personality disorder (24; 14%), and others (21; 12%). Alcohol dependence was diagnosed in 134 subjects (77%) and alcohol abuse in 40 (23%). Early onset conduct disorder (CD) was diagnosed in 42% (73/174) of the subjects.
Childhood Physical Abuse
Information on childhood exposure to physical abuse was obtained from multiple face-to-face interviews (i.e., a psychiatrist, psychologist, psychiatric nurse, and social worker interviewed each subject as part of a standard mental status examination). Structured questionnaires for family histories were sent to first-degree relatives, and collateral information was collected from health care and social service documents. CPA was defined as exposure to physical abuse before age 13 (n = 51; 29%). The abuse had to be pronouncedly traumatic (assessment by the subjects or relative) or reported as a recurrent problem, and sporadic abuse was not classified as an exposure event. Blinded to MAOA genotype, the classification was carried out by 2 different raters with an interrater reliability of 0.90 (Spearman’s rho correlation coefficient, p < 0.01). All subjects received a classification, but the classification of 2 subjects was determined through an open re-evaluation.
Alcohol Consumption
Alcohol consumption was measured before the follow-up with the Lifetime Drinking History (Skinner and Sheu, 1982) questionnaire, which is a structured interview where subjects are asked about patterns of alcohol consumption from the first year of regular drinking to the present. We divided the subject’s lifetime alcohol exposure by the number of years of drinking to form a variable describing the lifetime yearly mean alcohol consumption. Heavy drinking was defined as alcohol consumption ≥70 kg per year matching the upper quartile of the sample (n = 43).
The covariate “parental problem drinking” (100/174; 58%) was assessed from the same sources as CPA (described previously). Unambiguous records of excessive drinking of either parent scored as an event.
Assessment of Violent Behavior
The base line violent offenses (preceding the mental status examination) were generally serious, impulsive, and committed under the influence of alcohol. The occurrence of impulsive-aggressive behavior (115/174; 66%) and alcohol intoxication (155/174; 89%) during the violent crime were assessed blinded to outcome, from themental status examination reports, where the offender’s ability to control his behavior during the violent act was thoroughly discussed. A common subjective experience reported by the offenders was that of amnesia and loss of behavioral control during the act of violence. The most common convictions were manslaughter, attempted manslaughter, assault, or battery (61%), murder or attempted murder (19%), arson (16%), and rape (4%). Recidivism in violent behavior was assessed using data provided by the Legal Register Centre in August 2005. The distribution of the outcome acts of violence was manslaughter, attempted manslaughter, assault, or battery (81.2%), murder or attempted murder (7.3%), arson (7.3%), and rape (4.3%).
The total follow-up period (from the mental status examination to the August 2005 when criminal records were examined) was 11.7 years (140 months, range 85 to 182). We subtracted time spent in prison from the total follow-up time to provide a nonincarcerated follow-up period of 8.1 years (97 months, range = 3 to 182).
MAOA–LPR Genotyping
The MAOA-LPR was genotyped with PCR primer sequences: Forward 5′-(CCC AGG CTG CTC CAG AAA CATG 3)-3′ and Reverse 5′-(GTT CGG GAC CTG GGC AGT TGT G)-3′. Owing to the high GC content in the region where the MAOA-LPR is located, amplification was performed using Invitrogen’s Platinum Taq and PCRX Enhancer System kits, according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). A detailed description of the genotyping method appears elsewhere (Ducci et al., 2006).
As the study sample was composed of only men, the genotypes were grouped based on relative transcriptional activity into 2 categories: high activity (4 repeats) versus low activity (3 repeats). These 2 alleles accounted for 97% of the MAOA-LPR variety among the offenders (4 repeats, 0.56 and 3 repeats, 0.44). The distribution of MAOA-H and MAOA-L alleles is similar to those in other Caucasian male samples (Caspi et al., 2002; Gade et al., 1998; Manuck et al., 2000; Ni et al., 2007; Reif et al., 2007; Weder et al., 2009).
Statistical Analyses
The Pearson Chi-Square and Fisher’s exact tests were applied for frequency distribution comparisons. Correlations were examined with the Pearson and Spearman’s rho tests. ANOVA was used in alcohol consumption comparisons. Univariate analysis of covariance (ANCOVA) was used to examine interaction between MAOA genotype and CPA for alcohol consumption. Analyses were adjusted for age, and Bonferroni correction was used in post hoc multiple comparisons.
Four separate logistic regression models were constructed to examine risk of impulsive violence (dependent). The first model, adjusted for age, included MAOA genotype, heavy drinking, and CPA as independents, and interaction terms were tested. The same procedure was applied for both MAOA genotypes separately. This subdivision procedure was performed because research suggests that MAOA could contribute to inter-individual differences in vulnerability to noxious environments. To avoid spurious results caused by possible gene–environment correlations (rGE), we included early onset CD (evocative rGE factor) and parental problem drinking (passive rGE factor) as covariates in the logistic regression analyses. The rationale behind this, on one hand, was that the pure environmental effect of CPA could be biased by parental aggression arousal caused by the misbehaviors of the child and on the other hand, parental problem drinking could increase the level of physical abuse. Moreover, even though an association between at least one cluster B personality diagnosis (128/174, 74%) and recidivistic acts of violence (χ2 = 8.39, df = 1, p = 0.004) emerged in preliminary analyses, externalizing disorders were not included as covariates in the regression analyses, as early onset CD was strongly associated with cluster B personality disorders (χ2 = 20.60, df = 1, p < 0.001).
The fourth regression model was constructed to assess the effects of different exposure (heavy drinking and CPA) and covariate (CD and parental problem drinking) combinations on risk of acts of impulsive violence among MAOA-H offenders. The level of statistical significance was set at 0.05, and analyses were carried out with SPSS 16.0 (SPSS Inc., Chicago, IL).
RESULTS
Alcohol Consumption
The mean consumption of the whole sample was 52.4 kg (SD = 37.5). In a comparison of mean values of alcohol consumption between offenders with or without a history of CPA, no significant difference was observed (57 vs. 51 kg; F = 0.82, df = 1, p = 0.366). However, a significant interaction occurred between MAOA genotype and CPA (F = 4.8, df = 1, p = 0.029) (ANCOVA). A Bonferroni-corrected ANOVA comparison of alcohol consumption means (F = 3.0, df = 3, p = 0.034) showed that MAOA-H offenders with a history of CPA drank significantly more than MAOA-L offenders with CPA (p = 0.037). The other comparisons of mean alcohol consumption between groups failed to reach significance. The yearly mean consumption was as follows: MAOA-H with CPA (n = 31) 69.1 kg (SD ± 42.0), MAOA-L with CPA (20) 41.2 kg (SD ± 29.0), MAOA-H without CPA (66) 52.8 kg (SD ± 34.0), and MAOA-L without CPA (57) 48.8 kg (SD ± 36.9). In frequency distribution comparisons between offenders with and without CPA exposure separately within MAOA genotypes, heavy drinkers (≥70 kg per year) were more frequent among MAOA-H offenders with a history of CPA than among MAOA-H offenders without a history of CPA [48% (15/31) vs. 18% (12/66)]; χ2 = 9.6, df = 1, p = 0.002), whereas no difference emerged among MAOA-L offenders (Fig. 1 here).
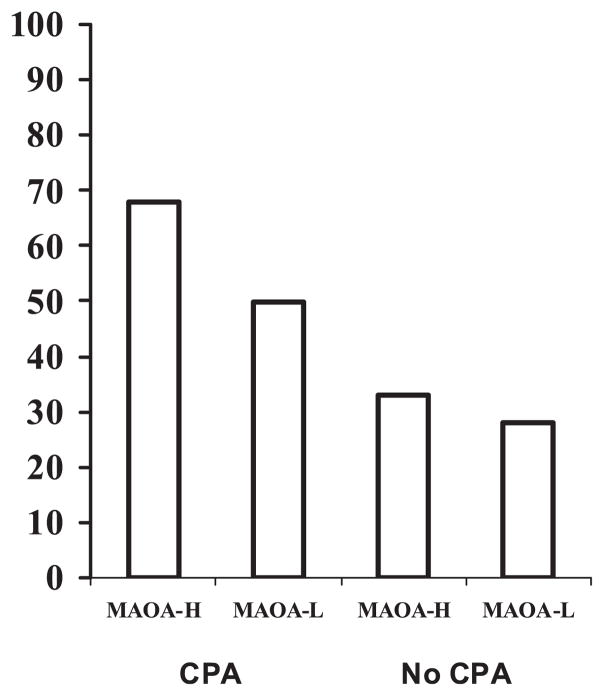
Fig. 1.
The frequency of recidivistic violence (%) in offenders with and without a history of childhood physical abuse (CPA, n = 51) divided into subgroups by monoamine oxidase A (MAOA) genotype. MAOA-H, high-activity genotype (n = 97); MAOA-L, low-activity genotype (n = 77).
Recidivistic Acts of Violence
Forty percent (69/174) of the offenders committed a new violent crime. The frequency of recidivism among the MAOA genotype subgroups with or without CPA was as follows (Fig. 1): MAOA-H with CPA 68% (21/31), MAOA-L with CPA 50% (10/20), MAOA-H without CPA 33% (22/66), and MAOA-L without CPA 28%(16/57).
The distribution of heavy drinking and CPA exposures in the MAOA genotype subgroups was examined before risk analyses were performed. Both heavy drinking and CPA were evenly distributed (χ2 = 1.15, df = 1, p = 0.284 and χ2 = 0.74, df = 1, p = 0.389), and age showed no correlation with genotype (r = −0 064, p = 0.404).
The independents in the regression models were tested for correlations, and the only weak correlation was found between heavy drinking and CPA in the MAOA-H subsample (r = 0.214, p = 0.036).
Table 1, model 1, presents that both heavy drinking and CPA showed positive main effects on risk of recidivistic acts of violence, whereas MAOA genotype showed no main effect. Model 1, with model fitting information χ2 = 26.3, df = 6, p < 0.001, and, with 71% predictive power, explained 19% of the variability of outcome (nagelkerke = 0.190). On stepwise inclusion of interaction terms, both MAOA*heavy drinking (b = −2.90, SE = 0.88, W = 10.9, p = 0.001) and MAOA*CPA (b = −3.16, SE = 0.93, W = 11.6, p =0.001) added risk to the model, whereas heavy drinking*CPA showed nonsignificant (b = −0.79, SE = 0.54, W = 2.1, p = 0.144).
Table 1.
Monoamine Oxidase A (MAOA) Genotype, Childhood Physical Abuse (CPA), and Heavy Drinking (≥70 kg per year) as Predictors of Recidivistic Acts of Violence
| Risk of violence
|
|||||
|---|---|---|---|---|---|
| b (SE) | W | p | OR | CI | |
| Model 1 | |||||
| MAOA (n = 174) | 0.37 (0.34) | 1.1 | 0.285 | 1.4 | 0.74–2.82 |
| Heavy drinking (43) | 0.93 (0.39) | 5.7 | 0.017 | 2.5 | 1.18–5.48 |
| CPA (51) | 1.28 (0.38) | 11.7 | 0.001 | 3.6 | 1.72–7.49 |
| Intercept | −2.50 (0.82) | 9.3 | 0.002 | ||
| Model 2 | |||||
| MAOA-H (97) | |||||
| Heavy drinking (27) | 1.64 (0.57) | 8.2 | 0.004 | 5.2 | 1.68–15.88 |
| CPA (31) | 1.66 (0.57) | 8.9 | 0.003 | 5.3 | 1.77–15.60 |
| Intercept | −3.90 (1.22) | 10.3 | 0.001 | ||
| Model 3 | |||||
| MAOA-L (77) | |||||
| Heavy drinking (16) | 0.04 (0.65) | 0.0 | 0.945 | 1.1 | 0.30–3.72 |
| CPA (20) | 0.96 (0.57) | 2.9 | 0.091 | 2.6 | 0.86–8.02 |
| Intercept | −0.12 (1.30) | 0.0 | 0.928 | ||
Parental problem drinking, early onset conduct disorder, and age were used as covariates in the logistic regression analyses.
MAOA-H, high activity genotype; MAOA-L, low activity genotype; b, regression coefficient; SE, standard error; W, Wald’s test; p, p-value; OR, odds ratio; CI, 95% confidence interval.
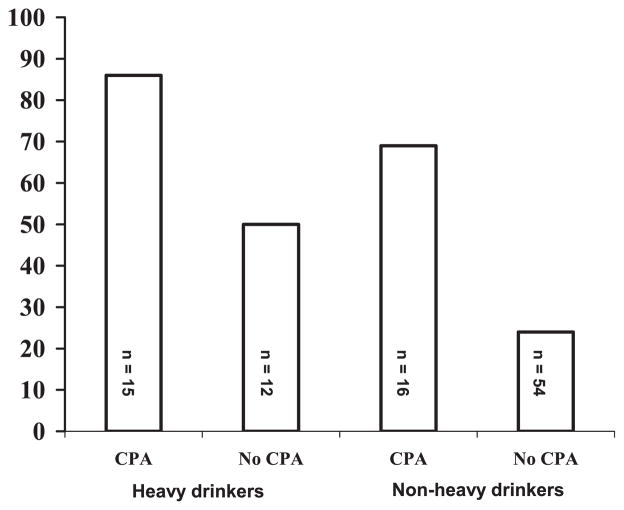
Table 1, model 2, shows that both heavy drinking and CPA increased the risk of acts of violence among MAOA-H offenders. Model 2, with model fitting information χ2 =26.5, df = 5, p = 0.001, and with 73% predictive power, explained 32% of the variability of outcome (nagelkerke = 0.320). Entry of the heavy drinking*CPA interaction term into the MAOA-H offender model failed to accumulate any risk to the model even though an interaction occurred (b = −3.45, SE = 1.25, W = 7.6, p = 0.006). Figure 2 displays the pattern of the interaction as it presents the frequency of recidivistic acts of violence among MAOA-H offenders divided into groups by history of heavy drinking and CPA. Still, in greater detail, the difference in frequency of recidivism between MAOA-H heavy drinkers with or without exposure to CPA was nonsignificant [86% (13/15) vs. 50% (6/12)]; χ2 = 4.3, df = 1, p = 0.087), whereas exposure to CPA showed important among MAOA-H nonheavy drinkers [69% (11/16) for those with a history of CPA and 24% (13/54) for those without CPA; χ2 = 10.9, df = 1, p = 0.002].
Fig. 2.
Frequency (%) of recidivistic acts of violence among 97 violent offenders carrying the high activity monoamine oxidase A genotype divided into subgroups by heavy drinking (≥70 kg per year) and childhood physical abuse (CPA).
Table 1, model 3 (model fitting information, χ2 = 7.5, df = 5, p = 0.183, 68% predictive power; nagelkerke = 0.131), shows that neither heavy drinking nor CPA affected the risk of acts of violence among MAOA-L offenders.
Table 2 shows some predictor combinations (heavy drinking, CPA, CD, and parental problem drinking) that resulted in a more precise classification than a random classification (50:50) of violent offenders into becoming recidivists or non-recidivists among 97 MAOA-H offenders. The classification was obtained from the classification table of model 4 (intercept, b = −2.06 SE = 0.64, W = 10.2, p = 0.001; model fitting information, χ2 = 22.5, df = 5, p < 0.001; 73% predictive power; nagelkerke = 0.277).
Table 2.
The Sensitivity and Specificity of Risk Factor Combinations to Predict Violent Outcome (yes/no) Among 97 Violent Offenders Carrying the High Activity Monoamine Oxidase A Genotype Alcoholic in a 8-Year Follow-Up
| Predicting acts of violence
|
|||||
|---|---|---|---|---|---|
| Heavy drinking | CPA | CD | Parental problem drinking | Correct classification (%) | |
| Yes | + | + | + | + | 89 |
| + | + | − | + | 87 | |
| + | + | + | − | 87 | |
| − | + | + | + | 67 | |
| − | + | + | − | 67 | |
| − | + | − | + | 63 | |
| − | + | − | − | 63 | |
| No | − | − | − | − | 77 |
| − | − | − | + | 77 | |
| − | − | + | − | 73 | |
| − | − | + | + | 73 | |
Heavy drinking ≥70 kg alcohol per year.
CPA, childhood physical abuse; CD, early onset conduct disorder.
DISCUSSION
These results suggest that MAOA genotype altered the effects of heavy drinking and CPA on the risk of severe recidivistic impulsive violent crimes during an 8-year nonincarcerated follow-up. MAOA, however, had no main effect on the risk. The independent and additive risk increases because of heavy drinking (OR 5.2, p = 0.004) and CPA (OR 5.3, p = 0.003) were statistically significant only among MAOA-H offenders, and not among MAOA-L offenders. The prediction that a MAOA-H offender with a history of both heavy drinking and CPA would become a recidivist proved correct in 87 to 89% of the cases, where the small 2% variation was because of occurrence of CD and/or parental problem drinking. In contrast, the prediction that an absence of these risk factors would lead to nonrecidivism proved to be correct in 73 to 77% of the cases. These figures of sensitivity and specificity may be considered high, given that the literature on long-term prediction of violent behavior has struggled to outperform a random classification because of methodological obstacles (Mossman, 1994). Previous meager prediction figures may depend on the fact that differing genotypes have rarely been examined conjunct to risk assessments.
Contrary to expectations, offenders with or without a history of CPA showed no important difference in adulthood alcohol consumption. Interestingly, however, after stratification into MAOA genotypes, high alcohol consumption was observed among MAOA-H offenders with a history of CPA, and this group had a high frequency of recidivistic acts of violence (Fig. 1).
The co-occurrence of heavy alcohol consumption and high frequency of recidivism among MAOA-H offenders with a history of CPA suggests that the high odds for recidivism caused by CPA among MAOA-H offenders (Table 1) may be mediated through excessive drinking. The rationale for this hypothesis was, on one hand, that childhood adversities have been associated with alcoholism (Brown and Anderson, 1991), and alcoholism, on the other hand, is linked to violence (Bye, 2007; Zhang et al., 1997). Moreover, the acute disinhibitory effect of alcohol may lead to a loss of behavioral control (Goldstein and Volkow, 2002), and some evidence suggests that alcohol consumption increases the risk of impulsive violent reconvictions among MAOA-H offenders (Tikkanen et al., 2009). Surprisingly, however, analyses revealed that even though an interaction occurred between heavy drinking and CPA in the logistic model among MAOA-H offenders, it added no risk, which implies that the effect of CPA was not mediated via the quantity of drinking. In fact, the difference in frequency of recidivism between MAOA-H offenders with or without an exposure to CPA was slightly greater among the nonheavy drinkers than among the heavy drinkers (Fig. 2). Moreover, high frequencies of recidivism (50 to 86%) cumulated into a minority that was comprised of individuals with a heavy drinking and/or CPA exposure history (n = 43), whereas the majority of MAOA-H offenders lacked these exposures (n = 54) and had a low frequency of recidivism (24%) (Fig. 2). This accumulation of recidivism into MAOA-H offenders with a heavy drinking and/or CPA exposure history is further accentuated, while the frequency of recidivism among MAOA-L offenders was generally low (34%). Such convergence of risk accumulation may in the future help secondary prevention measures and risk assessments to focus on distinct minorities—both high- and low-risk individuals—among violent offenders.
Furthermore, and despite the fact that our analyses suggest independent effects of heavy drinking and CPA on the risk for impulsive recidivistic violent crimes, it is possible that both effects are partially mediated via trait impulsivity, as childhood adversities are considered to lead to impulsive cluster B personality disorders with impulsive features (Johnson et al., 1999; Zanarini et al., 1997). Alcoholism/alcohol consumption also associates with novelty seeking that includes impulsivity (Grucza et al., 2006; Zhang et al., 1997). Given that an increasing body of evidence suggests that the MAOA-H, in particular, associates with impulsivity in healthy men (Cerasa et al., 2008; Manuck et al., 2000; Passamonti et al., 2006), impulsivity-related disorders such as BPD (Ni et al., 2007, 2009), attention deficit hyperactivity disorder (Manor et al., 2002), Tourette syndrome (Gade et al., 1998), and substance abuse (Gade et al., 1998), and that impulsive-aggressive behavior is linked to low central serotonin levels (Coccaro, 1989; Linnoila et al., 1983), heavy drinking and CPA exposures may have pushed MAOA-H offenders in our sample—with a predisposition toward impulsivity—over a certain threshold of impulsivity leading to impulsive acts of violence.
Our finding that MAOA-H individuals are prone to commit impulsive violent crimes after exposure to childhood adversity is seemingly in contrast with the finding by Caspi and colleagues (2002), who reported that MAOA-L individuals are at an increased risk of violent crimes after exposure to childhood maltreatment. However, both MAOA-H and MAOA-L individuals may present with different types of violence and antisocial behavior. We emphasize that our sample comprises antisocial alcoholics who committed impulsive acts of violence during alcohol intoxication (i.e., manslaughter, attempted manslaughter, assault, or battery). Such acts are defined as impulsive in the Finnish medicolegal institution, and the majority of these crimes were also impulsive judged by the mental status reports that specifically assess the ability to control behaviors during the crime. In contrast, MAOA-L has been connected to more premeditated nonalcohol-related violence and intent to harm others in both a community sample (Caspi et al., 2002) and a violent offender population (Reif et al., 2007). The type of antisocial personality (with or without comorbid alcoholism) may determine which MAOA genotype associates with violence in the same manner as gender difference seemed to determine which MAOA genotype was associated with problem behavior among adolescent boys and girls in the studies of Nilsson and and colleagues (2007) Nilsson and and colleagues (2008). Moreover, the quality and timing of childhood adversity may explain the mixed results in the field, as a recent finding by Weder and colleagues (2009) holds that different levels of childhood trauma may predispose victims to different violence-related features (aggression, rule-breaking, and inattention) in differing MAOA genotypes.
Finnish MAOA-H risk alcoholics exhibiting antisocial behavior, high alcohol consumption, and abnormal alcohol-related impulsive and uncontrolled violence might represent an etiologically distinct alcohol dependence subtype. Because the Finnish population may be considered an isolate population carrying a highly conserved genome (Kere, 2001), our sample probably differs genetically from other Caucasian samples. The statistically significant effects of heavy drinking and CPA among the genotype conferring high, rather than low, MAOA activity would be comprehensible if a different functional MAOA variant in linkage disequilibrium with MAOA-LPR were responsible for the effects observed. Gene by gene interactions may partly explain results, because emerging evidence shows that antisocial alcoholism is associated with abnormal genotypes (Belfer et al., 2006, 2007; Ducci et al., 2006, 2009; Enoch et al., 2006; Xu et al., 2007). Recent research also suggests that the MAOA-H may be a marker of a wider serotonergic dysfunction in BPD (Ni et al., 2007, 2009).
A limitation of this study is that CPA was examined retrospectively, but blinded to both outcome and genotype, and was defined as a dichotomous measure. On the other hand, childhood maltreatment has been shown to be a robust predictor of violent and antisocial behaviors, and most studies have observed an effect, despite differing definitions of maltreatment. To avoid spurious results, we adjusted the regression analyses for gene–environment correlations to obtain a pure maltreatment effect. Age was adjusted for, as the risk of impulsive recidivistic violent crimes among violent offenders (Tikkanen et al., 2009) and trait impulsivity among patients with BPD (Zanarini et al., 2007) typically varies over lifespan. An advantage of our sample, on the other hand, was that the high base rate of CPA and recidivistic violence gave power to detect interactive effects. Still, these results should be interpreted with the understanding that important pure gene by environment interactions are difficult to detect (Eaves, 2006; Jaffee and Price, 2007). Other strengths of this study were its large sample size, homogenous character, and reliable register-based criminal data. Moreover, that both the baseline and outcome acts of violence were severe and impulsive acts of violence narrow the examined aggression type to individuals that were extremely prone to repeat severe acts of violence, which is a clear distinction from other research that have measured aggressive personality features or single acts of violence.
In sum, our results suggest that alcoholic offenders carrying MAOA-H may be significantly more vulnerable to the negative effects of CPA and alcohol than carriers of the MAOA-L. These results should not, however, be applied to other populations because our sample of criminal alcoholics differs profoundly from the general population.
Acknowledgments
This research benefited from the support of the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, NIH.. Jari Tiihonen has been a Faculty member of the Lundbeck International Neuroscience Foundation since 1997, a consult/expert member of Advisory Board of Janssen-Cilag since 2006, a member of Eli Lilly Advisory Board since October 2006, has conducted research collaboration with Organon since 2007, and has received lecture fees from Janssen-Cilag, Eli Lilly, and Lundbeck. All other authors report no competing interests. No financial support was received from anyone benefiting from these results.
References
- [APA] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 1987. 3rd revised ed. [Google Scholar]
- Beitchman JH, Mik HM, Ehtesham S, Douglas L, Kennedy JL. MAOA and persistent, pervasive childhood aggression. Mol Psychiatry. 2004;9:546–547. doi: 10.1038/sj.mp.4001492. [DOI] [PubMed] [Google Scholar]
- Belfer I, Hipp H, Bollettino A, McKnight C, Evans C, Virkkunen M, Albaugh B, Max MB, Goldman D, Enoch MA. Alcoholism is associated with GALR3 but not two other galanin receptor genes. Genes Brain Behav. 2007;6:473–481. doi: 10.1111/j.1601-183X.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, Albaugh B, Virkkunen M, Yuan Q, Max MB, Goldman D, Enoch MA. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry. 2006;11:301–311. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am J Psychiatry. 1991;148:1423–1424. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, Van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Bye EK. Alcohol and violence: use of possible confounders in a time-series analysis. Addiction. 2007;102:369–376. doi: 10.1111/j.1360-0443.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Fera F, Passamonti L, Liguori M, Lanza P, Muglia M, Magariello A, Quattrone A. Ventro-lateral prefrontal activity during working memory is modulated by MAO A genetic variation. Brain Res. 2008;1201:114–121. doi: 10.1016/j.brainres.2008.01.048. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Central serotonin and impulsive aggression. Brit J Psychiatry. 1989;8:52–62. [PubMed] [Google Scholar]
- Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Yuan Q, Shen PH, White KV, Hodgkinson C, Albaugh B, Virkkunen M, Goldman D. HTR3 is associated with alcoholism with antisocial behavior and alpha EEG power—an intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol. 2009;43:73–84. doi: 10.1016/j.alcohol.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11:858–866. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- Eaves LJ. Genotype x environment interaction in psychopathology: fact or artifact? Twin Res Hum Genet. 2006;9:1–8. doi: 10.1375/183242706776403073. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141:599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade R, Muhleman D, Blake H, MacMurray J, Johnson P, Verde R, Saucier G, Comings DE. Correlation of length of VNTR alleles at the X-linked MAOA gene and phenotypic effect in Tourette syndrome and drug abuse. Mol Psychiatry. 1998;3:50–60. doi: 10.1038/sj.mp.4000326. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grucza RA, Cloninger R, Bucholz KK, Constantino JN, Schuckit MA, Dick DM, Bierut LJ. Novelty seeking as a moderater of familial risk for alcohol dependence. Alcohol Clin Exp Res. 2006;30:1176–1183. doi: 10.1111/j.1530-0277.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J. Childhood maltreatment increases risk tor personality disorders during early adulthood. Arch Gen Psychiatry. 1999;56:600–606. doi: 10.1001/archpsyc.56.7.600. [DOI] [PubMed] [Google Scholar]
- Kere J. Human population genetics: lessons from finland. Annu Rev Genomics Hum Genet. 2001;2:103–128. doi: 10.1146/annurev.genom.2.1.103. [DOI] [PubMed] [Google Scholar]
- Levy ER, Powell JF, Buckle VJ, Hsu YP, Breakefield XO, Craig IW. Localization of human monoamine oxidase-A gene to Xp11.23-11.4 by in situ hybridization: implications for Norrie disease. Genomics. 1989;5:368–370. doi: 10.1016/0888-7543(89)90072-4. [DOI] [PubMed] [Google Scholar]
- Linnoila M, De Jong J, Virkkunen M. Family history of alcoholism in violent offenders and impulsive fire setters. Arch Gen Psychiatry. 1989;46:613–616. doi: 10.1001/archpsyc.1989.01810070039006. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- Manor I, Tyano S, Mel E, Eisenberg J, Bachner-Melman R, Kotler M, Ebstein RP. Family-based and association studies of monoamine oxidase A and attention deficit hyperactivity disorder (ADHD): preferential transmission of the long promoter-region repeat and its association with impaired performance on a continuous performance test (TOVA) Mol Psychiatry. 2002;7:626–632. doi: 10.1038/sj.mp.4001037. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A may be associated with variability in aggression, impulsiveness, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Mossman D. Assessing predictions of violence: being accurate about accuracy. J Consult Clin Psychol. 1994;62:783–792. doi: 10.1037//0022-006x.62.4.783. [DOI] [PubMed] [Google Scholar]
- Ni X, Chan D, Chan K, McMain S, Kennedy JL. Serotonin genes and gene–gene interactions in borderline personality disorder in a matched case-control study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:128–133. doi: 10.1016/j.pnpbp.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Ni X, Sicard T, Bulgin N, Bismil R, Chan K, McMain S, Kennedy JL. Monoamine oxiades A gene is associated with borderline personality disorder. Psychiatr Genet. 2007;17:153–157. doi: 10.1097/YPG.0b013e328016831c. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Wargelius HL, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius HL, Sjoberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Olds D, Henderson CR, Cole R, Enckenrode J, Kitzman H, Luckey D, Pettitt L, Sidora K, Morris P, Powers J. Long-term effects of nurse home visitation on children’s criminal and antisocial behaviour: 15-year follow-up of randomized controlled trial. JAMA. 1998;280:1238–1244. doi: 10.1001/jama.280.14.1238. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fera F, Magariello A, Cerasa A, Gioia MC, Muglia M, Nicoletti G, Gallo O, Provinciali L, Quattrone A. Monoamine oxidase- a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol Psychiatry. 2006;59:334–340. doi: 10.1016/j.biopsych.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, Wenzler D, Jacob CP, Retz-Junginger P, Thome J, Lesch KP. Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacol. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, Rommelspacher H, Winterer G, Schmidt LG, Sander T. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Res. 1999;86:67–72. doi: 10.1016/s0165-1781(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Sander T, Kuhn S, Smolka M, Rommelspacher H, Samochowiec J, Lesch KP. Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. JNeural Transm. 2000;107:681–689. doi: 10.1007/s007020070069. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R. American Psychiatric Press; Washington, DC: 1990. Non-patient ed. [Google Scholar]
- Tikkanen R, Sjoberg RL, Ducci F, Goldman D, Holi M, Tiihonen J, Virkkunen M. Effects of MAOA-genotype, alcohol consumption, and aging on violent behavior. Alcohol Clin Exp Res. 2009;33:428–434. doi: 10.1111/j.1530-0277.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA genotype, maltreatment, and aggressive behavior: the changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Anderson TR, Neyer KM, Lamparella N, Jenkins G, Zhou Z, Yuan Q, Virkkunen M, Lipsky RH. Nucleotide sequence variation within the human tyrosine kinase B neurotrophin receptor gene: association with antisocial alcohol dependence. Pharmacogenomics. 2007;7:368–379. doi: 10.1038/sj.tpj.6500430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Reich DB, Silk KR, Hudson JI, McSweeney LB. The subsyndromal phenomenology of borderline personality disorder: a 10-year follow-up study. Am J Psychiatry. 2007;164:929–935. doi: 10.1176/ajp.2007.164.6.929. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, Levin A, Yong L, Frankenburg FR. Reported pathological childhood experiences associated with the development of borderline personality disorder. Am J Psychiatry. 1997;154:1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wieczorek WF, Welte JW. The nexus between alcohol and violent crime. Alcohol Clin Exp Res. 1997;21:1264–1271. [PubMed] [Google Scholar]