Abstract
Introduction
Better characterization of bone geometry in adolescents with anorexia nervosa (AN) may improve understanding of skeletal deficits in this population. Our objective was to determine whether hip cross-sectional geometry and bone strength were altered in adolescents with AN.
Methods
Measurements of the left total proximal femur and body composition were obtained in 85 adolescents with AN and 61 healthy controls by dual X-ray absorptiometry. The Hip Structural Analysis (HSA) program was used to determine aBMD, cross-sectional area (CSA), and section modulus (Z) at the femoral neck and shaft. Strength indices were calculated and corrected for lean mass.
Results
Femoral neck and shaft aBMD were lower in AN patients than healthy controls (−36% and −29%, p<0.001). In both regions, bone CSA and Z were lower in AN sufferers (−11 to −35%, p<0.001). While lean body mass correlated with HSA variables (r=0.48 to 0.58, p<0.001), body fat did not. AN sufferers had lower indices of both whole bone strength (−40%, p<0.001) and relative bone strength (−36%, p<0.001) than controls.
Conclusions
Anorexia nervosa sufferers had decreased resistance to axial (CSA) and bending loads (Z) compared with healthy controls. Differences in strength properties were significant even when adjusted for lean mass, suggesting that not only decreased mechanical loading, but also known metabolic differences are likely responsible for deficits in bone strength in these patients.
Keywords: Adolescents, Anorexia nervosa, Bone geometry, Bone strength, Malnutrition
Introduction
Adolescence is the critical period in a young woman’s life for bone mineral acquisition and attainment of peak bone mass [1]. Anorexia nervosa (AN), a disorder of malnutrition, intense fear of weight gain, and amenorrhea, is the third most common chronic disease among adolescent girls [2]. As a consequence of this disease, affected young women may never attain their peak bone mass [2–4]. Two clinical features of AN, estrogen deficiency and loss of body weight, are important risk factors for osteoporosis [5]. Multiple studies have demonstrated that over half of patients with AN are at risk of skeletal deficits that may not return to pre-illness levels, even following weight restoration and return of menses [6, 7]. The majority of these studies used dual-energy X-ray absorptiometry (DXA) measurements of areal bone mineral density (aBMD, g/cm2) and bone mineral content (BMC, g) as primary skeletal outcome measures. While DXA remains the preferred method of evaluating bone mass in children and adolescents due to its speed, precision, low radiation exposure, and availability of pediatric reference data, traditional outcomes of aBMD and BMC do not adequately describe the strength of bone. Bone strength depends on both the material and structural (geometric) properties of bone [8]. While material strength cannot yet be measured in vivo by non-invasive means, geometry can be measured and may reveal strength deficits not readily evident in conventional BMD or BMC [9].
Recent advances in bone densitometry make it possible to calculate specific measures of bone structural geometry from conventional DXA images. One such method is the Hip Structural Analysis (HSA) program, which uses properties of the DXA image to derive geometric measures that are commonly used in engineering evaluations of strength [10]. The HSA method measures bone geometry in narrow regions corresponding to thin cross-sectional slices of bone at specific locations through the proximal femur. The resulting structural variables provide clinically relevant indices of bone strength, and have been used to predict stress fractures in military recruits [9], explain gender and ethnicity differences in fracture rates [11], and evaluate bone adaptation to weight changes, hormone replacement, and exercise intervention [12, 13]. Significant structural changes can be seen after specific interventions despite only modest changes in BMD as measured by conventional methods of bone densitometry [12].
Better characterization of bone geometry in adolescents with AN may improve our understanding of bone adaptation to changes in mechanical loads and the hormonal milieu. Previous literature suggests that estradiol inhibits periosteal expansion and stimulates bone formation on the endocortical surface [3]. These associations could be either a direct effect of estradiol or an interaction with mechanical load response [14, 15]. Regardless, patients with AN who have both low estradiol levels (among other hormonal alterations) and reduced mechanical load (from low lean mass) would be expected to have altered bone geometry.
The objective of the current study, therefore, was to determine whether the geometric expression of DXA-derived bone mass suggested a deficiency in bone strength in young women with AN. We also sought to determine the relationships between anthropometric measurements and parameters of proximal femur cross-sectional geometry. We hypothesized that periosteal diameters would be greater due to estrogen deficiency, but that geometric strength would be lower in young women with AN compared with healthy young women.
Materials and methods
Participants
Female adolescents and young women aged 14 to 26 years with a diagnosis of AN (n=85) were recruited from the Eating Disorders Program at an outpatient adolescent medicine clinic. All met Diagnostic and Statistical Manual IV (DSM-IV) criteria for AN and were post-menarchal. All participants with AN had secondary amenorrhea; those less than 15 years old were at least 2 years post-menarchal. Patients receiving hormonal agents or other medications known to affect bone during the 3 months prior to study enrollment were excluded.
Data from the Penn State Young Women’s Health Study (YWHS) were used as control data. The YWHS was a longitudinal study of Caucasian adolescent girls attending school in central Pennsylvania. Details of recruitment methods have been previously reported [16]. Data from the participants from study visits 10 through 15 (corresponding to their ages 15 to 23 years) were utilized for comparison. All controls were post-menarchal at the time of the visits. Participants were excluded if they were taking medications known to affect bone health, such as contraceptive hormones (oral contraceptive pills, depot medroxyprogesterone), glucocorticoids, or anticonvulsants. Data from a total of 61 controls met the criteria for use in the data analysis.
All study procedures were reviewed and approved by the local institutional review boards. Informed consent was obtained from all participants over the age of 18 years or their parents. Minors (age <18 years) provided assent for participation.
Data collection
Data were collected in patients with AN at the baseline visit of a clinical trial. All participants completed a semi-structured interview for demographic information and health history, including information about medication use and menstrual history. Height (cm) was measured using a wall-mounted stadiometer. Weight (kg) was measured post-voiding, with participants wearing a hospital gown. The same stadiometer and calibrated scale were used for all measurements. Body mass index (BMI, kg/m2) was calculated, and BMI percentile was determined using published percentile tables [17].
Bone measurements
Participants with AN had aBMD and BMC measured by DXA using the QDR-4500 with Delphi upgrade (Hologic, Waltham, MA, USA). Measurements were performed at the left total proximal femur and the lumbar spine (L1–L4). BMD Z-scores were calculated using age- and gender-specific reference data for comparison [18, 19]. For participants less than age 20 years, pediatric normative data were used [20]. The in vitro precision for DXA measurements using the average of daily scans of standard phantoms over the previous 6 months was 0.35% at Children’s Hospital Boston. Controls had DXA images taken at the proximal femur using the QDR-2000 W bone densitometer (Hologic). The observed in vitro coefficient of variation was less than 0.7% for the day-to-day quality control scans [13].
Body composition
Body composition was also measured in all participants by DXA, including lean body mass (kg) and body fat, expressed as a percentage. To account for the use of two different Hologic machines to measure body composition, absolute values for lean body mass and body fat percentage from the QDR-4500 Delphi were corrected to values for the QDR-2000 W using published regression-generated equations [21]. The conversion equations reduce the discrepancy between scanners to a level that enables direct comparison of data obtained by the two instruments.
Bone geometry analyses
Proximal femur scans were analyzed for bone structure and cross-sectional geometry by use of the Hip Structure Analysis (HSA) program developed by Beck et al. [10], which is based upon principles first described by Martin and Burr [22]. The HSA software version 3.1 derives measurements of BMD and geometry of narrow cross-sections of bone from traditional DXA images. HSA v3.1 averages bone dimension and geometry measurements for a series of five parallel pixel mass profiles spaces ~1 mm apart along the bone axis. We report analyses from the narrow neck region (NN, across the femoral neck at its narrowest point) and the proximal femoral shaft region (S, across the shaft 1.5 times minimum neck width, distal to the intersection of the neck and shaft axes). HSA data are standardized to a common phantom, making the QDR-4500 Delphi and QDR-2000 W data equivalent.
At the two analysis regions, bone mineral density (BMD, g/cm2), bone cross-sectional area exclusive of soft tissue spaces (CSA, cm2), and cross-sectional moment of inertia (CSMI, cm4) were measured. Section modulus (Z, cm3), a measure of bone bending strength, was calculated as CSMI/dmax, where dmax = the maximum distance from the center of mass to the surface. CSA and section modulus are inversely related to stresses due to axial and bending loads respectively. Bone outer diameter was measured directly between the margins of the blur-corrected bone mass profile. Cortical thickness (cm) was estimated by modeling cortices of femoral shaft cross-sections as concentric circles. Models assume 100% of the measured mass is in the cortex for the femoral shaft, and endosteal (inner) diameter is estimated. The relative thickness of the femoral neck cortex was calculated as a buckling ratio. Details of the method were recently published [23].
Strength indices
Strength indices were calculated to evaluate whether bone strength was compromised in the young women with AN. The index is based on the general principle that the strength of a long bone scales as a section modulus over bone length [24]. A bone strength index (section modulus/height) was calculated, using height as a surrogate for bone length. Resulting values were multiplied by 1,000 for convenience. Relative bone strength (bone strength index/lean body mass) was also calculated to determine whether responses to load stimulus were different between the two groups.
Statistical analyses
Descriptive statistics (percentages, means, and standard deviations) were used to characterize the sample. Random effects regression models were used to compare anthropometric characteristics, age, and bone structural variables between groups while accounting for within participant variation due to the multiple observations per participant in the control group. Spearman correlation analyses were used to evaluate relationships between the anthropometric values and structural measures in the participants with AN. Analyses were performed separately for the narrow neck and shaft regions. Percentage differences in HSA variables and strength indices were calculated (mean value for AN sufferers − mean value for control subjects/mean value for AN sufferers). Statistical analyses were performed with SAS (SAS Institute, Cary, NC, USA). Level of significance was set at p value < 0.05.
Results
Descriptive statistics
We studied 85 adolescents and young women with AN and 61 healthy controls. Anthropometric measurements and information on demographic characteristics were obtained (Table 1). A total of 298 observations were included for the 61 controls; measurements were obtained on only one occasion for each participant with AN. There were no significant differences in age or height between the groups. As expected, patients with AN had significantly lower body weight, BMI, lean body mass, and percentage body fat. Group differences in body composition remained significant even after the conversion equation to correct for scanner differences was applied. Participants with AN had a median duration of illness of 18 months (range 3 to 138 months) and median duration of amenorrhea of 11 months (range 3 to 90 months).
Table 1.
Anthropometric measures and demographic characteristics of healthy female adolescents and adolescents with anorexia nervosa (AN). BMI body mass index
| Characteristics | AN patients | Controls | p value |
|---|---|---|---|
| Age (years) | 0;18.8±0.22 | 18.9±0.11 | NS |
| Height (cm) | 164.8±0.74 | 165.8±0.73 | NS |
| Weight (kg) | 47.7±0.85 | 59.7±0.77 | <0.0001 |
| BMI (kg/m2) | 17.5±0.28 | 21.6±0.24 | <0.0001 |
| Lean mass (kg) | 38.4±0.51a | 40.2±5.1 | 0.04 |
| Percentage body fat | 17.3±0.62a | 28.7±5.6 | <0.0001 |
Data are presented as mean ± SE from random effects model accounting for within-participant correlation unless otherwise indicated
Values from the Hologic QDR-4500 Delphi were converted to Hologic QDR-2000 values to allow for direct comparison of data between the two groups. Adjusted mean ± SE is presented
Bone mineral density
Conventional mean hip aBMD by DXA in the patients with AN was 0.847±0.176 g/cm2 (mean ± SD). The mean aBMD Z-score was −0.647±0.890, with a range of −2.7 to 1.5. Total femur BMD Z-scores were between −1.0 and −2.0 SD in 40% of patients, and ≤−2.0 SD in 4% of patients. At the lumbar spine, mean aBMD in the participants with AN was 0.884±0.092 g/cm2. The mean Z-score was −1.1±0.88 with a range of −3.1 to 0.8. Spinal BMD Z-scores were between −1.0 and −2.0 SD in 31% of patients, and ≤−2.0 SD in 21% of patients.
Bone dimensions and geometry
We found significant group differences in bone structural geometry (Tables 2 and 3). As expected, aBMD was significantly lower at both the narrow neck (−36.4%) and the femoral shaft (−29.6%) regions in participants with AN. Interestingly, despite having wider outer (periosteal) diameter at both the femoral neck (n.s.) and shaft (p<0.001) regions, the section modulus was lower in patients with AN (NN, −35.6%; S, −11.3%). The lower bone bending strength in participants with AN was due to wider inner (endocortical) diameters and lower bone CSA at both regions. Geometric differences led to lower cortical thickness and substantially higher buckling ratios (+38%) in both regions in participants with AN compared with healthy controls. The results did not change after adjusting for participant age or height.
Table 2.
Comparison of bone mineral density and bone geometry measurements in the narrow neck region between adolescents with AN and healthy controls. BMD bone mineral density, CSA bone cross-sectional area
| Variable | AN patients | Controls | Δ AN v. controls | p value |
|---|---|---|---|---|
| BMD (g/cm2) | 0.77±0.02 | 1.05±0.02 | −36.4% | <0.0001 |
| CSA (cm2) | 2.09±0.05 | 2.84±0.05 | −35.9% | <0.0001 |
| Section modulus (cm3) | 0.9±0.03 | 1.26±0.03 | −40% | <0.0001 |
| Outer diameter (cm) | 2.86±0.02 | 2.84±0.02 | +0.7% | 0.49 |
| Inner diameter (cm) | 2.57±0.02 | 2.43±0.02 | +5.4% | <0.0001 |
| Cortical thickness (cm) | 0.15±0.003 | 0.21±0.003 | −40% | <0.0001 |
| Buckling ratio | 10.8±0.24 | 7.01±0.24 | +35% | <0.0001 |
Data are presented as mean ± SE from random effects model accounting for within-participant correlation
Table 3.
Comparison of bone mineral density and bone geometry measures at the femoral shaft between adolescents with AN and healthy controls
| Variable | AN patients | Controls | Δ AN vs. controls | p value |
|---|---|---|---|---|
| BMD (g/cm2) | 1.08±0.02 | 1.43±0.02 | −32.4% | <0.0001 |
| CSA (cm2) | 2.96±0.06 | 3.50±0.06 | −18.2% | <0.0001 |
| Section modulus (cm3) | 1.51±0.04 | 1.67±0.04 | −10.6% | 0.005 |
| Outer diameter (cm) | 2.87±0.02 | 2.58±0.02 | +10.1% | <0.0001 |
| Inner diameter (cm) | 2.11±0.04 | 1.47±0.03 | +30.3% | <0.0001 |
| Cortical thickness (cm) | 0.38±0.01 | 0.56±0.01 | −47.4% | <0.0001 |
| Buckling Ratio | 4.14±0.09 | 2.39±0.09 | +42.3% | <0.0001 |
Data are presented as mean ± SE from random effects model accounting for within-participant correlation
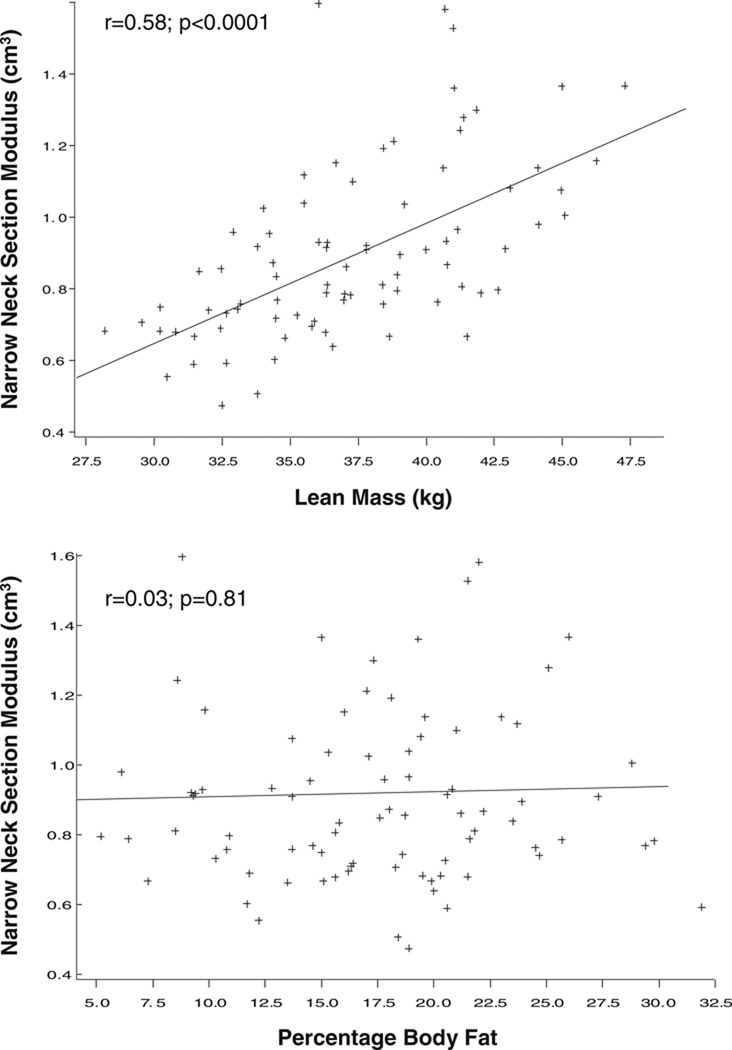
In patients with AN, both weight and height were correlated with bone CSA and section modulus at the narrow neck and shaft (r=0.26 to 0.57; p<0.01). Age did not predict structural variables in any hip region. There was a significant association between lean body mass and hip structural variables at the narrow neck and shaft (r=0.48 to 0.58), which remained significant after adjusting for height. The correlations were higher in the controls (r=0.66 to 0.77). Body fat percentage was not related to any bone parameter at either region (Fig. 1).
Fig. 1.
Correlation between anthropometric measures and narrow neck structural variables in patients with anorexia nervosa
In multivariate regression analyses, AN status remained a significant predictor of all hip structural variables at both the narrow neck and shaft regions (except narrow neck outer diameter) after controlling for age, height, and lean mass. The strongest model for predictors of section modulus included AN status, height, and lean mass (Table 4). Lean mass significantly influenced section modulus, bone cross-sectional area, buckling ratio, and cortical thickness at both regions. Height was an important predictor of inner diameter at both regions, and also for shaft buckling ratio, outer diameter, and cortical thickness.
Table 4.
Regression analyses of section modulus at the femoral narrow neck and shaft
| Models/predictors | Δ section modulus NN (cm3) AN vs. control |
p value | Δ section modulus shaft (cm3) AN vs. control |
p value |
|---|---|---|---|---|
| AN | −0.37 | <0.0001 | −0.16 | 0.005 |
| AN, adjusted for age | −0.37 | <0.0001 | −0.16 | 0.005 |
| AN, adjusted for height | −0.35 | <0.0001 | −0.13 | 0.008 |
| AN, adjusted for BMI | −0.29 | <0.0001 | −0.06 | 0.33 |
| AN, adjusted for height and age | −0.35 | <0.0001 | −0.14 | 0.007 |
| AN, adjusted for BMI and age | −0.29 | <0.0001 | −0.07 | 0.24 |
AN represents anorexia nervosa status (yes/no) Data are presented as difference in section modulus (cm3) per unit change of the covariate between patients with AN and controls
Strength indices
At both the narrow neck and shaft regions, patients with AN had a lower bone strength index than healthy controls (−40%, p < 0.001 and −10%, p=0.004 respectively). Even after normalizing to lean body mass, relative bone strength index remained significantly different between groups at both femoral sites (−36%, p<0.001 and −9%, p=0.03). Results did not change when the models were adjusted for participants’ age.
Discussion
We used hip structural analysis as a tool to measure bone cross-sectional geometry and to estimate bone strength in adolescents and young women with AN. We have shown that participants with AN have decreased resistance to axial (CSA) and bending loads (section modulus), and decreased cortical thickness at multiple regions of the hip compared with a healthy, age-matched control group. The buckling ratio, which indicates increased fracture risk in adult populations [25], was increased in participants with AN. While there are currently no data in chronically ill young women to predict future fracture risk, these results are intriguing. Participants with AN also had lower indices of both whole bone strength and relative bone strength compared with controls, indicating weaker bones. The differences in strength properties were significant even when adjusted for body size (height) and lean mass, a surrogate for the muscle loading forces that drive bone adaptation. This finding suggests that differences in bone geometry and deficits in bone strength in patients with AN are not only due to diminished lean mass and thus decreased muscle load, but also possibly related to hormonal differences between the two groups.
While DXA remains the standard method for bone density assessments in children and adolescents, conventional DXA measurements possess inherent limitations. Subtle changes in cross-sectional geometry can markedly affect structural properties in ways that are not clearly evident in aBMD. [26]. In the current study, we noted significant reductions in measurements of structural geometry in patients with AN compared with age-matched healthy controls. The same participants with AN had only moderately low measurements of aBMD at the hip, as reflected by their aBMD Z-scores. These findings highlight the importance of considering structural dimensions and not only density measurements in estimating bone strength.
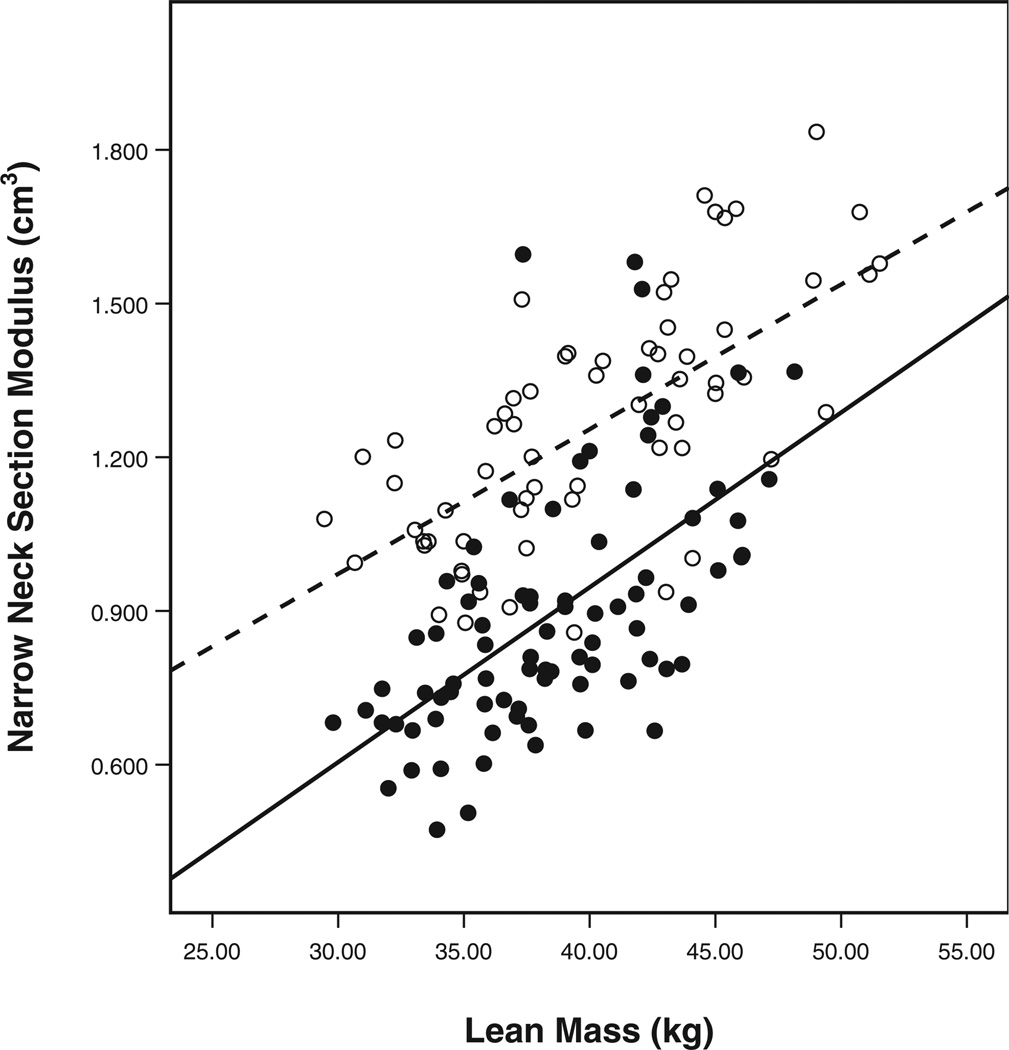
Variables of hip structural geometry were strongly correlated with anthropometric measurements, including height, weight, and BMI. Importantly, lean body mass was a significant predictor of CSA, section modulus, and cortical thickness, while total body fat percentage was not. Total lean body mass as measured by DXA appears to be a reliable surrogate for skeletal load [27]. Previous studies have documented that dynamic forces, rather than static loads, are the greatest stimuli for osteogenesis [28]. Thus, greater lean body mass would be expected to correlate with greater bone strength, consistent with our results and with previous work that has examined the “muscle-bone unit” in adolescent and young adult populations [29]. Interestingly, the effect of lean mass on section modulus was different between the two groups in our study (Fig. 2). A possible explanation for these results was provided in a recent study by Fricke et al. [30], who demonstrated that women with a present or former episode of AN have a lower set point for the acquisition of bone mass due to mechanical stimuli than unaffected females. This effect may be driven by a deficit in estrogen levels [15].
Fig. 2.
Differences in the effect of lean mass on section modulus between participants with anorexia nervosa and controls. Open circles controls, Solid line slope of the line (15.37) for controls, filled circles anorexia nervosa sufferers, dashed line slope of the line (0.99) for participants with anorexia nervosa
Consistent with our initial hypothesis, the participants with AN, who possessed decreased skeletal loading due to malnutrition and related loss of muscle mass, had increased inner and outer diameters, but decreased cortical thickness compared with controls. In addition to the effects of muscle mass, the widened subperiosteal diameter and diminished cortical thickness may be also related to the low estrogen and androgen levels observed in adolescents with AN [6]. Previous work supports an association between sex steroid levels and these structural variables [13], while biopsy data suggest that periosteal apposition is suppressed by estrogen [31].
This study has limitations related both to the study population and analyses. All participants were Caucasian, which could limit the generalizability of our findings. However, it is known that the majority of patients with AN in the USA are of Caucasian origin [32]. The HSA program provides a method of assessing bone structural parameters utilizing DXA technology. However, DXA scans are not optimized for measurements of bone geometry. There are inherent limitations in attempts to assess three-dimensional structure from two-dimensional images. The HSA program assumes that tissue mineralization is not different from that of average adults. In reality, it may be reduced in growing adolescent skeletons, leading to small underestimates of cross-sectional geometry. Assumptions regarding proportions of cortical vs. trabecular bone that are used to estimate cortical thickness and buckling ratio may also not be as applicable to adolescents. Material strength, or factors that influence it such as tissue mineralization, determine stress resistance, but cannot be reliably evaluated using DXA or any other current non-invasive method. Despite these limitations, these sources of error should not be limited to one participant group or the other.
In summary, in this sample of adolescent girls with AN, resistance to both axial and bending loads was compromised at the hip compared with healthy, age-matched controls. In adolescent girls with AN, lean body mass was strongly predictive of bone strength while body fat was not. This finding suggests a beneficial interaction between muscle and bone, and supports previous research emphasizing the importance of the muscle–bone unit. Estrogen and androgen deficiency also likely plays an important role in the geometric changes found in young women with AN. The results of this study highlight the need to take measures of bone geometry, as well as bone density, into account when evaluating bone health in patients with AN.
Acknowledgements
We gratefully acknowledge Julie Ringelheim, Natalie Glass, Suzanne Muggeo, Diane DiFabio, and Jessica Sexton for outstanding technical assistance; the excellent skill and care of the GCRC nurses at the Children’s Hospital Boston; and our patients who made this study possible. Funding for this study was provided by: RO1 HD043869 from the NICHD; NIH/NCRR Grant MO1-RR-2172 to the Children’s Hospital Boston General Clinical Research Center; Department of Defense, US Army Bone Health and Military Readiness Program; and Project 5-T71-MC-00009-14 from the Maternal and Child Health Bureau.
Footnotes
Conflict of interest statement The Hip Structure Analysis software developed by Dr. Beck has been licensed by Johns Hopkins University to Hologic, Inc. Dr. Beck has received research support from Eli Lilly, Aventis, NPS Pharmaceuticals, Amgen, and Merck, Inc. Dr. LeBoff has research support from Novartis., owns stock in Amgen, and has served on Roundtable discussions for Proctor and Gamble and Eli Lilly. All other authors have no conflicts of interest.
Contributor Information
A. D. DiVasta, Email: amy.divasta@childrens.harvard.edu, Division of Adolescent Medicine, Children’s Hospital Boston, 333 Longwood Avenue, Boston, MA 02115, USA.
T. J. Beck, Department of Radiology, Johns Hopkins University, Baltimore, MD 21287, USA
M. A. Petit, School of Kinesiology, University of Minnesota, Minneapolis, MN 55455, USA
H. A. Feldman, Clinical Research Program, Children’s Hospital Boston, 333 Longwood Avenue, Boston, MA 02115, USA
M. S. LeBoff, Skeletal Health and Osteoporosis Program, Brigham and Women’s Hospital, Boston, MA 02115, USA
C. M. Gordon, Division of Adolescent Medicine, Children’s Hospital Boston, 333 Longwood Avenue, Boston, MA 02115, USA Division of Endocrinology, Children’s Hospital Boston, 333 Longwood Avenue, Boston, MA 02115, USA.
References
- 1.Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991;73:555–563. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- 2.Silber TJ. Anorexia nervosa among children and adolescents. Adv Pediatr. 2005;52:49–76. doi: 10.1016/j.yapd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E, Szmukler GI, Formica C, Tsalamandris C, Mestrovic R. Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise. J Bone Miner Res. 1992;7:1467–1474. doi: 10.1002/jbmr.5650071215. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E, Karlsson MK, Duan Y. On exposure to anorexia nervosa, the temporal variation in axial and appendicular skeletal development predisposes to site-specific deficits in bone size and density: a cross-sectional study. J Bone Miner Res. 2000;15:2259–2265. doi: 10.1359/jbmr.2000.15.11.2259. [DOI] [PubMed] [Google Scholar]
- 5.Gordon CM, Nelson LM. Amenorrhea and bone health in adolescents and young women. Curr Opin Obstet Gynecol. 2003;15:377–384. doi: 10.1097/00001703-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141:64–70. doi: 10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- 7.Bachrach LK, Katzman DK, Litt IF, Guido D, Marcus R. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72:602–606. doi: 10.1210/jcem-72-3-602. [DOI] [PubMed] [Google Scholar]
- 8.Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 9.Beck TJ, Ruff CB, Mourtada FA, Shaffer RA, Maxwell-Williams K, Kao GL, et al. Dual-energy X-ray absorptiometry derived structural geometry for stress fracture prediction in male U.S. Marine Corps recruits. J Bone Miner Res. 1996;11:645–653. doi: 10.1002/jbmr.5650110512. [DOI] [PubMed] [Google Scholar]
- 10.Beck TJ, Ruff CB, Warden KE, Scott WW, Jr, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol. 1990;25:6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Wang XF, Duan Y, Beck TJ, Seeman E. Varying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old age. Bone. 2005;36:978–986. doi: 10.1016/j.bone.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Petit MA, McKay HA, MacKelvie KJ, Heinonen A, Khan KM, Beck TJ. A randomized school-based jumping intervention confers site and maturity-specific benefits on bone structural properties in girls: a hip structural analysis study. J Bone Miner Res. 2002;17:363–372. doi: 10.1359/jbmr.2002.17.3.363. [DOI] [PubMed] [Google Scholar]
- 13.Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study. Bone. 2004;35:750–759. doi: 10.1016/j.bone.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Beck TJ, Oreskovic TL, Stone KL, Ruff CB, Ensrud K, Nevitt MC, et al. Structural adaptation to changing skeletal load in the progression toward hip fragility: the study of osteoporotic fractures. J Bone Miner Res. 2001;16:1108–1119. doi: 10.1359/jbmr.2001.16.6.1108. [DOI] [PubMed] [Google Scholar]
- 15.Jarvinen TL, Kannus P, Sievanen H. Estrogen and bone—a reproductive and locomotive perspective. J Bone Miner Res. 2003;18:1921–1931. doi: 10.1359/jbmr.2003.18.11.1921. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd T, Andon MB, Rollings N, Martel JK, Landis JR, Demers LM, et al. Calcium supplementation and bone mineral density in adolescent girls. JAMA. 1993;270:841–844. [PubMed] [Google Scholar]
- 17.Center for Disease Control and Prevention. Surveillance Summaries, June 28, 2002. MMWR. 2002;2002 51 (No SS-4) [Google Scholar]
- 18.Kelly TL. Bone mineral density reference databases for American men and women. J Bone Miner Res. 1990;5:S249. [Google Scholar]
- 19.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5:389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 20.Zemel BS, Leonard MB, Kalkwarf HJ, Specker BL, Moyer-Mileur LJ, Shepherd JA, et al. Reference data for the whole body, lumbar spine, and proximal femur for American children relative to age, gender, and body size. J Bone Miner Res. 2004:S231. [Google Scholar]
- 21.Sakai Y, Ito H, Meno T, Numata M, Jingu S. Comparison of body composition measurements obtained by two fan-beam DXA instruments. J Clin Densitom. 2006;9:191–197. doi: 10.1016/j.jocd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Martin RB, Burr DB. Non-invasive measurement of long bone cross-sectional moment of inertia by photon absorptiometry. J Biomech. 1984;17:195–201. doi: 10.1016/0021-9290(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 23.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 24.Selker F, Carter DR. Scaling of long bone fracture strength with animal mass. J Biomech. 1989;22:1175–1183. doi: 10.1016/0021-9290(89)90219-4. [DOI] [PubMed] [Google Scholar]
- 25.Melton LJ, III, Beck TJ, Amin S, Khosla S, Achenbach SJ, Oberg AL, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos Int. 2005;16:460–467. doi: 10.1007/s00198-004-1820-1. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd T, Petit MA, Lin HM, Beck TJ. Lifestyle factors and the development of bone mass and bone strength in young women. J Pediatr. 2004;144:776–782. doi: 10.1016/j.jpeds.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–576. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897–905. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 29.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Fricke O, Tutlewski B, Stabrey A, Lehmkuhl G, Schoenau E. A cybernetic approach to osteoporosis in anorexia nervosa. J Musculoskelet Neuronal Interact. 2005;5:155–161. [PubMed] [Google Scholar]
- 31.Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2:90–96. doi: 10.1007/s11914-004-0016-0. [DOI] [PubMed] [Google Scholar]
- 32.Pike KM, Walsh BT. Ethnicity and eating disorders: implications for incidence and treatment. Psychopharmacol Bull. 1996;32:265–274. [PubMed] [Google Scholar]