Abstract
Mycobacterium tuberculosis causes widespread, persistent infection, often residing in macrophages that neither sterilize the bacilli nor allow them to cause disease. How macrophages restrict growth of pathogens is one of many aspects of human phagocyte biology whose study relies largely on macrophages differentiated from monocytes in vitro. However, such cells fail to recapitulate the phenotype of tissue macrophages in key respects, including that they support early, extensive replication of M. tuberculosis and die in several days. Here we found that human macrophages could survive infection, kill Mycobacterium bovis BCG, and severely limit the replication of M. tuberculosis for several weeks if differentiated in 40% human plasma under 5%–10% (physiologic) oxygen in the presence of GM-CSF and/or TNF-α followed by IFN-γ. Control was lost with fetal bovine serum, 20% oxygen, M-CSF, higher concentrations of cytokines, or premature exposure to IFN-γ. We believe that the new culture method will enable inquiries into the antimicrobial mechanisms of human macrophages.
Introduction
Tuberculosis is a major global health problem. Mycobacterium tuberculosis has humans as its only known natural host and macrophages as its predominant cellular niche. However, the study of M. tuberculosis pathobiology in vitro in its natural host species and cell type is hindered by methodologic shortcomings. If differentiation of primary human macrophages from blood monocytes faithfully recapitulated that in vivo, we would expect the macrophages to impose a prolonged non-replicating state on M. tuberculosis. This might be achieved at the cost of macrophage death and replacement, consistent with experiments in zebrafish with Mycobacterium marinum (1, 2) and in mice with Mycobacterium bovis var. BCG (3, 4) that revealed the granuloma to be a dynamic structure. In settings in vitro in which there is no macrophage replacement, the expected outcome would be prolonged limitation of M. tuberculosis replication followed by death of the macrophages and resumption of M. tuberculosis replication.
The evidence to date is equivocal that human monocyte-derived macrophages (MDMs) can exert prolonged control over M. tuberculosis’s replication in vitro. Investigators often culture the MDMs in aminoglycoside antibiotics before infection with mycobacteria, or even after the infection, in the belief that aminoglycosides only kill extracellular bacteria. However, pinocytosed aminoglycosides reach macrophage phagosomes via pinosome-phagosome fusion and contribute substantially to the cells’ apparent antibacterial activity (5, 6). The antimycobacterial effect of streptomycin can persist for over a week after its removal from macrophage cultures (J. Koo and C. Nathan, unpublished observations). To enumerate intracellular M. tuberculosis, investigators often rinse off extracellular bacteria when replacing media and before harvesting a monolayer. If M. tuberculosis kills the macrophages, wash steps can lead to the same reduction in CFU as if the macrophages killed the M. tuberculosis. Studies in which macrophages treated in a given way are said to “kill” M. tuberculosis sometimes actually report reduced rates of M. tuberculosis’s replication, in that the number of M. tuberculosis CFU at the time studied is greater than the number originally ingested. Many studies report that IFN-γ hastens replication of M. tuberculosis in MDMs (7). In vivo, however, IFN-γ is a non-redundant driver of mycobacterial control. Thus, the known genes whose loss-of-function mutations impart Mendelian susceptibility to mycobacterial disease (MSMD; OMIM 209950) are mostly involved in controlling the production of or response to IFN-γ (8, 9). Anti–IFN-γ autoantibodies can also confer susceptibility to tuberculosis (10), while administration of IFN-γ to patients with tuberculosis speeds bacterial clearance (11). Finally, almost all studies of interaction of M. tuberculosis with macrophages in vitro are confined to periods of a few days. In fact, in some prominent reports, replication or control of replication of M. tuberculosis in human macrophages is described in periods of observation as short as 4 hours, about one-fifth of M. tuberculosis’s replication time in microbiologic media. Latent tuberculosis, in contrast, can last a lifetime. In those who develop active tuberculosis, both progression of disease and chemotherapeutic cure usually proceed over months to years. Thus, it would be surprising if physiologically representative macrophages either allowed extensive replication of M. tuberculosis within a few hours or killed M. tuberculosis within a few hours.
Numerous experimental approaches to the in vitro differentiation of human monocytes have been highly informative for a wide range of questions. With respect to M. tuberculosis infection, however, methodologic limitations of existing protocols appear critical. Therefore, we extensively investigated numerous variables for the culture of human MDMs, using two cardinal criteria for physiologic relevance: the macrophages must survive infection by and control the replication of M. tuberculosis for at least 2 weeks. We achieved that standard by extensively modifying conventional methods, altering the source and amount of plasma proteins, the oxygen tension, and the timing, identity, and concentration of exogenous cytokines.
Results
Overview.
We assessed MDM viability by inspecting photomicrographs of every well in which MDM were cultured in this study. As revealed by counting of nuclei (12), monolayers that became confluent retained nearly all the nuclei added, supported a many-fold increase in the size of cells with single nuclei (13), and allowed sufficient fusion into multinucleated giant cells to reduce the total number of individual cells by a factor of approximately 5, as illustrated in Supplemental Figure 1A (supplemental material available online with this article; doi: 10.1172/JCI57235DS1). In contrast, subconfluence reflected limited cell survival, minimal cell enlargement, and/or cell detachment. CFU values are reported from monolayers that were 95%–100% confluent, with the following exceptions. In instances in which subconfluence was itself an important result, the estimated percent of subconfluence is reported, with “100” or “*” in the figures signifying complete destruction of the monolayer.
We first prioritized the ability of MDMs to kill BCG under biosafety level 2 (BSL2) conditions. Subsequently, we used more limited combinations of variables to optimize the ability of MDMs to restrict the replication of M. tuberculosis under BSL3 conditions. We used no antibiotics at any stage.
We did not wash the cultures at any time after adding cells or bacteria. The number of mycobacteria added was determined in retrospect as described in Methods and converted to a ratio, MOI, calculated as input CFU/input number of monocytes plated. We did not adjust the MOI for the decrease in number of individual MDMs as they fused.
Many of the experiments tested combinations of variables head-to-head and revealed that they interacted in combinatorial fashion. Rather than documenting every combination, subsequent sections summarize basic comparisons without showing data. We then illustrate the impact of selected variables under conditions determined to be otherwise optimal.
In the standard format we ultimately developed, cytokines were tested in two different time windows: from day 0 to 14, which we termed a period of “differentiation,” and from day 14 to the end of the experiment, which we called a period of “activation.” The activation period typically lasted 2 days before infection and then another 3 weeks following infection with BCG or 2 weeks following infection with M. tuberculosis (Supplemental Figure 1B). Although the terms “differentiation” and “activation” are arbitrary, they serve to underscore that cytokines had strikingly different effects when added in the two different periods. Because of the profound impact of O2 tension, we evaluated the role of cytokines in differentiation and activation in two rounds of experiments — first under 20% O2 and then under 5%–10% O2.
Over 3 years we initiated MDM cultures on 100 occasions from a total of 37 donors. MDMs derived on multiple independent occasions from a given donor behaved consistently when tested under the same conditions, but the results could be consistently different from those for MDMs from another donor. The final assay conditions reported are those that appeared optimal for MDMs from 24 donors tested head-to-head.
Cell isolation, plating density, culture vessels, and medium.
As noted above, voluminous data compiled in this series of studies will be summarized but not shown. Each variable stated to have been found optimal was used to obtain the results that are documented in the figures.
We isolated the mononuclear fraction (monocytes and lymphocytes, contaminated with platelets) by standard Ficoll-Paque discontinuous density separation. We plated the washed mononuclear fraction directly or subjected the cells to positive sorting with anti-CD14 antibody coupled to magnetic beads (Supplemental Figure 1B). Adding back the rest of the mononuclear cells was no better than using the CD14-positive cells alone. The donor’s PPD status was immaterial. We compared use of anti-CD14 antibody at the concentration recommended by the manufacturer or at 25% of that concentration, having demonstrated by flow cytometry that use of more dilute antibody isolated a relatively higher proportion of CD14bright cells and a relatively lower proportion of CD14dim cells, with the same total enrichment of CD14-positive cells (93% ± 5%). Results were comparable with both approaches, so we adopted the more economical method.
The CD14-positive, monocyte-enriched fraction was incubated in tissue culture plates from three different manufacturers. Results with plates from TPP were most reproducible. Results were comparable in 24- and 96-well plates. We chose 96-well plates for economy. The optimum plating density for CD14-positive monocytes was 8.5 × 104 cells per well in 200 μl (Supplemental Figure 1B), as in another study (14). It was critical to mitigate evaporation by filling the outer wells with sterile water and rigorously maintaining humidity in the incubators. Optimal results required completing manipulations in room air rapidly.
RPMI-1640 was a more effective base medium than DMEM. This was attributable at least in part to the higher concentration of glucose in the DMEM we tested (4 g/l) than in the RPMI-1640 we tested (2 g/l), because adding glucose to RPMI-1640 to equal the concentration in DMEM led to loss of viability of the MDMs. There was no advantage to MDM viability or control of mycobacterial replication if we supplemented the medium with extra l-arginine, pyruvate or 1,25-dihydroxyvitamin D, as long as we used 40% human plasma.
Effect of human plasma.
MDMs were differentiated for 2 weeks in RPMI containing various percentages of FCS and then infected with BCG. They failed to control bacterial replication. Moreover, MDM survival varied with the lot of FCS. In contrast, MDMs that were differentiated in human plasma in RPMI survived infection by and controlled replication of BCG. The optimal concentration of human plasma was 40%. Effects were equivalent when the plasma was fresh or stored at 4°C for up to 10 weeks and whether the plasma was autologous, heterologous, or pooled. For MDMs from certain donors, heterologous or pooled plasma was superior to autologous plasma.
Effect of incubation time.
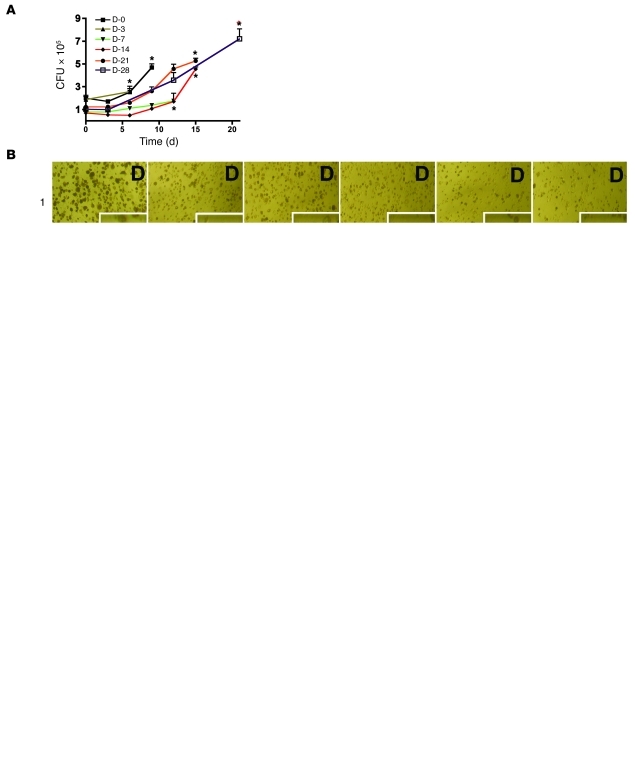
Figure 1A shows results for monocytes or MDMs in RPMI with 40% human plasma without added cytokines in 20% O2 that were infected with BCG after variable periods of differentiation. Monocytes incubated for 0 or 3 days before infection with BCG sustained replication of the bacillus beginning about 3 days later and died by day 6 or 9, respectively. Monocytes cultured for 1 week before infection largely restrained the growth of BCG for the next 12 days, but then died. MDMs allowed to differentiate for 2 weeks prior to infection survived for 12–14 days, restraining BCG’s replication for the first 9 days. Those allowed to differentiate for 3 or 4 weeks survived for a further 2 or 3 weeks after infection, respectively, but permitted more extensive replication of BCG. We chose 2 weeks of differentiation prior to infection as a standard approach and subsequently validated this in experiments with different O2 tensions and cytokines and with M. tuberculosis instead of BCG.
Figure 1. MDM control of BCG.
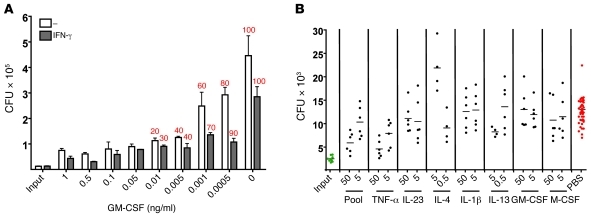
Effect of differentiation time, cytokines, and MOI. (A) Differentiation time. Cells were incubated in RPMI–40% human plasma for periods ranging from 0 to 28 days (D-0 to D-28) before infection with BCG (MOI of 0.3). Cells were lysed on the days following infection (indicated on the x axis.) and CFU determined. Asterisks denote host cell destruction. (B) Effects of IFN-γ during the activation period. MDMs differentiated for 14 days as in A were exposed to indicated concentrations of IFN-γ on day 14 and infected with BCG at the indicated MOI on day 16. Photomicrographs were recorded 21 days after infection in 1 experiment representative of 3, each with different donors. Scale bars: 100 μm; 25 μm, insets. (C) Effect of MOI on CFU. MDMs were differentiated and infected as in B. Cells were lysed for determination of CFU as in A. (D) Effect of cytokines during the differentiation period. MDMs were differentiated and infected as in C, except that where indicated they were exposed to GM-CSF, M-CSF, or GM-CSF plus IL-4 (50 ng/ml each) before infection (MOI of 0.1). Cells were lysed for determination of CFU as in A. Results were similar for 3 donors in B and 2 in C and D. The asterisks in “D” refer to cell destruction. (E) Schematic of cell culture. Monocytes were differentiated for 14 days with or without exogenous cytokines under various tensions of O2, activated beginning on day 14, infected on day 16, and lysed 2–8 weeks later for CFU. Blue stars denote changes of 30% of the medium.
In all experiments 30% of the medium in each well was replaced with fresh medium every 3–4 days. Refreshment was critical, and the optimal interval was 3–4 days. If a cytokine was tested, the replenishment medium included the cytokine for the indicated final concentration. By removing the uppermost 60 μl of the medium, we collected a negligible proportion of the added CFU. Thus, refreshment of the medium in this way did not artifactually impact the assessment of bacterial replication.
Effect of IFN-γ and MOI.
These two variables proved to be interrelated and so are discussed together. Including IFN-γ in the 2-week differentiation period led to death of BCG- or M. tuberculosis–infected MDM (data not shown). Therefore, monocytes were allowed to differentiate for 2 weeks without IFN-γ, exposed to IFN-γ for 2 days, and then infected with BCG, generally for 3 weeks (Supplemental Figure 1B). As shown in Figure 1B, a concentration of IFN-γ as low as 250 ng/ml was deleterious to MDM viability in the absence of BCG. In the presence of BCG, even lower concentrations of IFN-γ were deleterious. BCG was deleterious as well, and its toxicity increased in proportion to the MOI. Strikingly, however, very low concentrations of IFN-γ protected MDMs from the toxicity of BCG. The optimal concentration of IFN-γ was donor dependent. For the majority of donors, the optimal concentration of Actimmune (a clinically used IFN-γ preparation) ranged from 0.5 to 5 ng/ml, well below the concentration used in most studies.
Next we investigated the impact of MOI on MDM control of BCG. After 2 weeks of differentiation, MDMs were treated or not with 3 ng/ml IFN-γ, and wells were harvested at intervals over the next 4–6 weeks. With MOI in the range of 0.01–0.3, MDMs remained fully confluent. However, the curve relating MOI to the ability of the MDMs to restrain replication of BCG was bell-shaped, just as was the concentration-response effect of IFN-γ on MDM viability (Figure 1C and Supplemental Figure 1C). When the MOI was extremely low (0.03 or 0.01), BCG replicated extensively. When the MOI was 0.3 or 0.1, there was a less than 1 log10 increase in CFU after 3–5 weeks. Higher MOI led to earlier and more extensive death of the MDMs. We chose an MOI of 0.2 for most experiments.
In sum, the protective effect of IFN-γ, often considered the principal macrophage-activating factor (15), was strongly dependent on the time of its addition, its concentration, and the magnitude of the ensuing microbial challenge.
Effect of differentiation in M-CSF, GM-CSF, or GM-CSF plus IL-4.
MDMs were differentiated for 2 weeks in the presence or absence of 50 ng/ml of M-CSF, GM-CSF, or GM-CSF plus IL-4. The latter combination is often used to differentiate monocytes to dendritic cells. The MDMs were then “activated” or not with 3 ng/ml IFN-γ. Two days later they were infected with BCG at MOI 0.1 (Figure 1D) or 0.3 (Supplemental Figure 1D). Results at MOI of 0.1 and 0.3 were identical. M-CSF and GM-CSF had no effect on MDM viability or control of BCG replication, provided that the MDMs were differentiated in human plasma, which we confirmed contained M-CSF (data not shown). However, GM-CSF, but not M-CSF, could overcome the deleterious effect of differentiation in FBS. MDMs differentiated in the combination of GM-CSF plus IL-4 supported extensive replication of mycobacteria and died (Figure 1D). Paradoxically, in the cultures with dying MDMs, the number of BCG CFU appeared to decline drastically. This was an artifact due to the release of the BCG into the medium, where, after 3 days of bacteriostasis, and in contrast to M. tuberculosis, they died (data not shown). The deleterious effect of combined GM-CSF plus IL-4 was particularly conspicuous in human plasma–supplemented cultures.
From this point on we used the following framework for culture of MDMs: 2 weeks of differentiation in RPMI containing 40% human plasma, with replacement of 30% of the medium every 3–4 days, followed by activation of the cells 2 days before infection (Figure 1E).
In summary, a combination of the two additives most commonly used for culture of human monocytes — FBS and M-CSF — led to MDMs that were unable to control the replication of BCG and thus died. However, it would be easy to reach the opposite conclusion — that the MDM decreased BCG viability — when MDMs died and released BCG into the medium.
Impact of diverse cytokines on activation of MDMs to control BCG.
MDMs were cultured for 2 weeks in 20% O2 with 40% human plasma and then activated or not with IFN-γ alone at 3 ng/ml; with a panel of cytokines each tested alone at 0.5, 5, or 50 ng/ml or at those concentrations along with 3 ng/ml IFN-γ; or with all the cytokines except IL-4 and IL-13 pooled at 50 ng/ml each, both with and without 3 ng/ml IFN-γ. The panel included IL-1β, IL-4, IL-8, IL-12, IL-13, IL-15, IL-18, IL-22, IL-23, TGF-β, TNF-α, monocyte chemotactic protein–1 (MCP-1), M-CSF, GM-CSF, and VEGF. Tests with M-CSF and GM-CSF differed from those discussed earlier, in that the cytokines were now added during the activation period, rather than during the differentiation period.
Figure 2A summarizes results from 5 independent experiments comparing IFN-γ alone with vehicle control (PBS). Without IFN-γ, BCG replicated an average of 4-fold over 21 days in fully viable MDMs. Activation with IFN-γ restricted BCG’s replication to 2-fold. By comparison, unrestrained replication over the 3-week period would have led to approximately 4 × 106–fold expansion of BCG.
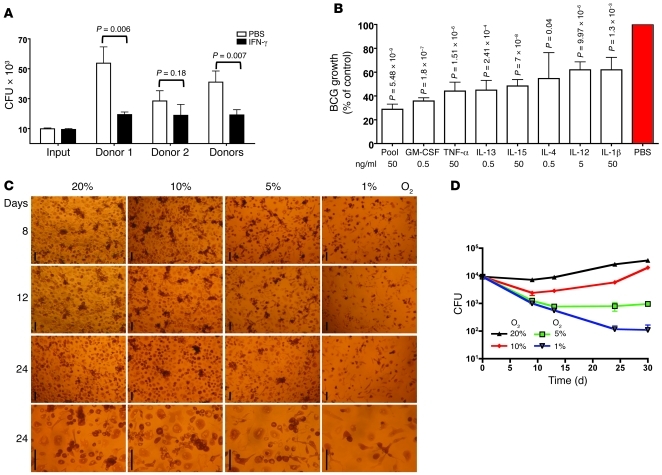
Figure 2. Interactive impact of O2 tension on MDM control of BCG.
(A and B) Bacteriostatic effect of MDMs cultured in 20% O2. MDMs differentiated with RPMI–40% human plasma (no added cytokines) for 14 days were treated or not on day 14 with (A) IFN-γ or (B) GM-CSF, TNF-α, IL-13, IL-15, IL-4, IL-12, or IL-1β; infected with BCG on day 16; and lysed 3 weeks later for determination of CFU. Results are representative of 5 experiments from 2 donors. In A, “Donors” refers to pooled results for both donors. In B, percentage of control of BCG growth is relative to MDMs receiving PBS instead of cytokines. (C) Morphology of MDMs after incubation in various concentrations of O2. Monocytes were incubated without exogenous cytokines for 14 days in the indicated concentrations of O2, then treated with or without IFN-γ (5 ng/ml) and infected with BCG (MOI of 0.1) on day 16. Pictures were taken 8, 12, and 24 days after infection. Scale bars: 100 μm. (D) Control of BCG as a function of O2 concentration. Experiments as in A comparing O2 concentrations of 20%, 10%, 5%, or 1%. CFU were determined at intervals over 30 days following infection. Results were essentially the same without (shown) or with added IFN-γ (5 ng/ml; data not shown). Results in D are representative of 7 independent experiments with cells from 2 donors.
Tested individually in the same 5 experiments, 7 of the 15 other cytokines also helped fully confluent cultures of MDMs restrict BCG’s replication, although none as well as IFN-γ (Figure 2B). Listed in decreasing order of effectiveness along with the concentration that proved optimal, these were GM-CSF (0.5 ng/ml), TNF-α (50 ng/ml), IL-13 (0.5 ng/ml), IL-15 (50 ng/ml), IL-4 (0.5 ng/ml), IL-12 (5 ng/ml), and IL-1β (50 ng/ml). Concentrations of IL-4 or IL-13 greater than 0.5 ng/ml led to destruction of the BCG-infected MDMs (Supplemental Figure 2, A and B). The other 8 cytokines tested singly led to bacterial counts similar to or higher than those in MDMs given no cytokines (Supplemental Figure 2, A and B). The combination of cytokines at 50 ng/ml gave results comparable to those using GM-CSF alone at 0.5 ng/ml (Figure 2B). The impact of IFN-γ was minimally affected by the addition of any of the other cytokines tested. With the cytokines that were weakly effective on their own, there were additive or slightly sub-additive effects, but no synergy, in combinations with IFN-γ (Supplemental Figure 2, C and D).
In summary, IFN-γ, GM-CSF, and TNF-α were the most effective cytokines of those tested during the activation period for enabling MDMs to survive infection by and control replication of BCG over a 3-week period.
Impact of O2 tension.
Nearly 20 years ago, Meylan et al. reported that human MDMs spread better, displayed a higher respiratory burst, and better controlled the replication of M. tuberculosis (which only increased in CFU by a factor of 0.39 log10 in 7 days) when the MDMs were cultured at physiologic tissue levels of O2 (5% O2; pO2 36 mmHg) than at ambient O2 (20%; pO2 140 mmHg), where the M. tuberculosis replicated by a factor of 1.17 log10 (16). In macrophage-free medium, the M. tuberculosis replicated equally well at both levels of O2 (16). Thus, as with lymphocytes (17) and fibroblasts (18), physiologic O2 levels can improve the functional performance of macrophages. Accordingly, we differentiated MDMs for 2 weeks and infected them with BCG for 8–24 days using 5% CO2 along with O2 at 20%, 10%, 5%, or 1% throughout. MDMs were fully confluent through the 3.5-week period of infection when the O2 was 20% or 10%. At 5% O2 the MDMs were sometimes slightly subconfluent. At 1% O2, the MDMs were smaller and markedly subconfluent (Figure 2C). Strikingly, while BCG replicated by 1 log10 over 3.5 weeks in MDMs with 20% O2, there was 0.5 log10 killing (that is, approximately 3.3-fold reduction in CFU below the number initially added) under 10% O2, 1 log10 killing at 5% O2, and 2 log10 killing at 1% O2 (Figure 2D). We chose 10% O2 for most subsequent experiments.
Next, by using a glovebox, we tested the impact of strictly and continuously maintaining 10% O2 at all times, including when adding IFN-γ, inoculating the cultures with BCG, and refreshing the medium every 3–4 days, compared with a less cumbersome approach in which we removed the cultures from low-O2 boxes long enough to perform these operations rapidly in room air. Brief exposure to room air made no difference (data not shown). Thereafter, we used the discontinuous method of incubation in low O2.
In summary, physiologic tissue levels of O2 enabled MDMs to kill BCG, while cells otherwise cultured identically at 20% O2 supported BCG’s extensive replication.
Revisiting the activation of MDMs for killing BCG.
Having selected 10% O2, 5% CO2 as the gas phase, we revisited the issue of activation of MDMs following a 2-week differentiation in RPMI with 40% autologous human plasma. During the next 2 days, the MDMs were exposed to IFN-γ alone, a panel of cytokines tested individually, or both and then infected with BCG at an MOI of 0.1. The cytokine exposure was continued throughout the next 3 weeks. The cytokine panel included the 15 listed earlier plus 10 more: IL-6, IL-10, IL-17, IL-17F, IL-32, CD40L, CX3CL1, WNT3a, leptin, and TNFSF11, each tested at 5 or 50 ng/ml, as well as a pool of all except IL-4 and IL-13, comprising 27 cytokines, at 50 ng/ml each. Figure 3A summarizes results from 5 independent experiments comparing IFN-γ alone with vehicle control (PBS). Without IFN-γ, BCG underwent an average of 0.3 replications over 21 days in fully viable MDMs. Activation with IFN-γ restricted BCG’s replication to the initial input or led to 2-fold killing. Tested individually in the same 5 experiments, 6 of the 28 cytokines helped fully confluent cultures of MDMs to kill BCG under 10% O2 (Figure 3B) (the optimal concentration is given in parentheses): IFN-γ (3 ng/ml), GM-CSF (50 ng/ml), TNF-α (50 ng/ml), IL-13 (5 ng/ml), and IL-4 (0.5 ng/ml). However, in some of these experiments, IL-4 or IL-13 led to partial destruction of the monolayers. Tested singly, the 22 other cytokines led to bacterial counts similar to or higher than in MDMs given no cytokines (Supplemental Figure 3, A and B).
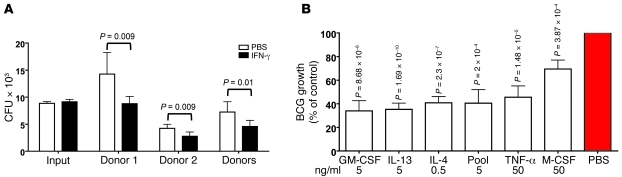
Figure 3. Effect of cytokines on MDM killing of BCG in 10% O2.
(A and B) MDMs differentiated in 10% O2 without added cytokines for 14 days were treated or not with IFN-γ (5 ng/ml) or other cytokines on day 14 and infected with BCG (MOI of 0.1) on day 16. CFU were measured 21 days after infection. (A) Number of CFU for each donor, pooled results, and input on day 0. (B) Percentage of control of growth compared with cells treated with PBS instead of cytokines. Experiments in A and B are representative of 7 independent experiments with cells from 2 donors.
In summary, 6 of 28 cytokines tested during the activation phase could enhance the ability of MDMs to kill BCG, but most of the MDMs’ anti-BCG activity was imparted by the use of physiologic tissue levels of O2. The effective cytokines included several associated with both classically and alternatively activated macrophages in the context of mouse immunology.
Turning to M. tuberculosis and revisiting activation.
Next, we set BCG aside and focused on the ability of MDMs to survive M. tuberculosis infection and restrict the replication of M. tuberculosis. We used MDMs “differentiated” for 2 weeks in RPMI with 40% human plasma under 10% O2 and 5% CO2. With most donors’ MDMs, differentiation for 2 weeks without added cytokines led to death of the MDMs during a subsequent 3-week infection with M. tuberculosis. Thus, to improve the efficiency of testing and to allow the comparison of differentiation with and without added cytokines that is presented in subsequent sections, we limited the infection period to 2 weeks. Figure 4A presents results from a donor whose MDMs responded to added IFN-γ during the activation phase. Figure 4B shows results from a donor whose cells acted as if they had already been exposed to IFN-γ and did not respond to the added cytokine. Such cultures may have produced their own IFN-γ (19), but this was not tested. In all cases, we observed a concentration-dependent response to GM-CSF, TNF-α, IL-12, and IL-15 (Figure 4, A and B). In contrast to BCG, we did not observe net killing of M. tuberculosis. Nevertheless, under 10% O2, MDMs survived a 2-week infection with M. tuberculosis and markedly limited the pathogen’s replication.
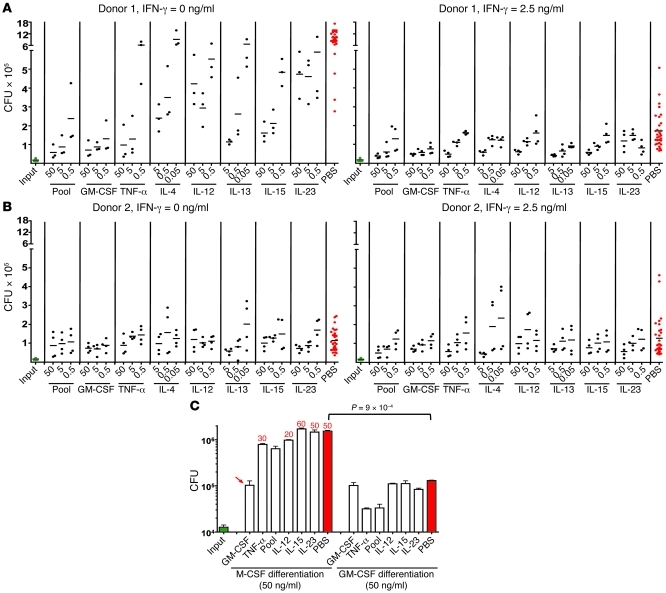
Figure 4. MDM restriction of M. tuberculosis replication in 10% O2: impact of cytokines in the activation and differentiation phases.
(A and B) Role of cytokines in the activation phase. MDMs from 2 donors were each tested twice in independent experiments, one of which is illustrated. MDMs were differentiated for 14 days in 10% O2, treated (right panels) or not (left panels) with IFN-γ (2.5 ng/ml) alone or with the other cytokines indicated, infected on day 16 with M. tuberculosis (MOI of 0.1), and lysed 2 weeks later for determination of CFU. (C) Comparison of GM-CSF and M-CSF in the differentiation phase. MDMs were differentiated as in A, but with M-CSF or GM-CSF (50 ng/ml each) during differentiation. Numbers above bars indicate estimated percentage destruction of the monolayers as recorded in photomicrographs on the day the CFU were determined. Absence of numbers indicates 100% confluent monolayers. Arrow indicates that the adverse effect of M-CSF during the first 14 days was overcome by inclusion of GM-CSF in the next 16 days. Results are from 1 experiment representative of 5 performed.
Infecting with M. tuberculosis after revisiting differentiation: contrasting effects of GM-CSF and M-CSF.
Results from MDMs differentiated in RPMI with 40% human plasma without exogenous cytokines are shown in Figure 4, A and B. As judged by limited cell enlargement, MDMs from 2 of 24 donors differentiated poorly without the aid of exogenous cytokines and died during the subsequent M. tuberculosis infection. We could prevent this by differentiating all 24 donors’ MDMs in either GM-CSF or TNF-α. Results with GM-CSF are described here and those with TNF-α in the next section.
First, we directly compared differentiation in GM-CSF and M-CSF, because M-CSF is widely used to differentiate mouse macrophages from bone marrow precursors and human MDMs from monocytes. The conditions were now substantially different from those in which we earlier made the comparison: culture in 10% O2 rather than 20% O2; activation with IFN-γ, GM-CSF, TNF-α, IL-12, IL-15, IL-23, or a pool of all 5; and infection with M. tuberculosis (MOI of 0.2) rather than BCG. As shown in Figure 4C and Supplemental Figure 4A, 2-week differentiation in the presence of M-CSF (50 ng/ml) led to subsequent destruction of the MDM monolayers in association with extensive proliferation of M. tuberculosis, no matter with what cytokines the MDMs were activated for 2 days before and then during the 2-week infection. There was one exception: GM-CSF activation at day 14 was able to revert the poor results seen with differentiation in M-CSF. Otherwise, MDMs differentiated with M-CSF allowed M. tuberculosis to replicate by a factor of approximately 100-fold by the time the monolayers were reduced to about 50% confluence.
In contrast, 2-week differentiation in the presence of GM-CSF (50 ng/ml) led to fully confluent monolayers throughout 2 weeks of M. tuberculosis infection. Replication of M. tuberculosis was limited to about 8-fold without IFN-γ and about 4-fold with IFN-γ (Figure 4C and Supplemental Figure 3A). The most effective cytokine during the activation phase was TNF-α. As shown in Figure 5A, which is representative of 5 independent experiments, as little as 0.5 pg/ml of GM-CSF in the 2-week differentiation period allowed morphologic differentiation of MDMs, but MDMs were only protected from destruction by M. tuberculosis over a subsequent 2-week period when the GM-CSF was used at concentrations of at least 50 pg/ml. From multiple experiments with different donors, we chose 0.5 ng/ml as the standard concentration for GM-CSF during the differentiation phase (Supplemental Figure 4B).
Figure 5. Impact of differentiation in GM-CSF and activation by various cytokines on MDM restriction of M. tuberculosis in 10% O2.
(A) Impact of GM-CSF concentration during differentiation. MDMs were differentiated for 14 days in 10% O2 with the indicated concentrations of GM-CSF. The first bar in each pair shows results for MDMs that were subsequently activated with IFN-γ (2.5 ng/ml) on day 14. The second bar pertains to cells not given IFN-γ. MDMs were infected on day 16, with the number of M. tuberculosis shown as “Input” (MOI of 0.1), and lysed 2 weeks later for determination of CFU. Numbers above bars indicate percentage destruction of monolayers as estimated from photomicrographs on the day of CFU collection. This experiment is representative of 5 performed. (B) Control of M. tuberculosis by MDMs differentiated in GM-CSF and activated with IFN-γ plus TNF-α after washing out uningested bacteria. Experiments were performed as in A, except that GM-CSF during the first 14 days was used at 0.5 ng/ml and the cells were then activated with IFN-γ (2.5 ng/ml) plus additional cytokines at concentrations shown in ng/ml. Four hours after infection with M. tuberculosis at the numbers shown as “input” (MOI of 0.1), extracellular mycobacteria were washed off to test the effect of a step typically included in macrophage infection experiments. Cells were lysed 2 weeks later to determine CFU. Results are representative of those from 3 donors, each analyzed in 2 independent experiments.
We validated these finding with MDMs from 3 donors in each of 2 experiments by performing differentiation with 0.5 ng/ml GM-CSF (Figure 5B and Supplemental Figure 3C). Their MDMs allowed only 1–3 divisions of M. tuberculosis over 2 weeks when activated with TNF-α, rather than the approximately 17 divisions that might be expected over that period given M. tuberculosis’s replication time of approximately 20 hours. In contrast, as little as 5 ng/ml IL-4 led to loss of control of the growth of M. tuberculosis. No further control was achieved by supplementing the viable M. tuberculosis with varied numbers of paraformaldehyde-fixed M. tuberculosis CFU (Supplemental Figure 3D) or by adding fresh autologous lymphocytes enriched for expression of CD2, CD3, CD4, or CD8 or remaining after depletion of CD14+ cells from the mononuclear fraction, 2 days prior to infection with M. tuberculosis (Supplemental Figure 3, E and F).
In summary, M-CSF, the cytokine most widely used for macrophage differentiation in vitro, was highly counterproductive for MDM control of virulent M. tuberculosis, just as seen when using 20% O2 and infecting the MDMs with BCG. In contrast, GM-CSF was a highly potent differentiation factor.
TNF-α can substitute for GM-CSF in the differentiation of MDMs that restrict replication of M. tuberculosis.
Next, we tested the “differentiating” effect of the other 27 cytokines in the panel that was tested earlier in the activation phase. Monocytes differentiated with IFN-γ (50 ng/ml) attained the morphologic hallmarks of differentiation for the first few days, but died over the following week. Several other cytokines supported morphologic differentiation (enlargement) of MDMs, namely IL-32 (50 ng/ml) (20), IL-15 (5–50 ng/ml) (21), and M-CSF (5–50 ng/ml), but the resulting MDMs were unable to control the growth of M. tuberculosis. Only TNF-α (optimal concentration, 0.5 ng/ml) provided similar results to those obtained with GM-CSF (data not shown).
To identify the best combination and concentration of the two most effective cytokines during the “differentiation” process, MDMs were differentiated with no exogenous cytokines or with TNF-α (0.5 or 5 ng/ml), GM-CSF (0.5 ng/ml), or GM-CSF (0.5 ng/ml) plus TNF-α (0.5 or 5 ng/ml) for 14 days under 10% O2 (Figure 6A and Supplemental Figure 4A) or 5% O2 (Figure 6B and Supplemental Figure 4B). On day 14, MDMs were activated with IFN-γ (2.5 ng/ml) or with 8 other cytokines. The MDMs were infected with M. tuberculosis on day 16, and CFU were determined 2 weeks later. Photomicrographs taken at the end of the experiment are shown in Figure 6C, Supplemental Figure 4C, and Supplemental Figure 4D. Under 10% O2, MDMs survived M. tuberculosis infection and limited M. tuberculosis replication to 1–2 divisions over 2 weeks, provided that the MDMs were differentiated in GM-CSF plus TNF-α and activated with IFN-γ or TNF-α. Under 5% O2, such cells restricted M. tuberculosis replication completely. For some donors, results were similar using only GM-CSF or only TNF-α for the differentiation phase, but MDMs from other donors required the combination (Supplemental Figure 4, A and B). Cultures in which MDMs were differentiated in only one of the cytokines that then failed to control M. tuberculosis replication confirmed the observation of Meylan et al. (16) that 5%–10% O2 in the gas phase did not impede M. tuberculosis’s replication (Supplemental Figure 4B).
Figure 6. Comparison of 10% and 5% O2, and use of GM-CSF and TNF-α in combination during differentiation for control and killing of M. tuberculosis by MDMs activated with various cytokines.
(A) CFU for cultures incubated in 10% O2. MDMs were differentiated for 14 days with no exogenous cytokines (left panel) or with GM-CSF plus TNF-α (0.5 ng/ml each) (right panel) under 10% O2. Cells were activated with the indicated cytokines (50 ng/ml each) on day 14, infected with M. tuberculosis (MOI of 0.1) on day 16, and lysed 2 weeks later for CFU. (B) CFU for cultures incubated in 5% O2. Experiments were performed as in A, except that the O2 concentration was further reduced. (C) Morphology of MDMs. Cells were prepared as in A and B, except that MDMs were differentiated with GM-CSF plus TNF-α (0.5 ng/ml each) for the first 14 days and then activated with TNF-α (50 ng/ml). Micrographs were taken 14 days after infection. Scale bars: 100 μm; 50 μm (insets). (D) MDM markers. MDMs were differentiated for 14 days with GM-CSF plus TNF-α (0.5 ng/ml each) (left panel) or M-CSF (50 ng/ml) (right panel) under 5% O2, then analyzed by flow cytometry. Percentages indicate the proportion of positive cells relative to the isotype control. Asterisk denotes a distinct subpopulation that was positive for CD40, although the median fluorescence of the whole population was close to that of the isotype control. Results are means for MDMs from 2 different donors in 1 of 3 independent experiments, each with 2 donors.
In summary, under 5%–10% O2, MDMs differentiated with very low concentrations of GM-CSF and TNF-α and then activated with very low concentrations of either IFN-γ or TNF-α could extensively restrict the replication of M. tuberculosis over a 2-week period without undergoing microscopically detectable cell death and without ever having been exposed to antibiotics.
Surface marker profiles of MDMs differentiated in different ways.
Next, we compared the cell surface marker profile of MDMs differentiated for 2 weeks using either the new regimen, GM-CSF (0.5 ng/ml) plus TNF-α (0.5 ng/ml), or a more conventional regimen, M-CSF (50 ng/ml). In two independent experiments with cells from different donors, flow cytometric analysis revealed no contamination by CD19+ B cells, CD3+ T cells, or CD56+ NK cells in either case. Of the 29 markers tested, the 20 that were positive revealed only two minor, qualitative differences for MDMs prepared by the different regimens (Figure 4D and Supplemental Figure 4E): small sets of CD62L+ (L-selectin) and CD40+ MDMs were detected after culture with GM-CSF plus TNF-α but not after culture with M-CSF. Moreover, at the quantitative level, there was only one substantive difference in marker expression: MDMs differentiated with M-CSF expressed higher levels of CD14 than those differentiated with GM-CSF plus TNF-α. In sum, surface marker profiling provided little clue to the striking functional distinctions between MDMs differentiated in these two ways.
Impact of microbiologic culture conditions on the susceptibility of M. tuberculosis to control by MDMs.
Having emphasized the use of physiologic O2 levels for culture of MDMs, we considered what impact physiologic conditions of O2 and pH might have on the M. tuberculosis with which the MDMs were infected. To explore this, we cultured M. tuberculosis for 1 week in 7H9 microbiologic medium at the customary pH of 6.8 under 20% O2 or in 7H9 at pH 5.5 (closer to the pH of the phagolysosome of activated macrophages) under 1% O2 before infecting MDMs. In two experiments with cells from different donors, control of M. tuberculosis replication was indistinguishable with either method of pre-culture (Supplemental Figure 5G).
Reexamination of the effect of exogenous vitamin D.
Under culture conditions we would now consider suboptimal, vitamin D has promoted the ability of human MDMs to control M. tuberculosis (22, 23). As noted earlier, we found no effect of adding 1,25-dihydroxyvitamin D to culture medium that contained 40% plasma. Having refined the conditions of differentiation and activation, we reexamined this question. MDMs from 2 different donors were differentiated for 2 weeks under 5% O2 with GM-CSF plus TNF-α in RPMI with 40% plasma and then activated or not for 2 days with 1,25-dihydroxyvitamin D (10 nM or 100 nM) before infection with M. tuberculosis or BCG (Supplemental Figure 5, I and J). Alternatively, MDMs were differentiated in 10% FCS for 14 days under 5% O2 with GM-CSF plus TNF-α (0.5 ng/ml each) with or without 1,25-dihydroxyvitamin D (50 nM or 100 nM), activated with IFN-γ on day 14, and infected with M. tuberculosis (MOI of 0.2) on day 16 (Supplemental Figure 5H). In both sets of experiments, MDMs were lysed 0, 3, and 7 days later, because cultures prepared in FBS did not survive infection longer. Addition of vitamin D did not affect the CFU (Supplemental Figure 5, H–J). In sum, it is likely that differentiation in 40% human plasma provided sufficient vitamin D, along with other factors.
Reproducibility of in vitro differentiation and activation of MDMs to restrict the replication of M. tuberculosis.
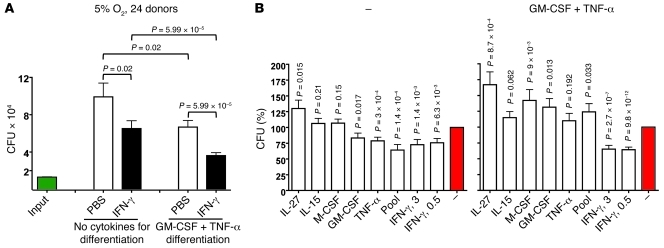
Throughout these studies we paid close attention to donor-to-donor variability. This led to a protocol designed to give comparable results with MDMs from as many donors as possible. To test to what degree this had been achieved, we applied two versions of the final conditions to cells from 24 donors: 10 men and 14 women of mixed European, Chinese, or African American descent (Figure 7, A and B). The experiments were conducted under 5% O2 for MDMs from all 24 donors and at 7.5% O2 for 16 of them (Supplemental Figure 5, A and B). Results under 5% O2 are pooled in Figure 7, A and B, comparing MDMs differentiated with no cytokines or with GM-CSF and TNF-α (0.5 ng/ml each) and activated or not with IFN-γ (2.5 ng/ml). Under 5% O2, MDMs from all donors tested that were differentiated with both GM-CSF and TNF-α and activated with IFN-γ survived infection as confluent monolayers and restricted the average replication of M. tuberculosis to 1–2 divisions (mean, 1.33 divisions) over 2 weeks (Figure 7A and Supplemental Figure 5C). Under 7.5% O2, the average extent of replication of M. tuberculosis was 2.5 divisions over 2 weeks (Supplemental Figure 5, A and B). After MDMs were differentiated with GM-CSF and TNF-α, activation by IFN-γ restricted the replication of M. tuberculosis with a high level of statistical significance (P = 6 × 10–5 in Figure 7A; P = 1 × 10–11 in Figure 7B). As alternative activating agents, both GM-CSF and TNF-α also helped confluent cultures of MDMs restrict replication of M. tuberculosis, but not as well as IFN-γ.
Figure 7. Reproducibility of control of M. tuberculosis replication by MDMs differentiated with GM-CSF and TNF-α in combination and activated with various cytokines in 5% O2.
(A) Reproducible control of M. tuberculosis with appropriately differentiated MDMs that were activated with IFN-γ at a physiologic tissue O2 tension. MDMs from 24 donors were differentiated in 5% O2 with no cytokines or with GM-CSF plus TNF-α (0.5 ng/ml each) for 14 days, exposed or not to IFN-γ (3 ng/ml), infected with M. tuberculosis on day 16 (MOI of 0.17), and lysed 2 weeks later for determination of CFU. Results from all donors were pooled to calculate averages. (B) Comparison of IFN-γ and other cytokines during the activation period. In the same experiments illustrated in A, MDMs were also activated with the indicated cytokines (each at 50 ng/ml, except for IFN-γ as indicated at 3 or 0.5 ng/ml). CFU counts from MDMs lysed 2 weeks infection are presented as percentage of those for the same donor’s MDMs given PBS instead of exogenous cytokines during the activation period.
Discussion
This study demonstrates that human monocytes can be differentiated in vitro into macrophages that reproducibly survive infection by and markedly slow the replication of M. tuberculosis over a 2-week period, without exposure to antibiotics at any time and without wash steps that risk conflating macrophage death or detachment with microbiologic control. The same MDMs kill BCG. To our knowledge, this degree of control of mycobacteria by human MDMs has not previously been reported.
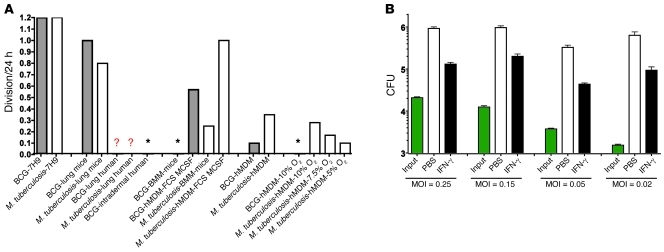
Figure 8A puts these results into perspective with reference to the approximate number of divisions that M. tuberculosis and BCG undergo in a 24-hour period. (In the following discussion, we use the terms “divisions” and “replications” to refer to the number of doublings it would require to produce the observed increase in CFU if there were no bacterial death. In reality, there may be a greater number of doublings that compensates for an unmeasured amount of bacterial death.) In media selected to support maximal rates of replication, these mycobacteria divide about once per 20 hours. In a recent study in which MDMs were differentiated with standard methods that included use of FBS and M-CSF, BCG replicated in the MDMs approximately 0.6 times per 24 hours (24). Our data confirm that result for those methods. In contrast, using our revised approach, MDMs allowed no net replication and instead reduced the number of viable BCG by about 90% over 3 weeks. The latter results are closer to those reported for BCG in activated mouse bone marrow–derived macrophages (moBMMs) over 5 days (24). Turning to M. tuberculosis, moBMMs that had not been exposed to antibiotics typically restricted mycobacterial replication to approximately 0.25 divisions per 24 hours, but then died around day 6 (A. Venugopal, P. Rath, and C. Nathan, unpublished observations). In contrast, human MDMs prepared as described here limited the growth of M. tuberculosis to approximately 0.1 division per 24 hours and survived longer than 2 weeks (Figure 8A). Considering both the extent and duration of control, this is to our knowledge the closest that human MDMs have come in vitro to mimicking the control of M. tuberculosis by macrophages in the immunocompetent human host.
Figure 8. Approximate net number of mycobacterial divisions in mice, in humans, and in their macrophages.
(A) Approximate net mycobacterial cell divisions per 24 hours for BCG (gray bars) or M. tuberculosis (white bars) in diverse settings. hMDM, human MDMs prepared under the most effective conditions illustrated in A; hMDM-MCSF-FBS, conventionally cultured human hMDM, hMDMs after about 1 week in 10% FCS with M-CSF in 20% O2; lung mice, logarithmic growth phase in the lungs of immunocompetent mice over the first 3 weeks following low-dose M. tuberculosis aerosol infection. Lung human, lungs in humans. Lung mice, lungs in mice. Asterisks, no net increase; mycobacteria are killed. Question mark indicates that no data are available, but it is expected that after a brief period of replication, there is no further net increase in more than 90% of immunocompetent people for M. tuberculosis and 100% for BCG. Figures for mycobacteria in host cells in vitro are based on periods of 3 weeks for BCG in hMDMs, 2 weeks for M. tuberculosis in hMDMs, and 1 week for BCG and M. tuberculosis in moBMMs. (B) Effect of MOI on the number of net divisions of M. tuberculosis in MDMs. MDMs were differentiated without exogenous cytokines in 10% O2 for 14 days, stimulated with IFN-γ (3 ng/ml) or not on day 14, infected with M. tuberculosis at the indicated MOI on day 16, and lysed 2 weeks later for determination of CFU (log10 scale). Results are representative of those with MDMs from 2 donors tested in 4 independent experiments.
The critical importance of physiologic tissue levels of O2 documented here confirms the report of Meylan et al. (16). Thus, as with lymphocytes (25) and fibroblasts (18), physiologic O2 levels can markedly improve the functional performance of macrophages. However, physiologic tissue levels of O2 alone were not sufficient for MDMs from some donors to restrain the replication of M. tuberculosis; these donors’ MDMs also required low levels of certain cytokines (Supplemental Figure 5, D and E).
It was striking that ultra-low inocula (MOI of 0.02) allowed M. tuberculosis to replicate faster than higher inocula until the M. tuberculosis attained a similar final intracellular burden (Figure 8B). Perhaps a similar phenomenon is involved during natural infection, where the pulmonary alveolar macrophages are thought to ingest low numbers of inhaled M. tuberculosis. If so, this might help M. tuberculosis establish a persistent infection before there has been time for the host to mount an adaptive immune response. The mechanism of this low-inoculum effect is unknown.
Several agents routinely used to differentiate and activate human macrophages in vitro proved detrimental altogether, or at conventional concentrations, or when applied at certain times, by the criteria of MDM resistance to mycobacteria and control of mycobacterial replication. For example, human MDMs derived in FBS were markedly inferior to those derived in human plasma with respect to these criteria. Such cells appear to be useful models of human MDM physiology in other assays. Similarly, M-CSF allowed for excellent morphologic differentiation of MDMs, but mycobacteria rapidly destroyed them. IFN-γ, a critical cytokine for MDM activation, was detrimental when applied during the period of differentiation. Moreover, excessive concentrations of IFN-γ hastened replication of M. tuberculosis in MDMs, even though lower concentrations had the opposite effect. The critical dependence of IFN-γ’s effects on timing and concentration may help explain seemingly discrepant findings in some earlier studies (7). More important, the contrasting effects of IFN-γ at different concentrations and times may help explain why IFN-γ is necessary for control of M. tuberculosis and is produced by T cells from immunocompetent people with latent M. tuberculosis infection that are challenged with antigens expressed by M. tuberculosis, and yet such people do not routinely eradicate latent M. tuberculosis infection.
Of the 28 cytokines tested, GM-CSF and/or TNF-α afforded the most successful differentiation, whereas IFN-γ and TNF-α afforded the best activation. IFN-γ and TNF-α are the two cytokines whose critical roles in the control of human tuberculosis are best documented by genetic deficiency states (9), acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ (10), administration of recombinant IFN-γ to treat multidrug-resistant tuberculosis (26), or administration of therapeutic agents that neutralize TNF-α in the treatment of inflammatory diseases (27).
There is still some way to go to achieve the goal of recapitulating the differentiation of human tissue macrophages in vitro. One shortcoming of the present method was that the MDMs did not express iNOS (A. Cunningham-Bussel, G. Vogt, and C. Nathan, unpublished observations). In contrast, macrophages in humans with inflammatory and infectious disorders, including tuberculosis, often express iNOS (28–31). Nonetheless, the methods presented here should prove useful in determining how MDMs control replication of M. tuberculosis and how M. tuberculosis eventually escapes control, as well as in studies of other aspects of the physiology of human macrophages.
Methods
Preparation of monocytes and MDMs by the optimized method.
Heparinized blood was collected by venipuncture from healthy donors who provided informed consent under an IRB-approved protocol. Blood was mixed with an equal volume of RPMI-1640 (GIBCO, Invitrogen) lacking phenol red and supplemented with 1 mM GlutaMAX (Invitrogen) and centrifuged above Ficoll-Paque (GE Healthcare) for 20 minutes at 500 g at 20°C. The diluted plasma above the PBMC layer was collected and centrifuged for 30 minutes at 2,000 g at 20°C. The supernatant was centrifuged again for 60 minutes at 2,000 g at 20°C. The final supernatant was stored at 4°C and used to prepare fresh medium throughout the experiment.
Monocytes were isolated from the mononuclear cell layer in the Ficoll-Paque centrifugation by positive immunomagnetic selection with antibody against CD14 (MACS; Miltenyi Biotec) according to the manufacturer’s instructions, with two exceptions. BSA was replaced by 7% autologous plasma-RPMI for purification because some lots of BSA proved toxic. Moreover, we used the anti-CD14 antibody at 25% of the concentration recommended by the manufacturer.
RPMI-1640 contained glucose at 2 g/l. The culture medium also contained human plasma at an optimal concentration of 40%. Effects were equivalent when the plasma was fresh or stored at 4°C for up to 10 weeks and whether the plasma was autologous, heterologous, or pooled. For MDMs from certain donors, heterologous or pooled plasma was superior to autologous plasma.
Every 3–4 days 30% of the medium in each well was replaced with fresh medium including cytokines. Refreshment was critical, and the optimal interval was 3–4 days.
The CD14-positive, monocyte-enriched fraction was incubated in tissue culture plates from TPP. Results were comparable in 24- and 96-well plates. In 96-well plates, the optimum plating density for CD14-positive monocytes was 8.5 × 104 cells per well in 200 μl. It was critical to mitigate evaporation by filling the outer wells with sterile water and rigorously maintaining humidity in the incubators. Optimal results required completing manipulations in room air rapidly.
Cells were differentiated for 2 weeks in RPMI with 40% human plasma with GM-CSF and TNF-α (0.5 ng/ml each) under 5%–10% O2 and 5% CO2 at 37°C in a humidified atmosphere in a chamber flushed with N2 under the control of a PRoOX sensor and ProCO2 regulator (BioSpherix). They were then activated for 2 days with IFN-γ (2.5 ng/ml) and then infected with M. tuberculosis at an MOI of 0.1–0.2 and followed for at least 2 weeks in the same atmosphere. The cytokines used for differentiation continued to be provided with the changes of 30% of medium every 3–4 days except the day of infection.
Cytokines.
We used IFN-γ (Actimmune) from Boehringer because its potency in this assay was higher than that of IFN-γ from another supplier (Supplemental Figure 5F). Other cytokines were from R&D Systems, with BSA as a carrier. Cytokines were diluted in PBS and frozen at –80°C in aliquots used once.
M. tuberculosis.
M. tuberculosis Erdman was grown at 37°C in Middlebrook 7H9 broth (Difco; BD) supplemented with 0.2% glycerol, 0.5% BSA, 0.2% dextrose, and 0.085% NaCl without detergent and frozen in log-phase (OD at 580 nm, 0.6–0.8) in the same medium with 20% glycerol. Inocula were prepared in MDM culture medium. Inocula were quantified retrospectively by dilution in PBS with 0.5% (final concentration) Triton X-100, plating on agar (Middlebrook 7H11, 10% oleic acid-albumin-dextrose-catalase enrichment; Difco, BD), and enumeration of CFU at 2 weeks.
Flow cytometry.
For analysis of cell surface marker expression, MDMs were differentiated for 2 weeks and then detached using trypsin (1.6 μg/ml) and EDTA (2 mM) in PBS. MDMs were treated with Fc receptor blocking agent (Miltenyi Biotec) and stained for 1 hour at 4°C by using antibodies and appropriate isotype controls from BD. Samples were analyzed on a BD LSR II flow cytometer.
Macrophage infections.
MDMs were infected at 16 days at an MOI of 0.1–0.2. We did not wash out extracellular bacteria, as pilot experiments demonstrated no difference in outcome whether or not MDMs were washed with warm medium 4 hours after inoculation. At the time points of interest, the appearance of each well was recorded by photomicroscopy. Then an aliquot of medium (100 μl) was removed and reserved. Macrophages were lysed by adding 100 μl PBS containing 1% Triton X-100 and mixing the well contents. The reserved medium from the same well was then pooled with the lysate, and bacteria were enumerated by plating duplicate serial dilutions on two agar plates each. Thus, there was no wash step prior to lysing macrophages, and all bacteria in the well were enumerated. Data from representative experiments show mean ± SD of 2–48 CFU values. Similar results were obtained in at least 3 experiments, with cells from the number of different donors stated.
Statistics.
Comparisons were analyzed with a 1-tailed Student’s t-test. A P value less than 0.05 was considered statistically significant.
Study approval.
Blood donors provided written, informed consent for the study “In Vitro Differentiation of Human Monocytes” as approved by the Weill Cornell Medical College–New York Presbyterian Hospital Institutional Review Board.
Supplementary Material
Acknowledgments
We thank the blood donors for their participation and are grateful for help from A. Cunningham-Bussel, G.E. Rehren, X. Guo, J. David Warren, L. Pedro Sorio de Carvalho, G. Lin, Xiuju Jiang, M.J. McConnell, and M. Fuortes (Weill Cornell Medical College); Sergei Rudchenko (Hospital for Special Surgery); Levi Beverly (Sloan Kettering Institute); and D. Bogunovic, Y. Itan, M. Vogt, and V. Tolyan-Vogt (The Rockefeller University); and for advice from S. Cohen (Stanford University School of Medicine). G. Vogt held a fellowship from the Fondation pour la Recherche Médicale (FRM). This work was supported by Department of Defense grant HDTRA01-06C-0039 (S. Cohen, principal investigator). The Department of Microbiology and Immunology is supported by the William Randolph Hearst Foundation.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(10):3889–3901. doi:10.1172/JCI57235.
References
- 1.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17(6):693–702. doi: 10.1016/S1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- 2.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136(1):37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills JW, Ryan L, LaCourse R, North RJ. Extensive Mycobacterium bovis BCG infection of liver parenchymal cells in immunocompromised mice. Infect Immun. 2001;69(5):3175–3180. doi: 10.1128/IAI.69.5.3175-3180.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28(2):271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tulkens P, Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978;27(4):415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- 6.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97(16):8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JP, Hayashi T, Datta SK, Kornbluth RS, Raz E, Guiney DG. CpG oligonucleotides partially inhibit growth of Mycobacterium tuberculosis, but not Salmonella or Listeria, in human monocyte-derived macrophages. FEMS Immunol Med Microbiol. 2005;45(2):303–310. doi: 10.1016/j.femsim.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 9.Filipe-Santos O, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Kampmann B, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115(9):2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson R, et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS One. 2009;4(9):e6984. doi: 10.1371/journal.pone.0006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawara A, Nathan CF, Cohn ZA. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawara A, Nathan CF. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- 14.Boechat N, et al. Culture at high density improves the ability of human macrophages to control mycobacterial growth. J Immunol. 2001;166(10):6203–6211. doi: 10.4049/jimmunol.166.10.6203. [DOI] [PubMed] [Google Scholar]
- 15.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meylan PR, Richman DD, Kornbluth RS. Reduced intracellular growth of mycobacteria in human macrophages cultivated at physiologic oxygen pressure. Am Rev Respir Dis. 1992;145(4 pt 1):947–953. doi: 10.1164/ajrccm/145.4_Pt_1.947. [DOI] [PubMed] [Google Scholar]
- 17.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci U S A. 2007;104(11):4547–4552. doi: 10.1073/pnas.0611732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5(8):741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darwich L, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 2009;126(3):386–393. doi: 10.1111/j.1365-2567.2008.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea MG, et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105(9):3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158(2):800–806. [PubMed] [Google Scholar]
- 22.Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Jordao L, Bleck CK, Mayorga L, Griffiths G, Anes E. On the killing of mycobacteria by macrophages. Cell Microbiol. 2008;10(2):529–548. doi: 10.1111/j.1462-5822.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 25.Meyron-Holtz EG, Ghosh MC, Rouault TA. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science. 2004;306(5704):2087–2090. doi: 10.1126/science.1103786. [DOI] [PubMed] [Google Scholar]
- 26.Condos R, et al. Recombinant gamma interferon stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infect Immun. 2003;71(4):2058–2064. doi: 10.1128/IAI.71.4.2058-2064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keane J, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 28.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100(10):2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans. 2009;37(pt 4):886–891. doi: 10.1042/BST0370886. [DOI] [PubMed] [Google Scholar]
- 30.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr Med Chem. 2009;16(19):2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 31.Nathan C. Role of iNOS in human host defense. Science. 2006;312(5782):1874–1875. doi: 10.1126/science.312.5782.1874b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.