Abstract
Astaxanthin (AX), which is produced by some marine animals, is a type of carotenoid that has antioxidative properties. In this study, we initially examined the effects of AX on the aging of a model organism C. elegans that has the conserved intracellular pathways related to mammalian longevity. The continuous treatments with AX (0.1 to 1 mM) from both the prereproductive and young adult stages extended the mean lifespans by about 16–30% in the wild-type and long-lived mutant age-1 of C. elegans. In contrast, the AX-dependent lifespan extension was not observed even in a daf-16 null mutant. Especially, the expression of genes encoding superoxide dismutases and catalases increased in two weeks after hatching, and the DAF-16 protein was translocated to the nucleus in the AX-exposed wild type. These results suggest that AX protects the cell organelle mitochondria and nucleus of the nematode, resulting in a lifespan extension via an Ins/IGF-1 signaling pathway during normal aging, at least in part.
1. Introduction
It has been understood that antioxidants and free radical scavengers decrease the intracellular reactive oxygen species (ROS) in treated experimental organisms and prolong their lifespans based on the free radical theory of aging [1, 2]. In the model organism nematode, Caenorhabditis elegans (C. elegans), there are many reports that dietary supplements, such as antioxidants and radical scavengers, extended the lifespan. C. elegans is an excellent experimental system to assess the pharmacological influence on intracellular aging pathways conserved between invertebrates and vertebrates [3, 4]. For example, it is conceivable that antioxidants, such as vitamin E, simply act to reduce the intracellular ROS in C. elegans [5]. The flavonoids, such as quercetin, are made to decrease the accumulation of the aging marker lipofuscin and localize the DAF-16 transcription factor, a homolog of mammalian FoxO, in the nucleus from the cytosol via an insulin/insulin-like growth factor-1 (Ins/IGF-1) signaling [6]. Oxaloacetate, the citric acid cycle metabolite, also increased the lifespan through an AMPK/FOXO-dependent pathway [7]. CoQ10, which is essential for the mitochondrial respiratory chain, reduced the superoxide anion levels mainly produced during electron transport [8]. In contrast, resveratrol that is a polyphenol found in red wine mimics calorie restriction by stimulating sirtuins, increasing the DNA stability, and extending the lifespan of metazoans [9–11]. Thus, molecular mechanisms of the supplemental lifespan extension are classified based on several intracellular pathways evolutionarily conserved from yeast to mammals.
Environmental effects on the nematode, which changes the DNA structure and repair, behavior, genetic recombination frequency, oxygen (O2) consumption, and lipofuscin accumulation over its lifespan, are very important when considering the lifespan extension [12]. It is estimated that the heritability of the lifespan in C. elegans is between 20% and 50%, and the remaining percentage is mainly due to environmental effects, including nutrients and pathogens in the medium and ROS resources such as O2 and ionizing radiation (IR) in atmosphere, on the lifespan [13]. Therefore, the environmental effects on the lifespan of worms are not negligible. During its growth, the worm continues to intake nutrients from the medium with or without the bacterium Escherichia coli (E. coli) as the food source. The length of the mean lifespan as a worm group is remarkably affected by the environmental nutrients in the culturing medium because the C. elegans genome has a nearly uniform base composition [14]. These environmental effects can be classified into factors that shorten or extend the nematode lifespan upon their exposure or lack of exposure.
On the other hand, the enzymatic antioxidant systems in C. elegans, for example, superoxide dismutase (SOD) and catalase, play an important role in protecting living cells from ROS. SOD and catalase scavenge the intracellular superoxide radical (∙O2 −) and hydrogen peroxide (H2O2), respectively. In C. elegans, five genes encoding these SODs (sod-1 to sod-5) and three genes encoding these catalases (ctl-1 to ctl-3) have been identified in the genome [15–21]. sod-1 and sod-5 genes encode cytosolic Cu/Zn SODs, sod-2 and sod-3 genes encode mitochondrial Mn SODs, and sod-4 gene encodes the homolog of the extracellular Cu/Zn SOD in mammals. ctl-1 and ctl-3 genes encode unusual cytosolic catalases, and ctl-2 gene encodes a peroxisomal catalase. In these genes, sod-3, sod-5, ctl-1, and ctl-2 are direct targets of the transcription factor DAF-16, which is a key regulator of the Ins/IGF-1 signaling pathway implicated in the normal aging process of C. elegans [22–24]. Expressions of these subsets of antioxidant genes in C. elegans are also induced by the exposure to dietary supplements via the Ins/IGF-1 signaling pathway [6].
Astaxanthin (AX), which is produced by marine animals, is a kind of carotenoid and shows a strong antioxidant activity that is attributed to the quenching of singlet oxygen (1O2) and the scavenging of lipid peroxidation by free radicals. In addition, AX inhibits the production of lipid peroxides in the animal cell membranes, and the antioxidant activity is about 2-fold more effective than β-carotene. The efficient antioxidant activity of AX is suggested to be due to the unique conjugated polyene structure of the terminal ring moiety [25]. It is reported that a marine carotenoid, fucoxanthin (FX), improved the insulin resistance and decreased the blood glucose level in mammals through the downregulation of the tumor necrosis factor-α [26]. Thus, the specific regulation of carotenoids containing AX and FX on the intracellular biomolecules is responsible for the characteristic chemical structures, which differ depending on the length of the polyene chain, a long conjugated double bond system forming the backbone of the molecule. We report the effects of AX, which has not only a strong antioxidant activity but also some biological activities that affect the nematode C. elegans lifespan.
2. Results
Continuous treatment with 0.1 to 1 mM AX from each stage of the first-stage larva (L1) or young adult in the hermaphrodite extended about 16–30% each mean of lifespan in the wild-type N2 and long-lived mutant age-1 of C. elegans (Figure 1 and Table 1). The AX-dependent lifespan extensions in N2 were more notable than these of the age-1 animals. Moreover, the maximum lifespan in N2 also increased significantly depending on the concentration of AX. In contrast, the AX-dependent increases in the mean and maximum lifespan were not statistically clear in a null allele of the daf-16 gene mutant, daf-16(mgDf50) animals. The wild-type lifespans measured using AX, which had not been solubilized in DMSO, were not significantly extended in a preliminary experiment (data not shown).
Figure 1.
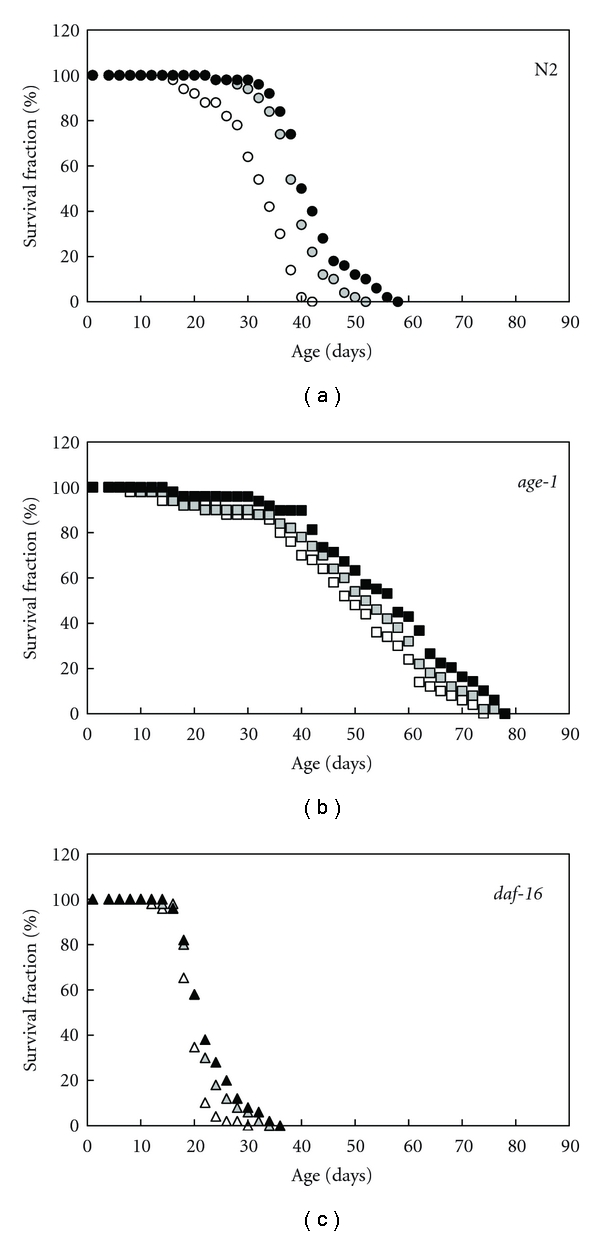
Survival curves at 20°C in wild-type N2, age-1(hx546), and daf-16(mgDf50) animals. About 100 animals were used in each experiment. Open circle, square, and triangle show controls, shaded circle, square, and triangle show the treatment with 0.1 mM AX, and closed circle, square, and triangle show the treatment with 1 mM AX. Means of lifespan ± standard deviation (SD) in control, 0.1 mM, and 1 mM AX were as Table 1.
Table 1.
Effect of AX on mean and maximum lifespans at 20°C in several age-related mutants.
| Strain (condition) | Mean lifespan (days) | Max. lifespan (days) | ||
|---|---|---|---|---|
| N2 (control) | 31.5 ± 6.4 | 25.5 ± 6.0 | 24.5 ± 5.1 | 43.8 ± 4.8 |
| N2 (0.1 mM AX) | 38.4 ± 5.3** | 32.2 ± 9.1** | 27.7 ± 5.7** | 51.6 ± 6.0* |
| N2 (1 mM AX) | 41.4 ± 6.5** | 32.8 ± 8.7** | 28.6 ± 6.4** | 53.3 ± 6.1* |
| age-1 (control) | 47.1 ± 15.1 | 40.1 ± 14.6 | 46.4 ± 14.7 | 77.6 ± 7.0 |
| age-1 (0.1 mM AX) | 50.2 ± 16.1 | 50.2 ± 16.8** | 50.5 ± 13.1* | 80.0 ± 7.8 |
| age-1 (1 mM AX) | 54.8 ± 14.7** | 49.8 ± 17.6** | 51.4 ± 12.9* | 82.6 ± 8.2 |
| daf-16 (control) | 19.2 ± 2.8 | 19.3 ± 2.9 | 18.0 ± 4.2 | 24.6 ± 2.6 |
| daf-16 (0.1 mM AX) | 21.2 ± 4.0 | 20.1 ± 2.8 | 18.6 ± 3.5 | 27.6 ± 3.5 |
| daf-16 (1 mM AX) | 22.0 ± 4.7 | 19.6 ± 3.5 | 19.5 ± 2.9 | 28.6 ± 3.8 |
Results about mean lifespan are indicated as means ± SD from three independent experiments. Results about maximum lifespan are expressed as means ± SD of more than six determinations. P values by t-test with an asterisk (controls versus AX-treated conditions) significantly differ as follows; *P < 0.05 and **P < 0.001.
On the other hand, the AX-dependent increases in the expression of some genes encoding antioxidant enzymes, such as SOD and catalase, were significantly observed in the wild-type N2 (Figure 2). Especially, the expression of the sod-3, sod-5, ctl-1, and ctl-2 genes in the AX-treated N2 was significantly increased within two weeks after the AX treatment. All these genes are targets of the DAF-16 transcription factor, and the expression is regulated via the Ins/IGF-1 signaling pathway associated with oxidative stress resistance and aging in C. elegans [22–24]. The AX-dependent increases in the expression levels of these genes were not yet observed in the 7-day-old animals (data not shown). Thus, it was recognized that there are the time lags until the AX-dependent rising in the expression of these genes. In these genes; however, there are the genes that were regulated by other transcription factors (e.g., role of SKN-1 in ctl-genes) [27]. Therefore, the inconsistency during the intrinsic catalase activities in this paper and the mRNA levels in the previous data may depend on the diversity in transcriptional regulation of ctl genes expression [28].
Figure 2.
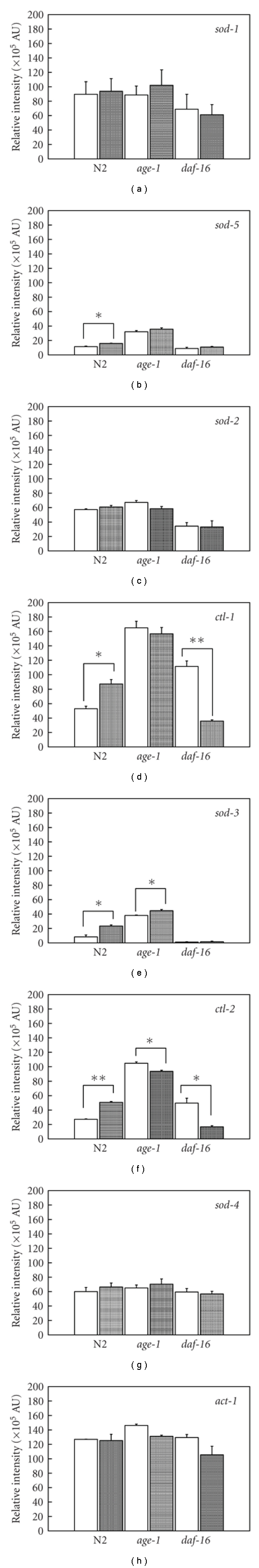
mRNA expression levels of sod and ctl genes in wild-type N2, age-1(hx546), and daf-16(mgDf50) animals using RT-PCR. Each mRNA of 14-day-old animals was prepared and analyzed. Panels indicate the quantitative data obtained using the luminescent image analyzer and AU in the panels indicates arbitrary unit. Data are means ± SD of three or more independent experiments. Left-hand open column and right-hand shaded column for each strain indicate values without and with 1 mM AX-exposure, respectively. Asterisk indicates significant difference during the values without and with AX exposure. P values, which were calculated using a two-tailed t-test for paired samples with unequal variances, are *P < 0.05 and **P < 0.005.
Furthermore, we found that AX still decreased the mitochondrial ∙O2 − production levels in the 14-day-old animals of the age-1 mutant but not the daf-16 null mutant. There was no significant difference in the mitochondrial ∙O2 − levels after 14 days from hatching in the AX-treated wild-type N2 compared with the 4-day-old animals. The AX treatment did not decrease but increased the mitochondrial ∙O2 − production levels of the 4-day-old animals of N2 and age-1 (Figure 3(a)). However, the AX-dependent increases in the mitochondrial ∙O2 − levels were not significant in the AX-treated 14-day-old animals of N2 and age-1 (Figure 3(b)). In general, dose ranging revealed that the mitochondrial ∙O2 − production levels per mg of mitochondrial protein in the 14-day-old animals, at approximately 150–300 × 103 the relative luminescence intensity unit (RLU) per second, was significantly enriched compared to the values in the 4-day-old animals (Figures 3(a) and 3(b)).
Figure 3.
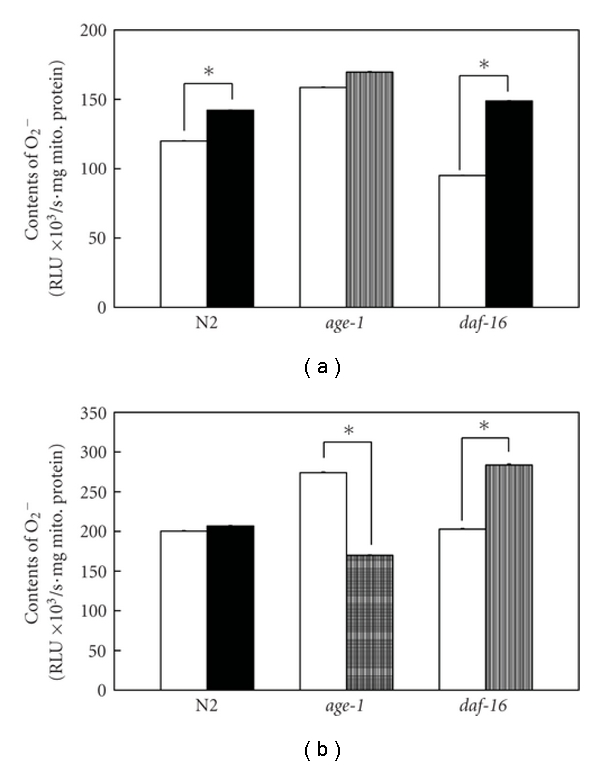
Mitochondrial ∙O2 − contents of various strains. Left-hand open bar for each strain of 4 days old (a) and 14 days old (b) indicates mitochondrial ∙O2 − level in vitro without 1 mM AX exposure, and right-hand shaded bar indicates values with AX exposure. Data are means ± standard error of the mean (SEM) of ten independent measurements. P values, which were calculated using a two-tailed t-test for paired samples with unequal variances, are *P < 0.001.
In the 4-day-old animals of wild-type N2, almost all DAF-16 protein was observed in the cytoplasm and not the nucleus. This phenomenon was also similar in the AX-exposed 4-day-old animals (Figures 4(a) and 4(b)). In contrast, the DAF-16 translocation into the nucleus had already been observed in the 4-day-old animals of age-1 with and without AX. The DAF-16-translocated nuclei were mainly in the epithelia, musculature, intestine, and part of the nervous system (Figures 4(c) and 4(d)). On the other hand, the DAF-16 was more localized in the nuclei of the 14-day-old animals of N2, and the translocation was significantly enhanced in the AX-exposed animals (Figures 4(e), 4(f), and Table 2). In the 14-day-old animals of age-1, more DAF-16 was translocated into the cytoplasm from the nucleus of the musculature and intestine compared to the 4-day-old animals (Figures 4(c), 4(d), 4(g), and 4(h)).
Figure 4.
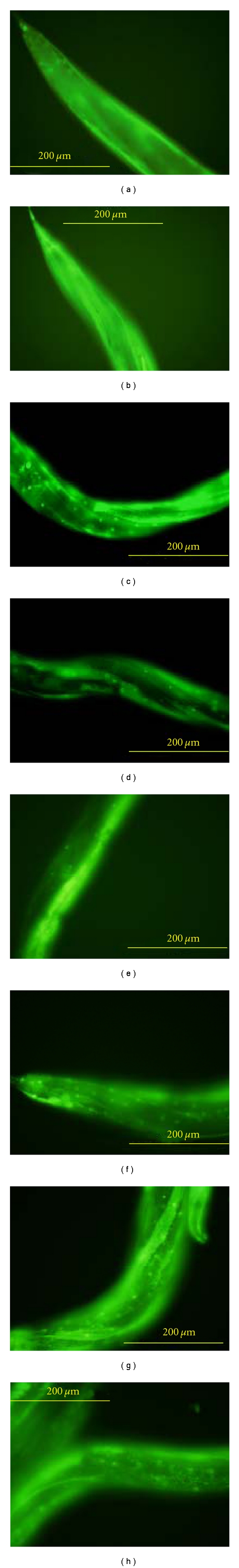
Localization of DAF-16::GFP in wild-type (a, b, e, and f) and age-1 animals (c, d, g, and h). Panels of (a, b, c, and d) show the 4-day-old animals, and panels of (e, f, g, and h) show the 14-day-old animals. Furthermore, Panels of (b, d, f, and h) show AX-exposed animals in each strain. Scale bar = 200 μm. Means of number of DAF-16-translocated nuclei ± SD in control and 1 mM AX were as Table 2.
Table 2.
Effect of AX on DAF-16 localization into the nucleus in several age-related mutants.
| Strain (condition) | Age (days) | Number of DAF-16-translocated nuclei (/unit area) |
|---|---|---|
| N2 (control) | 4 | N.D. |
| N2 (1 mM AX) | 4 | N.D. |
| N2 (control) | 14 | 3.4 ± 0.5 |
| N2 (1 mM AX) | 14 | 12.0 ± 2.0* |
| age-1 (control) | 4 | 12.6 ± 1.1 |
| age-1 (1 mM AX) | 4 | 13.6 ± 3.2 |
| age-1 (control) | 14 | 14.2 ± 2.3 |
| age-1 (1 mM AX) | 14 | 13.6 ± 1.3 |
DAF-16-translocated nuclei were counted mainly in the epithelia, musculature, and intestine. N.D. indicated not detected. Results about the number of DAF-16-translocated nuclei are indicated as means ± SD from more than five independent transgenic animals. P values by t-test with an asterisk (controls versus AX-treated condition) significantly differ as follow; *P < 0.001.
3. Discussion
Based on exposure of AX to several strains, we observed a longevity effect of about 16–30% in the wild-type N2 and long-lived mutant age-1 of C. elegans. In contrast, no significant differences in the AX-dependent lifespan extension were noted in a daf-16 null mutant. AX exposure to wild-type and age-1 animals mainly enhanced the mRNA expression of the DAF-16 target genes and increased the nuclear localization of the DAF-16 transcription factor. Furthermore, it was shown that AX also caused a decrease in the mitochondrial ROS production during the long-term exposure to these animals. This finding suggests that AX indirectly protects intracellular organelles, such as mitochondria and nuclei, from oxidative damage during normal aging because the AX molecules at the surface and inside the phospholipid membranes in intracellular organelles have dual activities to quench 1O2 and scavenge lipid peroxidation by free radicals [25]. That is, the mitochondria protected from oxidative damage leak less ROS during the mitochondrial respiration, and the nuclei protected from oxidative damage are more active for the gene expression in organisms. It is likely that the oxidative stress-induced expression of antioxidant genes has been continued at least until 14 days old in the AX-treated animals. Moreover, we propose that AX protects the intracellular organelles through the bioactivities at the phospholipid membranes of cells (including the mitochondrial and nuclear membranes) [25] and increases the expression of the DAF-16 target genes via the Ins/IGF-1 signaling pathway, at least in part, in the nematode C. elegans. As a result, AX increases the lifespans of the wild-type and long-lived age-1 mutant, which has activated the Ins/IGF-1 signaling. In particular, attention is directed to a more effective AX-dependent lifespan extension in the wild-type rather than the Ins/IGF-1 signaling-activated strain.
On the other hand, it is interesting that the functionality of the carotenoids (such as AX) is determined by its subcellular localization [29]. Studies using domestic animals have shown a significant uptake of orally fed carotenoids, for example, lutein and β-carotene, by the microsomes, cytosol, and nuclei of the circulating peripheral blood leukocytes with the mitochondria showing the highest uptake in the animals [30, 31]. In a recent paper, it was reported that AX had accumulated in the mitochondria of normal human mesangial cells cultured with a high concentration of glucose and reduced the production of the mitochondrial ROS-modified proteins [32]. The mitochondrial respiratory chain system utilizes approximately 85% of the oxygen consumed by the cell to generate ATP; therefore, an intracellular organelle mitochondrion is the most important source of ROS [33]. Thus, the mitochondria are a key player for lifespan determination in organisms based on the mitochondrial oxidative stress theory of aging [1, 2, 34]. Accordingly, the localization of the carotenoids in the mitochondria has been of particular relevance based on the previous reports. AX prevented the lipid hydroperoxide (LOOH) generation in membrane liposomes enriched with polyunsaturated fatty acids and improved the muscle lipid metabolism under the ROS generation in exercise groups of mice [35, 36]. Notably, McNulty et al. inferred that AX preserved the membrane structure and exhibited a significant antioxidant activity because AX showed a significant reduction in lipid peroxidation rather than other apolar carotenoids, such as lycopene and β-carotene [35]. In addition to this dual antioxidant capacity (quenching of 1O2 and scavenging of lipid peroxidation), the direct ∙O2 − scavenging efficiency of AX delivered in the DMSO vehicle was evaluated using an in vitro isolated human neutrophil assay [29]. Likewise, our study indicated that supplemental DMSO-dissolved AX delivered into the nematode plays a role regarding some antioxidant properties in the mitochondrial and nuclear membranes without modification of the constituent lipid structure under stressful conditions during normal aging. Of course, it is expected that these physical properties of AX against cellular and intracellular membranes are effective even in the daf-16 null mutant used in the current study. However, we consider that an imbalance during the production and quenching of ROS had occurred in the mitochondria of the AX-treated daf-16 mutant because not only the antioxidant genes but also the mitochondrial metabolic genes were regulated as the targets of DAF-16 transcription factor and related to the C. elegans lifespan and metabolism [37].
Recently, Miyashita has suggested that carotenoids have other novel biological activities, which are independent of the antioxidant properties. Modulation of the transcription activity of carotenoids is known to have an anticancer effect; however, the underlying mechanisms of this action still remain uncertain [26]. Moreover, the nonprovitamin A carotenoids (such as lutein, cantaxanthin, lycopen, and AX) are also capable of altering the patterns of gene and protein expressions and have a cellular function with a specific nutritional impact on the body [30, 38].
In summary, we conclude that AX taken into the subcellular organelles in nematode C. elegans consequently protects the cells at the surface and inside lipid-rich membranes against oxidative injury and functions to keep the optimal intracellular ROS balance mediated by regulation of the DAF-16 targets via the Ins/IGF-1 signaling pathway during normal aging. Hence, AX as a potential in vivo supplemental agent, extends the lifespan of nematodes not only by the direct antioxidant activities but also via the indirect AX-related activation of the Ins/IGF-1 signaling.
4. Methods
4.1. Materials
The C. elegans strains, wild-type N2 var. Bristol, age-1(hx546), and daf-16(mgDf50) were obtained from the Caenorhabditis Genetics Center at the University of Minnesota (Minneapolis, Minn, USA). The age-1(hx546) mutant is the first long-lived strain [39, 40], and the daf-16(mgDf50) mutant has a deficiency completely eliminating the daf-16 coding region [41]. Worms were grown at 20°C on nematode growth medium (NGM) agar plates with E. coli [42–44].
4.2. Measurements of AX-Treated Lifespan
The gravid hermaphrodites from the NGM agar plates were washed then dissolved in alkaline sodium hypochlorite in order to collect the eggs in utero. The released eggs were allowed to hatch by overnight incubation at 20°C in S buffer to the age synchronous cultures of the L1 stage larvae [44]. The lifespan of the hermaphrodites at 20°C was measured with or without AX crystalline (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif, USA) solubilized in dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, Mo, USA) (Figure 5) [29]. In order to prevent progeny production, 5-fluoro-2′-deoxyuridine (FUdR; Wako Pure Chemical Industries Ltd., Osaka, Japan) was added to the NGM agar plate at the final concentration of 40 μM after the animals had reached adulthood [43].
Figure 5.

AX-containing NGM plates for measurement of lifespan in nematode. Left-, middle-, and right-hand plates contain a red carotenoid pigment AX of 0, 0.1, and 1 mM, respectively. For its lipid solubility, nonesterified AX crystalline was delivered using a DMSO vehicle in NGM [29].
4.3. Quantitative RT-PCR for Antioxidant Enzymes
The poly(A)+ RNA of the animals cultured with or without AX was prepared, and then the cDNA was synthesized using a reverse transcription reaction [42]. The cDNA was used as a template for the subsequent polymerase chain reactions (PCR). We carried out the PCR for five sod and two ctl genes in the 14-day-old animals. Fragments of the PCR products were confirmed by agarose gel electrophoresis and ethidium bromide (EtBr). The fluorescence intensity of EtBr in the DNA fragments was half-quantitatively measured using a LAS-4000UVmini luminescent image analyzer (Fujifilm Co., Tokyo, Japan). The expression data were normalized to each transcript level of the act-1 gene (encoding the body wall and pharyngeal muscle actin protein) in N2, age-1(hx546), and daf-16(mgDf50).
4.4. Measurements of Mitochondrial ∙O2 − Production
For isolation of the mitochondria fraction, the 4- and 14-day-old animals were treated as previously described [28]. The mitochondria fraction was resuspended in the TE buffer. The protein content of each fraction was determined using a BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, Ill, USA). The mitochondrial ∙O2 − production was measured using the specific chemiluminescent probe, 2-methyl-6-p-methoxyphenylethynyl imidazopyrazinone (MPEC; ATTO Co., Tokyo, Japan) [45]. Forty μg of the intact mitochondria in 1 mL of the assay buffer containing 0.7 μM MPEC was placed in an AccuFLEX Lumi 400 luminometer (Aloka Co., Ltd., Tokyo, Japan), and the relative luminescence intensity per second was measured.
4.5. Subcellular Localization of DAF-16
To detect the intracellular DAF-16 activity, pGP30 vector (obtained from Dr. T. E. Johnson's laboratory), which has a construct fuged the daf-16 gene transcript a2 (daf-16a2) to gfp gene, was microinjected into each gonad of the wild-type and age-1 animals at 100 ng/μL with pRF4 containing the rol-6(su1006) gene. The presence or absence of DAF-16 localization into the nucleus of the 1 mM AX-exposed transgenic 4- and 14-day-old animals was observed using an Olympus Fluorescence Microscope with Digital Imaging System BX51TRF (Olympus Co., Tokyo, Japan).
Acknowledgments
This research was financially supported in part by a special research grant from Daito Bunka University (S. Yanase). The authors thank Dr. Kazunaga Yazawa from the Tokyo University of Marine Science and Technology and Mr. Jiro Takahashi from the Fuji Chemical Industry Co., Ltd. for their helpful suggestions. Furthermore, they are indebted to Dr. Thomas E. Johnson and Ms. Patricia Tedesco from the University of Colorado at Boulder for kindly distribution of pGP30 vector.
References
- 1.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Free radical theory of aging: effect of free radical reaction inhibitors on the mortality rate of male LAF mice. Journals of Gerontology. 1968;23(4):476–482. doi: 10.1093/geronj/23.4.476. [DOI] [PubMed] [Google Scholar]
- 3.Gill MS. Endocrine targets for pharmacological intervention in aging in Caenorhabditis elegans . Aging Cell. 2006;5(1):23–30. doi: 10.1111/j.1474-9726.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nature Reviews Drug Discovery. 2006;5(5):387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman BM, Geist MA. Effects of vitamin E on the nematode Caenorhabditis elegans . Age. 1983;6(1):1–4. [Google Scholar]
- 6.Kampkötter A, Nkwonkam CG, Zurawski RF, et al. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans . Toxicology. 2007;234(1-2):113–123. doi: 10.1016/j.tox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Williams DS, Cash A, Hamadani L, Diemer T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell. 2009;8(6):765–768. doi: 10.1111/j.1474-9726.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii N, Senoo-Matsuda N, Miyake K, et al. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mechanisms of Ageing and Development. 2004;125(1):41–46. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 10.Wood JG, Regina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 11.Venturini CD, Merlo S, Souto AA, Fernandes MDC, Gomez R, Rhoden CR. Resveratrol and red wine function as antioxidants in the central nervous system without cellular proliferative effects during experimental diabetes. Oxidative Medicine and Cellular Longevity. 2010;3(6):434–441. doi: 10.4161/oxim.3.6.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klass MR, Johnson TE. Nonmammalian Models for Aging Research. Basel, Switzerland: Karger; 1985. Caenorhabditis elegans; pp. 164–187. (Interdisciplinary Topics in Gerontology, Vol. 21). [Google Scholar]
- 13.Johnson TE, Wood WB. Genetic analysis of life-span in Caenorhabditis elegans . Proceedings of the National Academy of Sciences of the United States of America. 1982;79(21 I):6603–6607. doi: 10.1073/pnas.79.21.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sulston JE, Brenner S. The DNA of Caenorhabditis elegans . Genetics. 1974;77(1):95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans . Proceedings of the National Academy of Sciences of the United States of America. 1993;90(19):8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, Inokuma K, Yasuda K, Ishii N. Cloning, sequencing and mapping of a manganese superoxide dismutase gene of the nematode Caenorhabditis elegans . DNA Research. 1996;3(3):171–174. doi: 10.1093/dnares/3.3.171. [DOI] [PubMed] [Google Scholar]
- 17.Giglio AM, Hunter T, Bannister JV, Bannister WH, Hunter GJ. The copper/zinc superoxide dismutase gene of Caenorhabditis elegans . Biochemistry and Molecular Biology International. 1994;33(1):41–44. [PubMed] [Google Scholar]
- 18.Fujii M, Ishii N, Joguchi A, Yasuda K, Ayusawa D. A novel superoxide dismutase gene encoding membrane-bound and extracellular isoforms by alternative splicing in Caenorhabditis elegans . DNA Research. 1998;5(1):25–30. doi: 10.1093/dnares/5.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Jensen LT, Culotta VC. Activation of CuZn superoxide dismutases from Caenorhabditis elegans does not require the copper chaperone CCS. The Journal of Biological Chemistry. 2005;280(50):41373–41379. doi: 10.1074/jbc.M509142200. [DOI] [PubMed] [Google Scholar]
- 20.Taub J, Lau JF, Ma C, et al. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature. 1999;399(6732):162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 21.Petriv OI, Rachubinski RA. Lack of peroxisomal catalase causes a progeric phenotype in Caenorhabditis elegans . The Journal of Biological Chemistry. 2004;279(19):19996–20001. doi: 10.1074/jbc.M400207200. [DOI] [PubMed] [Google Scholar]
- 22.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochemical Journal. 2000;349(2):629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanase S, Onodera A, Tedesco P, Johnson TE, Ishii N. SOD-1 deletions in Caenorhabditis elegans alter the localization of intracellular reactive oxygen species and show molecular compensation. Journals of Gerontology—Series A. 2009;64(5):530–539. doi: 10.1093/gerona/glp020. [DOI] [PubMed] [Google Scholar]
- 24.Murphy CT, McCarroll SA, Bargmann CI, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans . Nature. 2003;424(6946):277–284. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 25.Goto S, Kogure K, Abe K, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochimica et Biophysica Acta. 2001;1512(2):251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita K. Food Factors for Health Promotion (Forum of Nutrition) Vol. 61. Basel, Switzerland: Karger; 2009. Function of marine carotinoids; pp. 136–146. [DOI] [PubMed] [Google Scholar]
- 27.Back P, Matthijssens F, Vlaeminck C, Braeckman BP, Vanfleteren JR. Effects of sod gene overexpression and deletion mutation on the expression profiles of reporter genes of major detoxification pathways in Caenorhabditis elegans . Experimental Gerontology. 2010;45(7-8):603–610. doi: 10.1016/j.exger.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Yanase S, Ishii N. Hyperoxia exposure induced hormesis decreases mitochondrial superoxide radical levels via Ins/IGF-1 signaling pathway in a long-lived age-1 mutant of Caenorhabditis elegans . Journal of Radiation Research. 2008;49(3):211–218. doi: 10.1269/jrr.07043. [DOI] [PubMed] [Google Scholar]
- 29.Cardounel AJ, Dumitrescu C, Zweier JL, Lockwood SF. Direct superoxide anion scavenging by a disodium disuccinate astaxanthin derivative: Relative efficacy of individual stereoisomers versus the statistical mixture of stereoisomers by electron paramagnetic resonance imaging. Biochemical and Biophysical Research Communications. 2003;307(3):704–712. doi: 10.1016/s0006-291x(03)01248-8. [DOI] [PubMed] [Google Scholar]
- 30.Chew BP, Park JS. Carotenoid action on the immune response. Journal of Nutrition. 2004;134(1):257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 31.Chew BP, Park JS, Weng BC, Wong TS, Hayek MG, Reinhart GA. Dietary β-carotene absorption by blood plasma and leukocytes in domestic cats. Journal of Nutrition. 2000;130(9):2322–2325. doi: 10.1093/jn/130.9.2322. [DOI] [PubMed] [Google Scholar]
- 32.Manabe E, Handa O, Naito Y, et al. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. Journal of Cellular Biochemistry. 2008;103(6):1925–1937. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- 33.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans . PLoS Biology. 2010;8(12) doi: 10.1371/journal.pbio.1000556. Article ID e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochimica et Biophysica Acta. 2007;1768(1):167–174. doi: 10.1016/j.bbamem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Aoi W, Naito Y, Takanami Y, et al. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochemical and Biophysical Research Communications. 2008;366(4):892–897. doi: 10.1016/j.bbrc.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300(5619):644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- 38.Park JS, Chew BP, Wong TS, Zhang JX, Magnuson NS. Dietary lutein but not astaxanthin or β-carotene increases pim-1 gene expression in murine lymphocytes. Nutrition and Cancer. 1999;33(2):206–212. doi: 10.1207/S15327914NC330214. [DOI] [PubMed] [Google Scholar]
- 39.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans . Nature. 1996;382(6591):536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 41.Ogg S, Paradis S, Gottlieb S, et al. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans . Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 42.Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mechanisms of Ageing and Development. 2002;123(12):1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 43.Ishii N, Takahashi K, Tomita S, et al. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans . Mutation Research. 1990;237(3-4):165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JA, Fleming JT. Methods in Cell Biology, Caenorhabditis elegans: Modern Biology Analysis of an Organism. Vol. 48. Millbrae, Calif, USA: Academic Press; 1995. Basic culture methods; pp. 4–29. [PubMed] [Google Scholar]
- 45.Shimomura O, Wu C, Murai A, Nakamura H. Evaluation of five imidazopyrazinone-type chemiluminescent superoxide probes and their application to the measurement of superoxide anion generated by Listeria monocytogenes. Analytical Biochemistry. 1998;258(2):230–235. doi: 10.1006/abio.1998.2607. [DOI] [PubMed] [Google Scholar]


