Abstract
Introduction
Thyroid cancer is an emerging public health concern. In the U.S., its incidence has doubled in the past decade, making it the 8th most commonly diagnosed neoplasm in 2010. Despite this alarming increase, most thyroid cancer patients benefit from conventional approaches (surgery, radioiodine, radiotherapy, TSH suppression with levothyroxine) and are often cured. Nevertheless, a minority have aggressive tumors resistant to cytotoxic and other historical therapies; these patients sorely need new treatment options.
Areas covered
Herein the biology and molecular characteristics of the common histological types of thyroid cancer are reviewed to provide context for subsequent discussion of recent developments and emerging therapeutics for advanced thyroid cancers.
Expert opinion
Several kinase inhibitors, especially those targeting VEGFR and/or RET, have already demonstrated promising activity in differentiated and medullary thyroid cancers (DTC, MTC). Although of minimal benefit in DTC and MTC, cytotoxic chemotherapy with anti-microtubule agents and/or anthracyclines in combination with intensity modulated radiation therapy appears to extend survival for patients with locoregionally-confined anaplastic thyroid cancer (ATC), but to have only modest benefit in metastatic ATC. Further discovery and development of novel agents and combinations of agents will be critical to further progress in treating advanced thyroid cancers of all histotypes.
Keywords: tyrosine kinase inhibitors, taxanes, differentiated thyroid cancer, medullary thyroid cancer, anaplastic thyroid cancer
1.0 INTRODUCTION
Despite a modest decline in the overall occurrence of cancer in the U.S., the incidence of thyroid cancer has more than doubled in the past decade. It is now the 8th most diagnosed cancer overall and the 5th most incident cancer in women in the U.S., making thyroid cancer an emerging public health concern.1–5 Furthermore, recent increases in thyroid cancer do not appear to be confined to the U.S.; for example, thyroid cancer is now the second most incident cancer in women in Saudi Arabia.6
Although historical therapeutic approaches have proven effective in treating most patients with early-stage thyroid cancers, those afflicted with radioiodine-refractory metastatic differentiated thyroid cancer (DTC), advanced medullary thyroid cancer (MTC) or anaplastic cancer (ATC) have, until recently, had few treatment options. Cytotoxic chemotherapy is largely ineffective for most patients with advanced disease. However, over the last decade, progress in better understanding the genetics and biology of thyroid cancers has created opportunities for new therapeutic approaches. Pharmaceutical companies have in parallel designed and synthesized a wide range of targeted agents. In this review, we discuss the characteristics of the three major histological sub-types of thyroid cancer (DTC, MTC and ATC) and data related to new and investigational agents with demonstrated efficacy or therapeutic promise in these cancers. Despite laudable progress in thyroid cancer therapeutics, many patients still die from advanced thyroid cancer, highlighting the imperative to discover and develop yet additional novel drugs and to critically evaluate them in rationally designed clinical trials.
2.0 BACKGROUND: THYROID CANCER ORIGINS, HISTOLOGIES AND CLASSIFICATION
Although malignancies can arise within any thyroid gland cells, this review focuses only upon those cancers that are derived from organ-specific thyroid cells (Figure 1), specifically excluding discussion of thyroid lymphomas, sarcomas and squamous cell carcinomas.
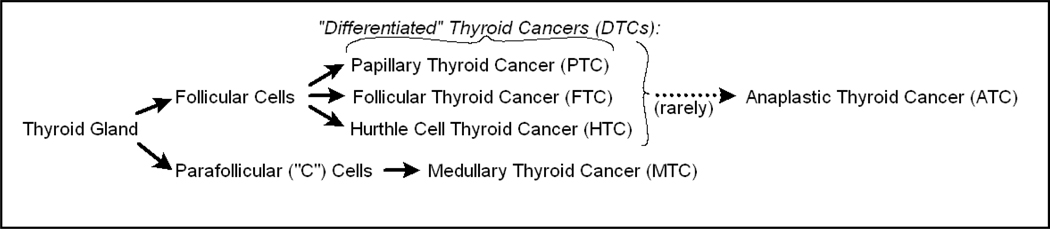
Figure 1.
Histological derivation of thyroid cancers.
Thyroid malignancies arise from follicular thyroid cells, which produce thyroglobulin (Tg) and express high levels of the sodium iodine transporter, NIS; or from parafollicular C-cells, which produce calcitonin and, when neoplastically transformed, carcinoembryonic antigen (CEA, Figure 1). Follicular cell-derived malignancies, collectively called “differentiated thyroid cancers” (DTCs), represent more than 90% of all thyroid neoplasms. DTCs are sub-classified as either papillary of follicular. Papillary thyroid cancer (PTC) is the most frequent, while follicular thyroid cancer (FTC) is less common but more aggressive. Hürthle cell thyroid cancer (HCC) is an uncommon but distinct and generally more aggressive type of thyroid cancer; The World Health Organization (WHO) now classifies it as a subtype of follicular cancer. In addition to HCC, other uncommon follicular cell-derived cancers associated with worse prognoses include “tall cell,” “insular,” “follicular variant PTC,” and “poorly differentiated”. It is unclear whether these uncommon tumors should be managed differently than other tumors in their class and they are, therefore, not elaborated upon here, saving to say that initial therapy for these variants also includes use of radioactive iodine.
Risk factors for DTC include prior exposure to ionizing radiation, either from radiotherapy used to treat acne, lymphomas or enlarged tonsils or thymus glands; exposure to fallout from nuclear reactors, i.e. such as from the Chernobyl radiation accident; and accidental exposure to radionuclides such as radioiodine; or exposure to radiation from other environmental sources. Autoimmune thyroiditis and/or chronic thyroid inflammation may also increase the risk for DTC. DTC is not commonly heritable; however, kindreds of “familial non-medullary thyroid cancer” have been described.
Early-stage DTCs, like the normal follicular thyroid cells from which they arise, usually express NIS and concentrate iodine; thus, early DTCs can be imaged and frequently also treated effectively with radioiodine (RAI). NIS expression and/or function is commonly lost in later stage DTCs, resulting in attenuated ability to concentrate RAI and RAI-resistance. Additionally, DTCs share with normal follicular cells the ability to produce thyroglobulin (Tg), a useful tumor biomarker in most patients, unless blocking (anti-Tg) antibodies develop.
Parafollicular-cell derived malignancies, classified as medullary thyroid cancers (MTCs), represent 5–8% of all thyroid cancers. It is critical to appreciate that neither parafollicular cells nor medullary thyroid cancer cells express NIS or have the ability to accumulate iodine. MTCs secrete calcitonin and CEA (and not Tg) and both are therefore potentially useful tumor markers in following extent of disease within individual patients.
Medullary thyroid cancers are more often heritable than DTCs, but the majority are sporadic. Hereditary MTCs, including those associated with Familial Medullary Thyroid Cancer Syndrome (FMTC) or with Multiple Endocrine Neoplasia 2 (MEN 2), harbor activating germ line mutations in the RET proto-oncogene - while the more common sporadic MTCs characteristically harbor tumor-specific activating RET mutations. The high frequency of RET mutations in MTC supports the currently accepted belief that RET activation is strongly implicated in MTC pathogenesis. Because of this biology, there has been great interest in assessing the clinical efficacy of new agents which target RET; indeed some believe that agents which target RET have ushered in a therapeutic revolution in the application of small molecule kinase inhibitors to treat thyroid cancers.
Rarely, normal follicular cells or differentiated thyroid cancers give rise to the third and most infrequent but most aggressive type of thyroid cancer, anaplastic thyroid cancer (ATC). ATC accounts for only 1–2% of thyroid cancers; however median overall survival is only about 5 months from the time of diagnosis.7, 8 Consequently, all anaplastic thyroid cancers are considered to be stage 4, distinguished only as stage 4A, 4B (locoregionally confined) or 4C (metastatic). ATC is rarely heritable.
3.0 GENETIC ALTERATIONS AND SIGNALING PATHWAYS IN THYROID CANCERS
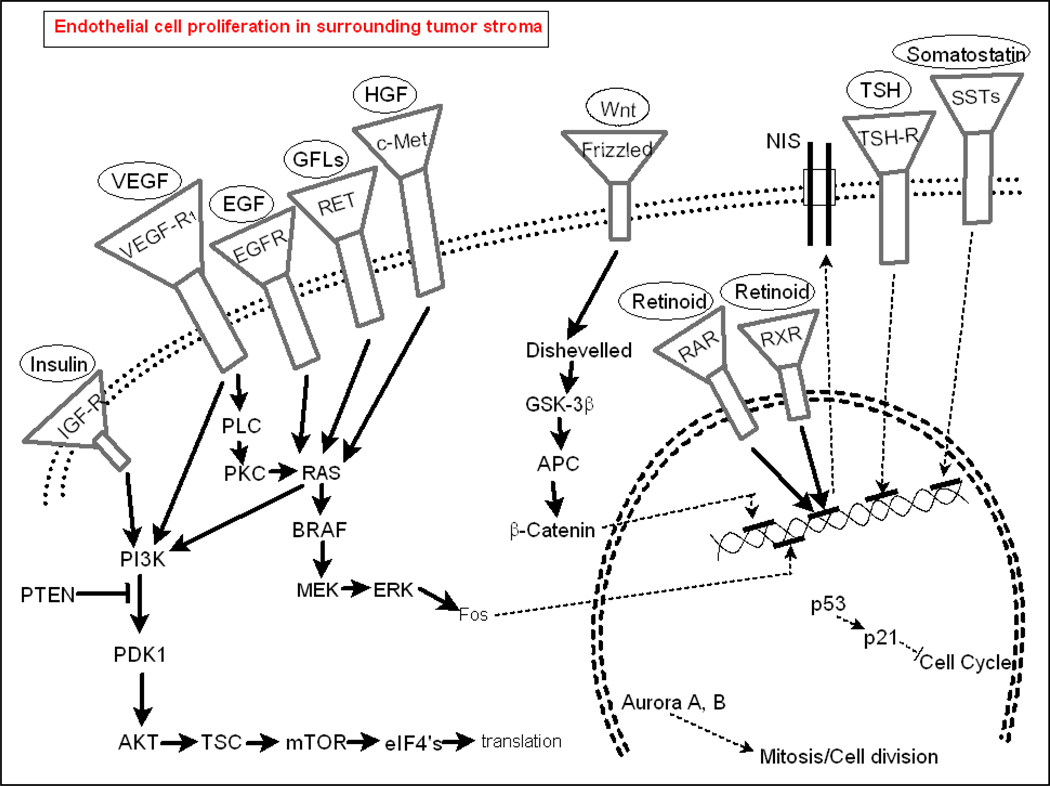
To provide a framework for understanding the development of therapeutics for thyroid cancers, it is useful to examine both genetic changes and dysregulated signaling pathways. Genetic mutations common in thyroid cancers are summarized in Table 1, whereas primary interactions between pathways are depicted in Figure 2.9–12 Mutations in thyroid cancers are common, heterogeneous, and vary by histotype. Most mutations in thyroid cancers are sporadic, but germ line mutations are frequent in hereditary cancers. Examples of germ line mutations include the PTEN mutation seen in Cowden’s syndrome-related follicular thyroid cancers, and the RET protoconcogene mutations seen in MEN2-associated MTCs and in FMTCs. Other mutations, deletions, and changes in gene expression are frequent in the three major thyroid cancer sub-types. Some of these are more histotype-specific (e.g. BRAFV600E mutation occurring most commonly in PTC) while others, such as activating mutations in epidermal growth factor receptor (EGF-R) and RAS, occur across histotypes.
TABLE 1.
| Endogenous Differentiated Thyroid Cancer-Specific Signaling Pathways | |||||
| Pathway | Histotypes | Cellular Effects | Therapeutic approach | ||
| Sodium iodide symporter (NIS) | DTC | ↑ Proliferation | Radioiodine | ||
| Thyrotropin/Thyroid Stimulating Hormone (TSH) | DTC | ↑ Proliferation | Suppressive dosage levothyroxine | ||
| Mutations in Thyroid Cancers | |||||
| Pathway | Activating/Inactivating | Histotypes | Prevalence | Cellular Effects | Targeted agents |
| RET, RET/PTC | Activating | MTC PTC |
>60% 25% |
↑Proliferation | Vandetanib |
| BRAFV600E | Activating | PTC ATC |
45% 26% |
↑ Proliferation | AZD6244, GSK1120212, GSK2118436 |
| PAX8/PPARγ | Disputed (activating or inactivating) | FTC | 45% | (complex) | Rosiglitazone. RS5444 |
| P53 | Inactivating | ATC | 55–70% | ↑ Proliferation | rAd-p53 |
| RAS | Activating | PTC FTC ATC |
10% 45% 22–55% |
↑ Proliferation | |
| PTEN | Inactivating | ATC | 12% | ↑ Proliferation | |
| EGF-R | Activating | PTC MTC ATC |
15% 35% (mets) 80% |
↑ Proliferation | Cetuximab, erlotinib, gefitinib, panitumumab |
| PI3K | Activating | ATC | 17% | ↑ Proliferation | NVP-BEZ235 |
| Axin-1 | Inactivating | ATC | 82% | ↑ Proliferation | |
| APC | Inactivating | ATC | 9% | ↑ Proliferation | |
| Additional Altered Signaling Pathways in Thyroid Cancers | |||||
| Pathway | Alteration | Histotypes | Cellular Effects | Targeted agents | |
| VEGF/VEGF-R | Up | PTC, FTC, MTC, ATC | ↑ Proliferation | Sunitinib, sorafenib, pazopanib | |
| mTOR | Up | ↑ Proliferation | Everolimus, Temsirolimus | ||
| β-Catenin | Up | ATC | ↑ Proliferation | ||
| Aurora A, B | Up | ATC | ↑ Proliferation | MLN8237 | |
| RAR, RXR | PTC, FTC | ↑ Proliferation | Acitretin, Bexarotene, retinoic acid | ||
| Somatostatin | Up | MTC, PTC | ↓ Proliferation | Octreotide/Lanreotide and radiotherapeutic conjugates | |
| IGF-R | Up | ↑ Proliferation | AG38A, AG1024, figitumumab, NVP-AEW541 | ||
Figure 2.
Predominant signaling pathways in normal thyroid cells and in thyroid cancers.
In parallel with the discovery of genetic mutations in thyroid cancers, much has been learned about aberrations in classical signal transduction that occur largely independent of known mutations. Examples of deregulated pathways are the VEGF/VEGF-R, PI3K/mTOR, B-catenin, aurora kinase, somatostatin and IGF-R pathways. Recent evidence suggests that tumorigenic stem cells may initiate follicular, papillary and anaplastic thyroid cancers.13Consequently, embryonic signal transduction pathways may also be important in thyroid cancers; thus, re-expression and up-regulation of non-classical/developmental signaling pathways may additionally contribute to thyroid cancer pathogenesis. Some mutations or dysregulated and aberrant signal transduction pathways appear to have a pathogenic role in neoplastic transformation, while others appear to occur after neoplastic transformation and therefore likely, instead, contribute primarily to cancer progression.
4.0 THERAPEUTIC APPROACHES TARGETING “UNIQUE” ENDOGENOUS FOLICULAR THYROID CELL SIGNALING PATHWAYS
In DTC not amenable to surgical cure, two endogenous follicular thyroid cell signaling pathways can be often readily exploited to therapeutic advantage. These pathways, the TSH pathway and the NIS pathway, deserve special attention because they are targets for the treatment of both early stage and advanced differentiated thyroid cancers. Thyrotropin, also known as TSH (thyroid stimulating hormone), often stimulates follicular cell proliferation in early DTC. To offset this effect, administration of levothyroxine, at high enough doses to suppress TSH levels below <0.1 mIU/L, has become standard-of-care initial treatment for patients with metastatic DTC. Therapies which target the TSH pathway by mechanisms other than inducing TSH suppression with levothyroxine have not yet been brought to the clinic, yet may hold promise. For example, loss of the TSH receptor (TSH-R loss) has been associated with a more aggressive DTC phenotype - and TSH-R reconstitution has been shown to slow DTC cell line growth in vitro and in vivo,14 thereby providing preliminary preclinical evidence supporting the future clinical translation of this approach.
As noted, most DTCs, at least initially also express the sodium iodide symporter, NIS, a thyroid follicular cell-specific transporter that results in intracellular accumulation of iodine. As a result, radioiodine (RAI) has also become a standard-of-care initial therapy in advanced DTC. After multiple recurrences, however, DTCs commonly become RAI-resistant, necessitating consideration of alternative approaches as reviewed below. MTCs and ATCs are neither regulated by TSH nor express functional NIS, making potential TSH suppressive and RAI therapy irrelevant. An effort to induce NIS expression in MTC, however, using appropriately engineered viral therapeutics, appears promising. Pre-clinical studies with a NIS-expressing adenovirus have shown that re-induction of NIS expression is possible. Proof of principle was demonstrated in vitro and in vivo; xenografts of RAI-refractory MTC cells were virally transfected and subsequently treated with RAI - with anti-tumor effects demonstrated.15 Several viral therapies which target increasing NIS are now also in clinical trials in non-thyroid cancers.16,17 Consequently, there is ongoing interest in the future translation of this approach to both MTC and in RAI-resistant DTC.
5.0 KINASE INHIBITORS AS THYROID CANCER THERAPEUTICS
Advances in the understanding of molecular events contributory to the pathogenesis and progression of thyroid cancers, as summarized above, have led to the hypothesis that inhibition of several different kinases may have therapeutic utility in these cancers. Whereas inhibition of some kinases such as vascular endothelial growth factor receptors 1–3 (VEGF-R1-3) might be predicted to be helpful in treating multiple histologies, inhibition of others (e.g. RET, BRAF) would seem instead perhaps to have more histotype/mutation-specific effects.
Table 2 lists, in alphabetical order, tyrosine and other kinase inhibitors which are currently or will soon be in clinical trials; data about these agents are presented in the table and discussed in the text. These agents each inhibit multiple kinases in pre-clinical models, as indicated in Table 2, but are active against fewer kinases when given to humans at clinically-relevant/achievable dosages, as described in the text. The toxicity profiles of these inhibitors, like the kinases which they inhibit, overlap considerably. Most of these agents cause fatigue, hypertension, nausea/vomiting, skin rash and/or hand-foot syndrome; they can also cause elevations of transaminases, pancreatic enzymes and thyroid stimulating hormone (TSH). Nonetheless, because each uniquely inhibits kinases, treatment-emergent adverse events vary by agent. Moreover, host-related factors also seem to contribute to differences in inter-patient tolerability. In the text which follows, emphasis is placed upon those TKIs which the authors believe to have the most compelling preliminary data in support of therapeutic promise in thyroid cancers.
TABLE 2.
Kinase Inhibitors: Investigational Agents and Results of Clinical Trials
| Agent | Manufacturer | Targets | ORR (%) | SDR (%) |
PFS/OS (mos) |
References | |
|---|---|---|---|---|---|---|---|
| Axitinib (AG-013736) | Pfizer | VEGF-R1-3, PDGF-Rβ, c-Kit | |||||
| phase 2 advanced (n=30 PTC, 15 FTC, 11 MTC, 2 ATC, 2 other) | DTC=31% MTC=18% ATC=50% |
DTC=42% MTC=27% ATC=0% |
18.1/na | 18 | |||
| AZD6244 (selumetinib) | AstraZeneca | MEK1/2 | |||||
| phase 2 RAI-resistant progressive PTC (n=32) | Overall=3% | Overall=66% | 13.4/na | 19 | |||
| Cediranib (Recentin, AZD2171) | AstraZeneca | VEGF-R1-3, PDGF-Rα/β c-Kit | |||||
| CEP-751 | Cephalon | RET, FLT3, TrkA-C | |||||
| E7080 | Eisai | VEGF-R1-3, PDGF-Rβ, c-Kit, FGF-R, SCF-R | |||||
| phase 1 incl. TC (preliminary)(n=?) | 3 PRs | 28 | |||||
| Gefitinib (Iressa, AZD1839) | AstraZeneca | EGF-R | |||||
| phase 2, advanced, RAI failure (n=11 PTC, 6 FTC, 4 MTC, 5 ATC, 1 HTC) | Overall=0% | Overall=48% | 3.7/ 17.5 | 4 | |||
| Imatinib (Gleevec, STI571) | Novartis | RET, Bcr-abl, c-Kit, PDGF-Rβ | |||||
| phase 2 metastatic MTC (n=5) | MTC=0% | MTC=80% | 5 | ||||
| phase 2 metastatic progressive MTC (n=8) | MTC=0% | MTC=88% | 6/na | 6 | |||
| Lestaurtinib (CEP-701) | Cephalon | RET, FLT3, JAK2, TrkA-C | |||||
| Motesanib (AMG 706) | Amgen/Takeba | VEGF-R1-3, RET, PDGF-Rβ, c-Kit, FLT3, JAK2, TrkA-C | |||||
| phase 1 (n=2 PTC, 1 FTC, 1 MTC, 1 ATC, 1 HTC, 1 F/PTC) | PTC=50% FTC=100% MTC=100% (others=0%) |
HTC=100% FTC=100% others=0% |
7 | ||||
| phase 2 advanced, progressive, RAI-resistant DTC; 57 PTC, 15 FTC, 17 HTC, 4 other | Overall=14% PTC=12% FTC=17% |
DTC=67% | 10/na | 22 | |||
| phase 2 advanced, progressive, RAI-resistant MTC; 91 eval pts | Overall=2% | Overall=81% | 12/na | 23 | |||
| Pazopanib (Votrient, GW786034) | GlaxoSmithKline | VEGF-R1-3, PDGF-Rα/β, c-Kit | |||||
| phase 2 progressive, RAI-resistant DTC, MTC, ATC (n=37 DTC) | Overall=49% FTC=73% PTC=33% HTC=45% |
11.7/na | 24 | ||||
| PLX4032 (RO5185426) | Roche/Plexxikon | BRAF (V600E mut > wt) | |||||
| phase 1 solid tumor; 3 PTC w/BRAF mut (preliminary) | PTC=33% | PTC=67% | 8+ months | 26 | |||
| PP1, PP2 (pyrazolo-pyrimidines) | TKI: Src family kinases (LCK, CSK, p38, p38β2, CK1δ), mutant RET (C634R, C634R/V804G) > RET | ||||||
| RPI-1 | Cell Therapeutics | RET, c-Met | |||||
| Semaxanib (SU5416) | Pharmacia / SUGEN | VEGF-R2, c-Kit, c-Kit mutants (D814Y, D814V, D818Y) | |||||
| Sorafenib (Nexavar, BAY 43-9006) | Bayer/Onyx | VEGF-R2-3, RET, PDGF-Rβ, BRAF (wt and V599E mut), c-Kit, FGF-R1, p38 | |||||
| phase 2 metastatic, RAI-resistant (n=41 PTC, 11 FTC/HTC, 4 ATC) | PTC=15% FTC/HTC=0% ATC=0% |
PTC=61% FTC=82% ATC=25% |
PTC=10-16/na FTC=4.5/na | 27, 28, 29 | |||
| phase 2 metastatic or advanced MTC (n=15 sporadic, 5 hereditary) | MTC(sporadic)=6% MTC(hereditary)=20% |
sporadic=88% hereditary=80% |
17.9/na | 30 | |||
| phase 2 progressive post-RAI, metastatic DTC (n=13 PTC, 15 FTC, 4 other) | Overall=25% PTC=22% FTC=49% other=67% |
Overall=34% PTC=67% FTC=36% other=0% |
14.5/na | ||||
| pilot metastatic MTC (n=5) | MTC=40% | MTC=60% | 31 | ||||
| phase 2 ATC post-chemoTx (n=15) | ATC=13% | ATC=27% | 5.1/na | 27, 32 | |||
| phase 2 metastatic, progressive, RAI-resistant (n=18 PTC, 9 FTC/HTC, 1 MTC, 2 ATC) | Overall=23% PTC=22% FTC=33% MTC=0% ATC=0% |
Overall=53% PTC=61% FTC=44% MTC=100% ATC=0% |
17.9/na | ||||
| phase 1 + tipifarnib in solid tumors incl. MTC (n=6) (preliminary) | Overall=50% | ? | 33, 34 | ||||
| phase 2 + tipifarnib; advanced TC, metastatic DTC (n=22 DTC, 13 MTC) | DTC=4.5% MTC=38% |
DTC=36% MTC=31% |
18/na | 33, 34 | |||
| Sunitinib (Sutent, SU011248) | Pfizer | VEGF-R1-3, PDGF-R, c-Kit, RET, FLT3, CSF-1R | |||||
| phase 2 progressive/refractory post chemoTx, surgery, or RAI (n=31 DTC, 23 MTC) | DTC=14% MTC=35% |
DTC=68% MTC=57% |
38, 39 | ||||
| phase 2 progressive/refractory post-RAI (n=12 PTC, 8 MTC) | PTC=8% MTC=12% |
PTC=67% MTC=88% |
33, 40 | ||||
| phase 1 solid tumor, (n=1 PTC [post-RAI], 1 FTC [RAI-res.], both progressive, metastatic) | PTC=100% FTC=0% |
PTC=0% FTC=100% |
|||||
| Vandetanib (Zactima, ZD6474) | AstraZeneca | VEGF-R1-3, RET, EGF-R | |||||
| phase 2 advanced hereditary MTC (w/ RET germline mut) (n=30) | MTC=20% | MTC=53% | 27.9/na | 41 | |||
| phase 2 advanced hereditary MTC (RET germline mut) (n=19) | MTC=16% | MTC=32% | 42, 43 | ||||
| phase 3 advanced MTC, hereditary or sporadic (n=231) | MTC=45% | 20.5 months | 44 | ||||
| Vatalanib (PTK787, ZK222584) | Bayer Schering/Novartis | VEGF-R1-3, PDGF-Rβ, c-Kit, cFMS | |||||
| XL184 (cabozantinib) | Exelixis | VEGF-R2, RET, MET | |||||
| phase 1 advanced MTC (n=37) | MTC=29% | MTC=51% | 45 | ||||
| XL281 | Exelixis | BRAF (wt and V600E mut) | |||||
| phase 1 FTC (n=10) (preliminary) | FTC=0% | FTC=20% | 27 | ||||
KEY:
na = not assessed
ORR = Overall Response Rate
SDR = Stable Disease Rate
PFS/OS = Progression-free Survival/Overall Survival
5.1 Axitinib (AG-01376, Pfizer) is an inhibitor of VEGF-R-1-3. A non-randomized phase 2 trial in patients with advanced thyroid cancers demonstrated activity and reasonable tolerability.18 Response rates in patients with differentiated thyroid cancer (papillary thyroid cancer, n=30; follicular cancer, n=15) and medullary thyroid cancer (n=11) were 31% and 18% respectively. One of two patients with anaplastic thyroid cancer also had a response. The stable disease rates were 42% in DTC and 27% in MTC. Common adverse events were fatigue (50%), diarrhea (48%), nausea (33%), hypertension (28%), mucositis (25%), weight loss (25%), vomiting (13%), and hand-foot syndrome (15%); toxicities were primarily grade 1–2.
5.2 AZD 6244 (ARRY-142886, selumetnib, Astra-Zeneca) is the first MEK (mitogen-activator protein kinase) 1/2 inhibitor to be tested in thyroid cancer. In a phase 2 trial in patients with RAI-resistant PTC (n=32,) overall response rate was 3% and stable disease was documented in 66%; median progression free survival (PFS) was 13.4 months,19 comparing favorably with PFS achieved in response to inhibitors of VEGFRs. Adverse events included rash (69%), fatigue (49%), diarrhea (49%), and peripheral edema (36%), some of which were grade 3–4. In phase 1 evaluation, transient blurred vision was additionally noted at higher doses.19, 20, 21
5.3 Motesanib (AMG 706, Amgen/Takeva) inhibits VEGFR1-3 and platelet-derived growth factor receptors alpha/beta (PDGF-Rα/β) apparently exclusively at relevant clinical doses. In a phase 2 trial in patients with advanced or metastatic progressive RAI-resistant differentiated thyroid cancer (57 papillary, 15 follicular, 17 Hürthle, 4 other), the overall response rate was 14% and the stable disease rate was 67%.22 In a phase 2 trial in advanced or metastatic progressive medullary thyroid cancer, the overall response rate was only 2%, but the stable disease rate was 81%; median PFS was 12 months.23 Toxicities included diarrhea (59%), hypertension (56%), fatigue (46%), weight loss (40%), abdominal pain (30%), nausea (28%) and hemorrhage (14%); grade 3–4 events were hypertension (25%) and diarrhea (13%).
5.4 Pazopanib (GW786034, Votrient, GlaxoSmithKline) predominantly inhibits VEGF-R1-3, PDGF-Rα/β and cKit. A phase 2 trial in RAI-resistant differentiated medullary and anaplastic thyroid cancers is ongoing. Results from a cohort of patients with differentiated thyroid cancer were recently published;24 pazopanib administration resulted in a 49% overall response rate (73%, 33% and 45% in FTC, PTC and HCC, respectively, albeit response by histological subtype was not a pre-specified end point). A positive correlation between higher plasma pazopanib levels in cycle 1 and response was also observed; this finding will likely lead to another trial in which pazopanib doses will be individualized according to plasma PK levels (personal communication, Dr. Keith Bible). Commonly observed adverse events were fatigue, diarrhea, hypertension, and transaminase elevations. Profound treatment-emergent hypopigmentation, presumably a reflection of dual cKit and PDGF-R/PDGF pathway inhibition, was observed in some patients.25 Unpublished data, from an interim analysis, showed that pazopanib also exibited clinical activity in MTC, but produced only transient clinical benefit in ATC (Keith Bible, personal communication).
5.5 PLX4032 (RO5185426, Roche, Plexicon) inhibits both wildtype and V600-mutated BRAF, the latter with greater potency in humans. In a phase 1 dose escalation study which included 3 patients with PTC and 49 patients with melanoma, all 3 PTC patients responded. Progression-free survival was eight or more months in the patients with PTC. Frequent grade 2 and 3 adverse events were arthralgia, fatigue and rash.26
5.6 Sorafenib (Nexavar, BAY43-9006, Bayer-Onyx) inhibits VEGFR2-3, RET, c-Kit, fibroblast growth factor receptor 1 (FGF-R1) and p38 in humans at relevant doses. Although the clinical effects of sorafenib on BRAF inhibition were initially believed to be prominent, this is now uncertain. Its broad-spectrum anti-kinase activity has nevertheless spurned multiple phase 2 clinical trials. In 3 phase 2 trials in patients with metastatic RAI-resistant thyroid cancer, overall response rates seemed to vary by histologic subtype, as in the case of pazopanib.27,28, 29 Responses were seen in 15–22% of patients with PTC and in 31–49% of patients with FTC. In one trial, 6 of 41 patients with PTC (15%) attained a PR and 23 (56%) had stable disease lasting >6 months; patients with other histotypes of thyroid cancer did not have responses. In DTC, mean thyroglobulin levels decreased by >25%. Paired tumor biopsies were obtained in ten patients with this histology; 4 (40%) demonstrated reductions in VEGF-R and ERK phosphorylation as well as in VEGF expression. Most adverse events were grade 1–2; grade 3 adverse events included hand-foot syndrome, musculoskeletal pain, and fatigue.27
Two small trials also demonstrated sorafenib efficacy in medullary thyroid cancer. In the first, 15 patients with sporadic and 9 with hereditary MTC were evaluable;30 1 patient with sporadic and 2 patients with hereditary MTCs experienced PRs. 88% of patients with sporadic medullary cancer and 80% of patients with hereditary medullary cancer had stable disease; overall progression-free survival was an encouraging 17.9 months. In the second pilot trial, 2 of 5 MTC patients had responses.31
Despite promising results in DTC and MTC results, sorafenib, like pazopanib, has been disappointing in ATC. In that population, 13% attained PRs and 27% had stable disease. Adverse events included significant cardiovascular toxicity,27 grade 3–4 lymphopenia, rash, weight loss, chest pain and dyspnea.32
Sorafenib in combination and tipifarnib, a farnesyl transferase inhibitor, has also been evaluated in patients with differentiated and medullary thyroid cancer,33,34 but results will not be presented in this paper as further clinical development of tipifarnib is unlikely.35
Treatment-emergent keratoacanthosis and cutaneous squamous cell carcinoma have been documented in patients who have received sorafenib. There is uncertainty about whether this may be due to primary treatment effect (e.g. BRAF or other kinase inhibition); improved detection due to heightened surveillance; induced skin pigmentation changes with photosensitization or perhaps a combination of these factors.36, 37 These adverse events have also been observed in patients who have received Exelixis’s XL-281, a specific inhibitor of wild-type and mutant BRAF. Keratocanthosis and squamous cell carcinoma of the skin have also occurred in patients treated with other TKIs which do not inhibit BRAF.
5.7 Sunitinib (Sutent, SU011248, Pfizer) inhibits VEGF-R1-3, c-Kit and PDGF-Rα/βat doses administered in humans. It has been evaluated in phase 2 trials in patients with progressive refractory DTC and MTC, with overall response rates of 14% and 35% and stable disease rates of 68% and 57%, respectively.38, 39 In a small trial of sunitinib in 12 patients with progressive PTC or MTC33,40 responses were also seen. Common adverse events were fatigue, diarrhea, hand-foot syndrome, neutropenia and hypertension (which was often severe).38
5.8 Vandetanib (Zactima, ZD6474, AstraZeneca) inhibits VEGF-R1-3, RET and EGF-R at clinically relevant doses. In a phase 2 trial in advanced MTC with RET germ line mutations (MEN2A or MEN2B, n=30), overall response rate was 20% and stable disease rate was 53%.41 In another phase 2 trial, the overall response rate was 16%, but the stable disease rate was 32%.42, 43 In a phase 3 registration trial, 231 patients with MTC were randomized to receive vandetanib or placebo; overall response rate in vandetanib-treated patients was 45%, with progression-free survival dramatically improved in patients in comparison to placebo-treated patients.44, Severe toxicities included QTc prolongation, rash and diarrhea; less severe, but more common, toxicities included fatigue, nausea, hypertension, and low-grade rash or diarrhea.42 On the basis of these results, vandetanib has recently been approved by the U.S. FDA for use in advanced, progressive and symptomatic MTC.
5.9 XL184 (Carbozantinib, Exelixis) inhibits VEGF-R2, RET and MET in humans at relevant doses. A phase 1 dose-finding study enrolling patients with various cancers included an expansion cohort of 37 patients with MTC. Almost all patients in the expansion cohort had some tumor shrinkage, with 29% attaining a partial response.45
6.0 OTHER AGENTS WITH ACTIVITY IN THYROID CANCER
In addition to tyrosine kinase inhibitors, several additional classes of pharmaceuticals have demonstrated efficacy in thyroid cancers, while others seem rationale candidates for future clinical development. Many of the latter are now being evaluated in clinical trials. In Table 3, we provide a list of these classes and subclasses, enumerating important drug(s) in each class and supplying basic information about them; further data about selected agents are presented additionally in the text below. We focus on angiogenesis inhibitors, histone deacetylase inhibitors, nuclear receptor agonists (i.e. PPARγ receptor and retinoid receptor agonists,) mitosis inhibitors and novel tumor vasculature inhibitors.
Table 3.
Other Investigational Agents: Results of Clinical Trials
| Class | Agent | Manufacturer | Mechanisms/Targets |
|---|---|---|---|
| Angiogenesis Inhibitors | Thalidomide1,3,4 (Thalomid) | Celgene | induces apoptosis; angiogenesis inhibitor (VEGF, βFGF); inhibits IL-6 expression |
| *Lenalidomide1,3,4 (Revlimid) | Celgene | induces apoptosis; inhibits angiogenesis; inhibits IL-6 expression | |
| Epigenetic Modulating Agents: Histone Deacetylase Inhibitors | Romidepsin1,3,4 (depsipeptide) | Celgene | HDAC inhibitor |
| Valproic acid1,3,4 (divalproex, depakote) | (generic/various) | HDAC inhibitor | |
| Vorinostat1,3 (Zolinza, SAHA) | Merck/Patheon | HDAC inhibitor | |
| LBH-5894 (Panobinostat) | Novartis | HDAC inhibitor, Notch inducer | |
| Epigenetic Modulating Agents: Hypomethylating Agents | Azacytidine1,3 (5-azacytidine, Vidaza) | Celgene | hypomethylating agent |
| Decitabine 1,4 (Dacogen) | Eisai | hypomethylating agent | |
| Nuclear Receptor Agonists: PPARγ | Rosiglitazone1,4 (Avandia) | GSK | PPARγ agonist |
| RS54443 | Sankyo | PPARγ agonist | |
| Nuclear Receptor Agonists: Retinoid | Bexarotene1,4 (Targretin) | Eisai | binds retinoid X receptors (RXRs), induces apoptosis |
| Mitosis Inhibitors: Inhibitors of Disassembly | *Paclitaxel1,3,4 (Taxol) | Bristol-Myers | stabilizes tubulin polymers, inhibiting disassembly; binds Bcl-2 and promotes apoptosis |
| *Docetaxel1,3,4 (Taxotere) | Sanofi-Aventis | disrupts microtubule assembly, inhibits disassembly | |
| Mitosis Inhibitors: Inhibitors of Assembly | Fosbretabulin3,4 (combretastatin A-4-P) | Oxygene | disengages VE-cadherin, depolymerizes tubulin |
| AKT Pathway Inhibitors: PI3K and mTOR Inhibitors | NVP-BEZ2355 | Novartis | dual PI3K/mTOR inhibitor |
| AKT Pathway Inhibitors: mTOR.TORC1 Inhibitors | Temsirolimus1,4 (Torisel, CCI-779) | Wyeth/Pfizer | mTOR inhibitor |
| Everolimus1,4 (Afinitor, Certican, Zortress) | Novartis | mTOR inhibitor | |
| Alkylating Agents | *Carboplatin1,3,4 (Carboplatin Hexal, paraplat, paraplatin) | (generic) | alkylating agent |
| *Cisplatin1,3,4 | (generic) | alkylating agent | |
| Antimetabolites | Capecitabine1,3,4 (Xeloda) | Roche | prevents pyrimidine biosynthesis |
| Pemetrexed1,4 (Alimta) | Eli Lilly | folate antimetabolite, inhibits purine/pyrimidine biosynthesis | |
| Topoisomerase Inhibitors | *Doxorubicin1,2,3,4 (Adriamycin, hydroxydaunorubicin) | (generic/ various) | topoisomerase II inhibitor and DNA intercalator |
| Irinotecan1,3 (Camptosar, CPT-111) | (generic) | topoisomerase I | |
| Unique | Irofulven3 (MGI-114, HMAF, 6-hydroxymethylacylfulvene) | MGI Pharma | Selective inducer of apoptosis |
| Tumor Vasculature Inhibitors | VB-1114 | Vascular Biogenics | targets endothelial cells in tumor vasculature |
| NGR-TNF4 | MolMed | Tumor homing peptide directed against CD13 on tumor blood vessels fused with tumor necrosis factor (TNF); increases vascular permeability, disrupts endothelial adhesion | |
| Proteosome Inhibitors | Bortezomib1,4 (Velcade, PS-341) | Millennium/Takeda | inhibits 26S proteasome, which in turn disrupts NF-kB-mediated cell survival |
| Heat Shock Protein Inhibitors | 17-AAG3 (Tanespimycin, 17-allylamino-17-demethoxygeldanamycin) | Kosan | HSP90 inhibitor |
| Monoclonal Antibodies | Yttrium Y 90 Monoclonal Antibody MN-14 (90Y-MN-14)3 | antibody against tumor-associated carcinoembryonic antigen (CEA) | |
| Radioimmunotherapy | CEA-131I-based radioimmunotherapy3 | antibody against tumor-associated carcinoembryonic antigen (CEA) labeled with iodine-131 | |
| Cancer Vaccines | Dendritic cell vaccine3,4 | Natural Killer (NK) cell activator | |
| Ras peptide vaccine3 | ras mutant peptide-directed inhibitor | ||
| Immune Modulators | aldesleukin3 (Proleukin, recombinant IL-2) | Prometheus | Cytokine immune activator |
| Sargramostim1,4 (GM-CSF, Leukine) | Bayer | improves function of antigen presenting cells by activation/recruitment of dendritic cells, macrophages, monocytes | |
| Interferon α-2b1, 3 (Intron A) | (various) | Cytokine immune activator |
In general clinical use for thyroid cancer (off-label or on-label)
FDA-approved for use in non-thyroid cancer(s) or benign condition(s)
FDA-approved for use in thyroid cancer
Completed single agent phase 1b or phase 2 testing in thyroid cancer
In ongoing clinical trials in thyroid cancer
Not yet in clinical trials in thyroid cancer/preclinical data available
6.1 Angiogenesis Inhibitors (Other Than TKIs)
Multiple angiogenesis inhibitors have been evaluated in thyroid cancers, with several trials involving thalidomide or lenalidomide. Thalidomide (Celgene) was reported to have anti-angiogenic properties in 1994;46 it blocks vascular endothelial factor, modulates tumor necrosis factor alpha, inhibits the actions of basic fibroblast growth factor and alters cytokine production and activity. Although its actions in cancer are believed to relate to its inhibition of angiogenesis, some mechanisms underlying its antineoplastic activity are incompletely defined. Thalidomide was evaluated in a phase 2 trial of 28 patients with advanced or metastatic RAI-unresponsive DTC, MTC, and a few uncommon histological subtypes of thyroid cancer; the overall response rate was 19%, and stable disease was attained in 32%. Common adverse events were, in descending order of frequency, fatigue, hypersomnolence, peripheral neuropathy, constipation, dizziness and vasovagal episodes.47
Lenalidomide (Celgene), a less neurotoxic derivative of thalidomide, was also evaluated in patients with metastatic, progressive RAI-unresponsive thyroid cancer;48 22% of patients had a tumor response and 44% had tumor stabilization. The adverse event profile included grade 3–4 thrombocytopenia, neutropenia and pulmonary emboli.
6.2 Epigenetic Modulating Agents
Epigenetic changes are frequent in thyroid cancers, providing a rationale for testing histone deacetylase inhibitors and hypomethylating agents in these malignancies. Histone deacetylase inhibitors (HDACIs) alter the chromatin acetylation state, modulating transcription of the ~2% of the human genome regulating growth, differentiation and apoptosis.33 Hypomethylating agents can lead to re-expression of previously methylated/repressed genes in thyroid cancers, potentially thereby altering thyroid cancer pathogenesis and progression.
6.2.1 Histone Deacetylase Inhibitors
The HDACIs valproic acid, vorinostat and, more recently, romidepsin, have been evaluated in thyroid cancers. Valproic acid, an anti-epileptic and mood-stabilizing drug, was tested in thyroid cancer cell lines in the mid-2000’s and found to modulate cell growth.49, 50 More recently, it was shown to inhibit tubulin acetylation and potentiate the activity of paclitaxel in an anaplastic thyroid cell line.51
Vorinostat (Merck), which inhibits all histone deacetylase sub-types, was evaluated in a phase 2 trial in metastatic, RAI-refractory DTC. Although there was a 71% stable disease rate, there were no objective responses. Drug-related adverse events included fatigue, dehydration, ataxia, thrombosis and thrombocytopenia. Further clinical trials with Vorinostat monotherapy are deemed unlikely.52
Romidepsin (Celgene,) a cyclic peptide HDACI, selectively inhibits 4 histone deacetylase sub-types, induces cellular differentiation, causes cell cycle arrest, depletes heat shock protein-90-dependent oncoproteins and is anti-angiogenic. In a phase 1 trial, 6 of 9 patients with RAI-refractory thyroid cancer had disease stabilization, but none responded and none had evidence of treatment-induced enhancement of RAI-uptake. Adverse events were primarily hematologic, but nausea and vomiting were also frequent.53 In a phase 2 trial, 10 of 20 patients with progressive metastatic DTC had tumor stabilization but no responses were observed. Overall survival, however, was 36 months. Significant cardiac toxicity and thromboembolism were observed, including one grade 4 pulmonary embolism.54
Panobinostat (LBH-589, Novartis) is a newer histone deaceylase inhibitor55 which has the unique additional property of upregulating the Notch pathway.56 Notch is an interesting candidate molecular target in MTC, as it is downregulated in MTC, with antiproliferative effects noted when its expression is restored.57 In a phase 1 trial in advanced solid tumors, 6 of 13 patients had stable disease, but no responses were seen. Increased acetylation was observed even at the lowest dose tested, indicating that the intended target had been affected despite low clinical activity. Adverse events included prolonged grade 2 thrombocytopenia, grade 3 neutropenia, anemia and hypoglycemia.58 Collectively, results from HDAC inhibitor monotherapy in thyroid cancers have, unfortunately, been disappointing.
6.2.2 Hypomethlyating Agents
The hypomethylating agents azacytidine and decitabine have also been evaluated in thyroid cancers. In some cell lines, 5-azacytidine (Celgene) reversed NIS methylation, caused re-expression of NIS and increased radioiodine uptake.59 A phase 1 trial of 5-azacytidine in patients with RAI-unresponsive thyroid cancer was conducted, but the results were never presented in manuscript form. Clinical trials of decitabine (SuperGen), a less toxic and more potent hypomethylating agent than azacytidine, are ongoing.
6.3 Nuclear Receptor Agonists
Two types of nuclear receptor agonists, peroxisome proliferator-activated receptor gamma (PPARγ) agonists and retinoid receptor agonists have generated interest among thyroid cancer researchers.
6.3.1 PPARγ Agonists
Peroxisome proliferator activated receptor (PPAR) is a nuclear receptor with three isoforms; the PPARγ isoform in particular is known to regulate in cellular growth and differentiation. PPARγ agonist treatment of thyroid cancer lines has resulted in growth inhibition and apoptosois,60 and combination with paclitaxel has demonstrated in vivo efficacy in ATC models.61
Rosiglitazone (GlaxoSmithKline) has been evaluated in 17 patients with metastatic RAI-resistant DTC. Although none of the patients had a response, the stable disease rate was 46% with evidence of increased RAI-uptake in several patients.62
In pre-clinical evaluation, RS5444 (Sankyo) and paclitaxel were synergistic in vivo.61 This observation led to a phase 1 dose-escalation trial of the combination in patients with ATC.63 One patient, in that trial, had a sustained tumor response. Furthermore, patients who received the higher doses of the RS5444 and paclitaxel had longer time to tumor progression (TTP) than did patients who received lower doses. By far, the most common and concerning drug-related adverse events were fluid retention and edema. These preliminary data suggest that further clinical trials of PPARγ agonists and taxanes in combination may be warranted in ATC.
6.3.2 Retinoid Receptor Agonists
Research related to retinoids, specifically retinoic acid, has highlighted the importance of retinoid signaling in thyroid cancers. Two clinical trials with retinoic acid showed that it could restore RAI-sensitivity in thyroid tumors. These trials provided proof of principle that retinoid signaling plays a role in NIS expression and RAI-uptake. In a study of 53 patients with RAI-insensitive DTC, retinoic acid increased RAI uptake in 9 (17%).64 In another study, 16 patients whose tumors no longer had RAI-uptake, as documented by post-RAI treatment scans, were treated with retinoic acid followed by RAI; 3 patients had tumor responses and another 4 patients had disease stabilization; PFS was 26.5months.65 In addition to its affect on NIS/RAI-uptake, retinoic acid may decrease thyroid cell proliferation via another mechanism, attenuation of VEGF secretion.66
In part based on the above observations, nuclear retinoid X receptor (RXR) and nuclear retinoid receptor A (RAR) agonists have also generated interest as potential thyroid cancer therapeutic agents. In particular, bexarotene (Targretin, Eisai), a synthetic RXR agonist, was evaluated in 11 patients with RAI-unresponsive recurrent/metastatic thyroid cancer; there were no clinical responses. SPECT (single proton emission computed tomography) imaging demonstrated treatment-induced increases in tumor RAI uptake in 8 patients; the significance of this, however, is called into question because other imaging techniques failed to consistently confirm the SPECT imaging findings.67 A phase II trial of bexarotene in poorly differentiated thyroid cancer is ongoing (clinicaltrials.gov identifier NCT00718770).
6.4 Mitosis/Microtubule Inhibitors
While targeted agents have largely supplanted cytotoxic chemotherapy for treatment of advanced DTC, cytotoxic chemotherapy is still being evaluated for treatment of ATC and sometimes MTC. Cisplatin, doxorubicin, etoposide, peplomycin - alone or in combinations, with or without radiation therapy - have historically demonstrated only modest activity in advanced ATC. In this section, only agents which inhibit mitosis by inhibition of microtubule disassembly (paclitaxel and docetaxel) and/or assembly (fosbretabulin) are discussed, as they have been the focus of most ATC studies to date.
Paclitaxel (Taxol, BMS) monotherapy was assessed in a phase 2 trial in ATC, in which it produced limited and transient responses in 53% of patients.68 Docetaxel (Sanofi-Aventis) has also produced occasional limited responses in advanced ATC.69
More recently, a report suggested that docetaxel in combination with radiotherapy may increase overall survival in patients with locoregionally-confined ATC.70 Another report showed that a regimen containing both a taxane and an anthracycline administered with intensity modulated radiotherapy appeared to dramatically increase overall survival, but this benefit was limited to patients who had loco-regionally confined ATC.71 In a phase 1 trial of high dose docetaxel in combination with high dose gefitinib, a patient with anaplastic thyroid cancer attained a partial response which lasted 4 months; this regimen continues to be tested.72 A randomized phase II trial comparing the effects of paclitaxel ± pazopanib in combination with intensity modulated radiotherapy in ATC is ongoing (RTOG, clinicaltrials.gov identifier NCT01236547).
The dephosphorylated metabolite of fosbretabulin (OXiGENE), a tubulin inhibitor which disrupts both microtubule assembly and disassembly, appears to selectively inhibit growth of proliferating endothelial cells in tumors. In a phase 1 trial, a patient with ATC attained a prolonged response which was maintained for 4 years73 prompting a phase 2 trial in ATC.74 In the phase 2 trial, median overall survival was 4.7 months with 34% of patients alive at 6 months. Adverse events including lymphopenia, headache, tumor pain and QTc prolongation were frequent but generally not severe. A phase II trial assessing paclitaxel + carboplatin ± combretastatin A4 phosphate in advanced ATC has also been undertaken (clinicaltrials.gov identifier NCT00507429) but results have not yet been published.
6.5 PI3 Kinase Pathway Inhibitors
Genetic alterations or mutations of PIK3CA, Ras, and/or PTEN as well as PIK3CA amplifications are common in aggressive PTC as well as in ATC75–78 making targeting the PI3K/PTEN/AKT/mTOR also of potential therapeutic relevance in these cancers. Two mTOR inhibitors, both approved by the US Food and Drug Administration (FDA) for treatment of some non-thyroid cancers, are now being tested alone and in combination in patients with thyroid cancers. Temsirolimus (Wyeth), FDA-approved for the treatment of renal cancer, causes the following adverse events: fatigue, skin rash, stomatitis, anemia, lymphopenia, hyperglycemia and hypophosphatemia. Everolimus (Novartis), FDA-approved for the treatment of renal cancer and ependymal giant cell astrocytoma associated with tuberous sclerosis, causes similar adverse events as temsirolimus but also causes asthenia and cough. NVP-BEZ235 (Novartis) inhibits both mTOR and PI3 Kinase and is also now in thyroid cancer clinical trials.
In some thyroid cancer cell lines, a remarkable synergism was observed between vorinostat and temsirolimus.79 Similarly, synergism has been observed when rapamycin is combined with MEK inhibition, indicating that combinations of mTOR inhibitors and other targeted agents may also have promise in thyroid cancers.80
6.6 Cytotoxic Chemotherapy Other than Taxanes
Doxorubicin is the only cytotoxic agent which the U.S. Food and Drug Administration (FDA) has approved for treatment of thyroid cancer. Unfortunately, the activity of doxorubicin is modest and limited primarily to ATC. In general, cytotoxics have minimal efficacy in DTC and should not be administered alone in these cancers. Some chemotherapeutic regimens, however, have limited activity in MTC. In particular, 2 of 7 patients treated with cyclophosphamide, vincristine and dacarbazine attained durable tumor regression.81 Other regimens used in neuroendocrine differentiation tumors have also shown activity in MTC.82 Although an attractive consideration, combining these regimens with novel agents has proven difficult due to heightened toxicities. For example, a phase 2 trial of doxorubicin in combination with interferon alpha 2b was stopped early because of severe toxicity as well as low response rate; nearly three-fourths of patients developed grade 3–4 neutropenia with fatigue, nausea/vomiting, anorexia, mucositis; neurologic symptoms were also common and severe.83 Significant toxicities have also been encountered in trials combining TKIs and cytotoxics; a notable exception to this is co-administration of paclitaxel and pazopanib, which was shown to be tolerable in a phase 1 trial;84 that combination is now being tested in a phase 2 trial in ATC in combination with radiation therapy (clinicaltrials.gov identifier NCT01236547).
Irofulven (MGI Pharma) is a natural-product derivative which interacts with DNA in a unique way to inhibit DNA synthesis. It was evaluated in a phase 1 trial in multiple neoplasms. A patient with thyroid cancer who participated in the trial had a tumor response, raising the question of whether irofulven should be evaluated in thyroid cancer-specific trials.85
6.7 Tumor Vasculature Inhibitors
Two novel tumor vasculature inhibitors are under investigation in treating thyroid cancers. NGR-TNF is a fusion product of the tumor homing peptide, CNGRC, which targets tumor necrosis factor to CD13 expressing cells, and tumor necrosis factor (TNF). In a phase 1 trial, common toxicities included chills, fever, nausea, constipation, diarrhea, anorexia and hypotension.86 In another phase 1 trial, NGR-hTNF was combined with doxorubicin; the combination had an acceptable toxicity profile and 2 of 15 patients attained partial tumor responses with an additional 9 patients experiencing stable disease.87
Vascular Biogenics-111 (VB-111), is a novel non-replicating adenovirus type 5 construct targeted to endothelial cells, with expression of FAS ligand (and resulting apoptosis) induced in response to TNF stimulation; it is intended to trigger endothelial cell death only in the tumor microenvironment.88 Activity in a patient with PTC was seen in a phase 1 trial of VBL-111. In that trial, there was evidence of improving TTP when patients were given repeated doses of the agent. A phase 2 trial in DTC is also now ongoing (JP Morgan Healthcare Conference, 2010; personal communication, Pamela Harris and Keith Bible).
6.8 Proteosome Inhibitors
Bortezomib (Velcade, Millennium), an inhibitor of the 26s proteasome that disrupts NF-κB-mediated cell survival, is FDA-approved for treatment of multiple myeloma and mantle cell lymphoma and is now in clinical trials in thyroid cancers. Common toxicities are anemia, leucopenia, thrombocytopenia, nausea, diarrhea, vomiting, peripheral neuropathy, fever fatigue and dyspnea.
6.9 Heat Shock Protein (HSP) Inhibitors
17-AAG (Tanespimycin, Geldanamycin) is the only HSP inhibitor studied to date in thyroid cancers. 17-AAG binds to HSP-90 and facilitates its degradation; this, in turn, causes degradation of client proteins, including mutated p53, BcR-Abl, AKT, RAF-1 and B-RAF. The sensitivity of thyroid cancer cells to 17-AAG seems dependent on levels of Hsp90 expression rather than on histologic type per se.89 Pre-clinical data also suggest that it increases RAI uptake by decreasing its efflux.90 The results of the first 17-AAG thyroid cancer trial will be released soon (personal correspondence Keith Bible).
6.10 Other/Additional Emerging Therapeutics
CEA-131 I-based radioimmunotherapy has undergone preliminary evaluation in patients with MTC; treated patients have had an apparent improvement in overall survival relative to historical controls,91 providing incentive for further study of this approach.
Cancer vaccines have also undergone preliminary human testing, primarily in patients with MTC. Dendritic vaccinations, which result in natural killer cell activation, have been administered in the absence of substantive toxicity in MTC. In one trial using this approach in 7 patients with MTC, the overall response rate was 14.3% and the stable disease rate was 57%; in another trial of 10 patients, overall response rate was 30% as was the stable disease rate.92, 93 RAS peptide vaccination is also being tested.
Several immune modulators, including aldesleukin (recently evaluated in solid tumors including thyroid cancers, clinicaltrials.gov identifier NCT00019331), GM-CSF and interferon α-2b have also been preliminarily tested in thyroid cancer with results not yet reported. Several forms of gene therapy are additionally in development for thyroid cancer.94 A favored approach is gene transfer of pro-drug activating enzymes in combination with administration of pro-drugs, which are subsequently converted into suicide-inducing drugs.95
In the future, agents that target the Notch, Hedgehog and WNT embryonic signaling pathways are likely to be tested in thyroid cancer, as they have been implicated as important in thyroid cancer pathogenesis.13
7.0 EXPERT OPINION
For the first time in decades, progress is being made in treating advanced thyroid cancers. Some kinase inhibitors, especially those targeting VEGF-R and/or RET, have already demonstrated preliminary safety and efficacy in advanced differentiated and medullary thyroid cancers and have begun to change clinical practice. This is illustrated by the recent U.S. FDA approval of vandetanib for use in progressive, symptomatic, metastatic MTC. Other investigational agents and regimens have also demonstrated preliminary evidence of efficacy and safety in thyroid cancers, albeit not yet as dramatically as has been the case for kinase inhibitors. Much is still uncertain, however, with regard to the precise extents of benefits and liabilities of these agents. Further, as most patients with advanced DTC and MTC fair well and have overall good prognoses, issues related to patient selection are of particular importance in advancing therapies for these cancers.
Kinase Inhibitors
It is important to emphasize that there are no data yet indicating survival benefit from TKI use in advanced thyroid cancers. Nevertheless, on the basis of encouraging but limited available data oncologists and endocrinologists are increasingly utilizing TKIs to treat advanced thyroid cancers. This phenomena is illustrated by the fact that the number one off-label use of pazopanib is for treatment of thyroid cancer (pazopanib was FDA approved for renal cancer in 2009). We caution, however, that off-label prescribing of TKIs is very likely to impede clinical research and progress and should only be considered after patients have been appropriately encouraged to enroll in high-quality thyroid cancer trials. Indeed, benefit attained from TKI use in thyroid cancers is most often relatively short-lived (months to a few years in duration) and TKI monotherapy does not cure thyroid cancer. Clearly, future clinical trials, and not off-label treatment, are needed to advance our understanding of their roles in these cancers.
As uniform dosing of TKIs across all patients apparently yields quite disparate plasma levels (and therefore response rates) in thyroid cancer patients, optimization of tyrosine kinase monotherapy may additionally be achievable by intra-patient PK-driven dose modification, as is presently being critically evaluated in the case of pazopanib. Hopefully, thyroid cancer therapy will be optimized based upon lessons learned from earlier clinical trials. For example, it appears that responses to some TKIs may be more likely and durable in follicular thyroid cancers in than in papillary thyroid cancers; in the future, it is therefore likely that not only histotypes, but subsets of histotypes, will be important considerations in choosing the most appropriate treatment for individual patients.
Further optimization of monotherapy may ultimately be attained by targeting each patient’s unique tumor mutational profile, not just by targeting tumor histology or histologic subtype. For instance many, but not all, patients with PTC have a therapeutically targetable activating mutation in BRAFV600E; detection of this mutation may therefore help define who is most likely to benefit from agents which inhibit the BRAFV600E. Such an approach, however, necessitates performing tumor pre-therapy biopsies (and sometimes multiple tumor biopsies); these procedures are costly and invasive, can limit enrollment in clinical trials and are often impractical to perform in clinical practice. Nonetheless, this approach seems critical to accomplish the goals of personalized medicine with targeted antineoplastics in the 21st century. Ultimately, performing and analyzing serial biopsies may actually prove not only helpful to patients in terms of anti-tumor activity, but also cost-effective for patients, insurers and the health care system as a whole. In particular, the cost of monotherapy with kinase inhibitors and other novel agents in the United States is currently between $60,000 to $250,000/year/patient, making it an economic necessity to individualize their use.
Other Investigational Agents and Investigational Regimens
Cytotoxic chemotherapy, which was previously thought to have no role in the treatment of DTC and only a minimal role in the treatment of MTC and ATC, has recently re-emerged as of potential therapeutic utility, most especially in combination with intensity modulated radiation therapy in the initial treatment of Stages 4A and 4B ATC. For example, preliminary evidence demonstrating that combining anti-microtubule inhibitors, anthracyclines and intensity modulated radiotherapy appears to substantially improve overall survival of patients with loco-regional ATC should encourage further clinical trials involving cytotoxics. Additional trials combining cytotoxic chemotherapy and novel agent(s) similar to the ongoing RTOG trail examining IMRT + paclitaxel ± pazopanib in ATC (clinicaltrials.gov identifier NCT01236547) are also needed.
A wide array of other agents, such as nuclear receptor agonists, tumor vasculature inhibitors, PI3 kinase inhibitors and epigenetic modulating agents have already demonstrated, or are likely to demonstrate, efficacy in thyroid cancers. Novel combinations of kinase inhibitors with novel non-TKI inhibitors are currently being explored and are expected to yield promising results. For example, 5 trials with mTOR inhibitors and other agents are ongoing - 3 of these combining an mTOR inhibitor with sorafenib.
Summary
Over the past decade, significant progress has been made in the evolution of treatment options for advanced thyroid cancers. Nonetheless, the sobering reality is that each of these options has serious limitations. Perhaps the real achievement of recent investigative efforts lies not only in incremental improvements attained to date, but importantly also in our realization that progress can be made at all in advancing therapies for patients afflicted with uncommon and previously neglected advanced thyroid cancers. Investigators around the world are encouraged to take notice and participate in yet additional endeavors to identify still better treatment options.
ARTICLE HIGHLIGHTS.
Systemic therapies (e.g. cytotoxic chemotherapy) have, in the past, demonstrated only limited efficacy in patients with advanced differentiated and medullary thyroid cancers, prompting studies of novel targeted therapeutics in these cancers.
Small molecule inhibitors of RET, a kinase subject to activating mutation in medullary thyroid cancer, have demonstrated clinical activity in MTC. Vandetanib is now approved by the U.S. FDA for use in advanced, symptomatic and progressive MTC.
Small molecule kinase inhibitors, especially those which inhibit VEGF-Rs have emerged as agents with both preclinical and also promising clinical activity in differentiated and medullary thyroid cancers.
Therapeutics targeting inhibition of microtubule function (especially taxanes) or topoisomerase II (especially doxorubicin) have demonstrated preliminary efficacy in anaplastic thyroid cancer and may, either alone or in combination with radiotherapy and/or novel targeted therapeutics, have an expanding role in ATC management.
Abbreviation List
- ATC
anaplastic thyroid cancer
- DTC
differentiated thyroid cancer
- MTC
medullary thyroid cancer
- PTC
papillary thyroid cancer
- FTC
follicular thyroid cancer
- FDA
Food and Drug Administration
- TSH
thyrotropin/thyroid stimulating hormone
- VEGF-R
vascular endothelial growth factor receptor
- RET
rearranged during transfection (gene or protein)
- Tg
thyroglobulin
- CEA
carcinoembryonic antigen
- WHO
World Health Organization
- NIS
sodium iodine symporter
- FMTC
familial medullary thyroid cancer
- MEN
multiple endocrine neoplasia
- RAI
radioiodine
- TSH-R
thyroid stimulating hormone receptor
- PTEN
phosphatase and tensin
- BRAF
v-raf murine sarcoma viral oncogene homolog B1 (gene or associated protein)
- MEK
mitogen-activator protein kinase ½
- PFS
progression free survival
- PDGFR
platelet-derived growth factor receptor
- HCC
Hürthle cell carcinoma
- PK
protein kinase
- PR
progression rate
- ERK
extracellular signal-regulated kinase
- TKI
tyrosine kinase inhibitor
- PPAR
peroxisome proliferator activated receptor
- HDACI
histone deacetylase inhibitors
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- SPECT
single photon emission commuted tomography
- PIK3CA
phosphoinositide-3-kinase, catalytic, alpha polypeptide
- Ras
retrovirus associated sequence (gene or protein)
- mTOR
mammalian target of rapamycin
- AKT
v-akt murine thymoma viral oncogene homolog 1 (gene or associated protein)
- PI3
phosphoinositide-3 (gene or protein)
- DNA
deoxyribonucleic acid
- NGR-TNF
NGR-tumor necrosis factor
- NGR
peptide GNGRAHA, targeting aminopeptidase N (CD13) and the integrin αvβ3 (CD51/CD61)
- CD13
aminopeptidase N (APN)
- TNF
tumor necrosis factor
- TTP
thrombotic thrombocytopenic purpura
- HSP
heat shock protein
Footnotes
Declaration of interests:
Dr. Harris is a full-time employee at the National Cancer Institute, National Institutes of Health, Bethesda, Maryland; she has no conflict of interests.
Dr Bible states no conflict of interests and has received no funding in preparation of this manuscript.
REFERENCES
- 1.Cancer Facts and Figures. 2009 [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Hao Y, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 6.Al-Eid HA, SO Cancer Incidence Report Saudi Arabia. 2003 [Google Scholar]
- 7.McIver B, Hay ID, Giuffrida DF, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 8.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clinical Oncol (R Coll Radiol) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21 Suppl 2:S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Antona C, Pallares J, Montero-Conde C, Inglada-Perez L, Castelblanco E, Landa I, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr Relat Cancer. 2010;17:7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 11.Schiff BA, McMurphy AB, Jasser SA, et al. Epidermal growth factor receptor (EGFR) is overexpressed in anaplastic thyroid cancer, and the EGFR inhibitor gefitinib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res. 2004;10:8594–8602. doi: 10.1158/1078-0432.CCR-04-0690. [DOI] [PubMed] [Google Scholar]
- 12.Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V, et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann S, Maschuw K, Hassan I, et al. Functional thyrotropin receptor attenuates malignant phenotype of follicular thyroid cancer cells. Endocrine. 2006;30:129–138. doi: 10.1385/ENDO:30:1:129. [DOI] [PubMed] [Google Scholar]
- 15.Spitzweg C, Baker CH, Bergert ER, et al. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum Gene Ther. 2007;18:916–924. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
- 16.Myers RM, Greiner SM, Harvey ME, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82:700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trujillo MA, Oneal MJ, McDonough S, et al. A probasin promoter, conditionally replicating adenovirus that expresses the sodium iodide symporter (NIS) for radiovirotherapy of prostate cancer. Gene Ther. 2010;17:1325–1332. doi: 10.1038/gt.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas AS. Phase II study and tissue correlative studies of AZD6244 (ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma (IRPTC) and papillary thyroid carcinoma (PTC) with follicular elements; ASCO Annual Meeting; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow L. A First in Human Dose-Ranging Study to Assess the Pharmacokinetics, Pharmacodynamics, and Toxicities of the MEK Inhibitor, ARRY-142886 (AZD6244). Patients with Advanced Solid Malignancies; AACR-NCI-EORTC International Conference on Moledular Targets and Cancer Therapeutics.2005. [Google Scholar]
- 22.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 23.Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27:3794–3801. doi: 10.1200/JCO.2008.18.7815. [DOI] [PubMed] [Google Scholar]
- 24. Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. * Of importance, because pazopanib was found to have impressive clinical activity in advanced radioiodine-refractory DTC - and because pazopanib is commercially available and approved by the US FDA for use in renal cell carcinoma.
- 25.Sideras K, Menefee ME, Burton JK, et al. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. J Clin Oncol. 2010;28:e312–e313. doi: 10.1200/JCO.2009.26.4432. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. J Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. * Of importance, as sorafenib was reported to have substantive clinical activity in advanced radioiodine-refractory DTC - and because sorafenib is commercially available and approved by the US for use in renal cell carcinoma.
- 28. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. * Of importance, as sorafenib was reported to have substantive clinical activity in advanced radioiodine-refractory DTC - and because sorafenib is commercially available and approved by the US for use in renal cell carcinoma.
- 29. Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–931. doi: 10.1530/EJE-09-0702. * Of importance, as sorafenib was reported to have clinical activity in advanced MTC - and because sorafenib is commercially available and approved by the US for use in renal cell carcinoma.
- 30. Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–2330. doi: 10.1200/JCO.2009.25.0068. * Of importance, as sorafenib was reported to have clinical activity in advanced MTC - and because sorafenib is commercially available and approved by the US for use in renal cell carcinoma.
- 31.Kober F, Hermann M, Handler A, Krotla G. Effect of sorafenib in symptomatic metastatic medullary thyroid cancer; ASCO Annual Meeting; 2007. [Google Scholar]
- 32.Nagaiah G, Fu P, Wasman J, et al. Phase II trial of sorafenib (bay 43–9006) in patients with advanced anaplastic carcinoma of the thyroid (ATC); ASCO Annual Meeting; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman SI. Targeted therapy of thyroid cancer. Biochem Pharmacol. 2010;80(5):592–601. doi: 10.1016/j.bcp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Chintala L, Kurzrock R, Fu S, et al. Phase I study of tipifarnib and sorafenib in patients with biopsiable advanced cancer (NCI protocol 7156); ASCO Annual Meeting; 2008. [Google Scholar]
- 35.Hong DS, Cabanillas ME, Wheler J, et al. Inhibition of the Ras/Raf/MEK/ERK and RET Kinase Pathways with the Combination of the Multikinase Inhibitor Sorafenib and the Farnesyltransferase Inhibitor Tipifarnib in Medullary and Differentiated Thyroid Malignancies. J Clin Endocrinol Metab. 2011;96:997–1005. doi: 10.1210/jc.2010-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–e61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 37.Smith KJ, Haley H, Hamza S, Skelton HG. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009;35:1766–1770. doi: 10.1111/j.1524-4725.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 38.Cohen E, Needles B, Cullen K, et al. Phase 2 study of sunitinib in refractory thyroid cancer; ASCO Annual Meeting; 2008. [Google Scholar]
- 39.De Souza J, Busaidy N, Zimrin A, et al. Phase II trial of sunitinib in medullary thryoid cancer (MTC); ASCO Annual Meeting; 2010. [Google Scholar]
- 40.Ravaud A, de la Fouchardiere C, Courbon F, et al. Sunitinib in patients with refractory advanced thyroid cancer: the THYSU phase II trial; ASCO Annual Meeting; 2008. [Google Scholar]
- 41. Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28:767–772. doi: 10.1200/JCO.2009.23.6604. ** Of high importance, as vandetanib was reported to have substantive clinical activity in advanced MTC, ultimately leading to a phase III trial and to US FDA approval of vandetanib for use in advanced progressive and symptomatic MTC.
- 42. FDA Briefing Document, Oncology Drug Advisory Committee Meeting. 2010 ** Of high importance, as this document details the US FDA approval of vandetanib for use in advanced progressive and symptomatic MTC.
- 43.Haddad R, Krebs A, Vasselli J, et al. A phase II open-label study of vandetanib in patients with locally advanced or metastatic hereditary medullary thyroid cancer; ASCO Annual Meeting; 2008. [Google Scholar]
- 44. Wells S, Robinson B, Gagel R, et al. Vandetanib (VAN) in locally advanced or metastatic medullary thyroid cancer (MTC): A randomized, double-blind phase III trial (ZETA); ASCO Annual Meeting; 2010. ** Of high importance, because of the report of improved progression-free survival from the use of vandetanib in advanced MTC
- 45.Kurzrock R, Cohen E, Sherman S, et al. Long-term results in a cohort of medullary thyroid cancer (MTC) patients (pts) in a phase I study of XL184 (BMS 907351), an oral inhibitor of MET, VEGFR2, and RET; ASCO Annual Meeting; 2010. [Google Scholar]
- 46.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis; Proceedings of the National Academy of Sciences of the United States of America; 1994. pp. 4082–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ain KB, Lee C, Williams KD. Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid. 2007;17:663–670. doi: 10.1089/thy.2006.0289. [DOI] [PubMed] [Google Scholar]
- 48.Ain K, Lee C, Holbrook K, et al. Phase II study of lenalidomide in distantly metastatic, rapidly progressive, and radioiodine-unresponsive thyroid carcinomas: preliminary results; ASCO Annual Meeting; 2008. [Google Scholar]
- 49.Catalano MG, Fortunati N, Pugliese M, et al. Valproic acid induces apoptosis and cell cycle arrest in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2005;90:1383–1389. doi: 10.1210/jc.2004-1355. [DOI] [PubMed] [Google Scholar]
- 50.Fortunati N, Catalano MG, Arena K, et al. Valproic acid induces the expression of the Na symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004;89:1006–1009. doi: 10.1210/jc.2003-031407. [DOI] [PubMed] [Google Scholar]
- 51.Catalano MG, Poli R, Pugliese M, et al. Valproic acid enhances tubulin acetylation and apoptotic activity of paclitaxel on anaplastic thyroid cancer cell lines. Endocr Relat Cancer. 2007;14:839–845. doi: 10.1677/ERC-07-0096. [DOI] [PubMed] [Google Scholar]
- 52.Ringel M, Kloos R, Arbogast D, et al. Phase II study of oral histone deacetylase inhibitor SAHA in patients with metastatic thyroid cancer. 77th Annual Meeting of the American Thyroid Association; Phoenix, AZ. 2006. [Google Scholar]
- 53.Piekarz R, Luchenko V, Draper D, et al. Phase I trial of romidepsin, a histone deacetylase inhibitor, given on days one, three and five in patients with thyroid and other advanced cancers; ASCO Annual Meeting; 2008. [Google Scholar]
- 54.Sherman E, Fury M, Tuttle R, et al. Phase II study of depsipeptide (DEP) in radioiodine (RAI)-refractory metastatic nonmedullary thryoid carcinoma; ASCO Annual Meeting; 2009. [Google Scholar]
- 55.Catalano MG, Pugliese M, Gargantini E, et al. Cytotoxic activity of the histone deacetylase inhibitor panobinostat (LBH589) in anaplastic thyroid cancer in vitro and in vivo. Int J Cancer. 2011 doi: 10.1002/ijc.26057. [e pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.LaBonte MJ, Wilson PM, Fazzone W, et al. DNA microarray profiling of genes differentially regulated by the histone deacetylase inhibitors vorinostat and LBH589 in colon cancer cell lines. BMC Med Genomics. 2009;2:67. doi: 10.1186/1755-8794-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jaskula-Sztul R, Pisarnturakit P, Landowski M, et al. Expression of the Active Notch1 Decreases MTC Tumor Growth In Vivo. J Surg Res. 2011 doi: 10.1016/j.jss.2011.03.035. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck J, Fischer T, Rowinsky E, et al. Phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of LBH589A: A novel histone deacetylase inhibitor; ASCO Annual Meeting; 2004. [Google Scholar]
- 59.Venkataraman GM, Yatin M, Marcinek R, Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Nasymporter gene methylation status. J Clin Endocrinol Metab. 1999;84:2449–2457. doi: 10.1210/jcem.84.7.5815. [DOI] [PubMed] [Google Scholar]
- 60.Ohta K, Endo T, Haraguchi K, et al. Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:2170–2177. doi: 10.1210/jcem.86.5.7493. [DOI] [PubMed] [Google Scholar]
- 61.Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity PPARgamma agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25:2304–2317. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 62.Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140:960–966. doi: 10.1016/j.surg.2006.07.038. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 63.Smallridge R, Copland J, Brose M, et al. Phase I study of CS-7017, an oral PPAR-g agonist, in combination with paclitaxel in advanced anaplastic thyroid cancer. AACR 102nd Annual Meeting; Orlando, FL. 2011. [Google Scholar]
- 64.Handkiewicz-Junak D, Roskosz J, Hasse-Lazar K, et al. 13-cis-retinoic acid re-differentiation therapy and recombinant human thyrotropin-aided radioiodine treatment of non-Functional metastatic thyroid cancer: a single-center, 53-patient phase 2 study. Thyroid Res. 2009;2(1):8. doi: 10.1186/1756-6614-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coelho SM, Vaisman F, Buescu A, et al. Follow-up of patients treated with retinoic acid for the control of radioiodine non-responsive advanced thyroid carcinoma. Braz J Med Biol Res. 2011;44:73–77. doi: 10.1590/s0100-879x2010007500120. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann S, Rockenstein A, Ramaswamy A, et al. Retinoic acid inhibits angiogenesis and tumor growth of thyroid cancer cells. Mol Cell Endocrinol. 2007;264:74–81. doi: 10.1016/j.mce.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Liu YY, Stokkel MP, Pereira AM, et al. Bexarotene increases uptake of radioiodide in metastases of differentiated thyroid carcinoma. Eur J Endocrinol. 2006;154:525–531. doi: 10.1530/eje.1.02123. [DOI] [PubMed] [Google Scholar]
- 68. Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587–594. doi: 10.1089/thy.2000.10.587. ** Of high importance, as this study presents results of a prospective clinical trial demonstrating paclitaxel to have clinical activity in advanced ATC.
- 69.Kawada K, Kitagawa K, Kamei S, et al. The feasibility study of docetaxel in patients with anaplastic thyroid cancer. Jpn J Clin Oncol. 2010;40:596–599. doi: 10.1093/jjco/hyq025. [DOI] [PubMed] [Google Scholar]
- 70.Troch M, Koperek O, Scheuba C, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95:E54–E57. doi: 10.1210/jc.2009-2827. [DOI] [PubMed] [Google Scholar]
- 71.Foote RL, Molina JR, Kasperbauer JL, et al. Enhanced survival in locoregionally confined anaplastic thyroid carcinoma: a single-institution experience using aggressive multimodal therapy. Thyroid. 2011;21:25–30. doi: 10.1089/thy.2010.0220. [DOI] [PubMed] [Google Scholar]
- 72.Fury MG, Solit DB, Su YB, Rosen N, Sirotnak FM, Smith RP, et al. A phase I trial of intermittent high-dose gefitinib and fixed-dose docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2007;59:467–475. doi: 10.1007/s00280-006-0286-6. [DOI] [PubMed] [Google Scholar]
- 73.Dowlati A, Robertson K, Cooney M, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–3416. [PubMed] [Google Scholar]
- 74.Mooney CJ, Nagaiah G, Fu P, et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid. 2009;19:233–240. doi: 10.1089/thy.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia-Rostan G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 76.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 77.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 78.Santarpia L, El-Naggar AK, Cote GJ, et al. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93:278–284. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 79.Hou P, Bojdani E, Xing M. Induction of thyroid gene expression and radioiodine uptake in thyroid cancer cells by targeting major signaling pathways. J Clin Endocrinol Metab. 2010;95:820–828. doi: 10.1210/jc.2009-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin N, Jiang T, Rosen DM, et al. Dual inhibition of mitogen-activated protein kinase kinase and mammalian target of rapamycin in differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2009;94:4107–4112. doi: 10.1210/jc.2009-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu LT, Averbuch SD, Ball DW, et al. Treatment of advanced medullary thyroid carcinoma with a combination of cyclophosphamide, vincristine, and dacarbazine. Cancer. 1994;73:432–436. doi: 10.1002/1097-0142(19940115)73:2<432::aid-cncr2820730231>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 82.Nocera M, Baudin E, Pellegriti G, et al. Treatment of advanced medullary thyroid cancer with an alternating combination of doxorubicin-streptozocin and 5 FU-dacarbazine. Groupe d'Etude des Tumeurs a Calcitonine (GETC) Br J Cancer. 2000;83:715–718. doi: 10.1054/bjoc.2000.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Argiris A, Agarwala SS, Karamouzis MV, et al. A phase II trial of doxorubicin and interferon alpha 2b in advanced, non-medullary thyroid cancer. Invest New Drugs. 2008;26:183–188. doi: 10.1007/s10637-007-9091-2. [DOI] [PubMed] [Google Scholar]
- 84.Tan AR, Dowlati A, Jones SF, et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. Oncologist. 2010;15:1253–1261. doi: 10.1634/theoncologist.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alexandre J, Kahatt C, Bertheault-Cvitkovic F, et al. A phase I and pharmacokinetic study of irofulven and capecitabine administered every 2 weeks in patients with advanced solid tumors. Invest New Drugs. 2007;25:453–462. doi: 10.1007/s10637-007-9071-6. [DOI] [PubMed] [Google Scholar]
- 86.Heerschap A, Fiedler W, Marreaud S, et al. A phase I study of NGR-TNF, a novel vascular targeting agent, in patients with refractory solid tumors (EORTC 16041); ASCO Annual Meeting; 2007. [Google Scholar]
- 87.Gregorc V, Santoro A, Bennicelli E, et al. Phase Ib study of NGR-hTNF, a selective vascular targeting agent, administered at low doses in combination with doxorubicin to patients with advanced solid tumours. Br J Cancer. 2009;101:219–224. doi: 10.1038/sj.bjc.6605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peled M, Shaish A, Greenberger S, et al. Antiangiogenic systemic gene therapy combined with doxorubicin administration induced caspase 8 and 9-mediated apoptosis in endothelial cells and an anti-metastasis effect. Cancer Gene Ther. 2008;15:535–542. doi: 10.1038/cgt.2008.20. [DOI] [PubMed] [Google Scholar]
- 89.Braga-Basaria M, Hardy E, Gottfried R, et al. 17-Allylamino-17-demethoxygeldanamycin activity against thyroid cancer cell lines correlates with heat shock protein 90 levels. J Endocrinol Metab. 2004;89:2982–2988. doi: 10.1210/jc.2003-031767. [DOI] [PubMed] [Google Scholar]
- 90.Marsee DK, Venkateswaran A, Tao H, et al. Inhibition of heat shock protein 90, a novel RET/PTC1-associated protein, increases radioiodide accumulation in thyroid cells. J Biol Chem. 2004;279:43990–43997. doi: 10.1074/jbc.M407503200. [DOI] [PubMed] [Google Scholar]
- 91.Chatal JF, Campion L, Kraeber-Bodere F, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006;24:1705–1711. doi: 10.1200/JCO.2005.04.4917. [DOI] [PubMed] [Google Scholar]
- 92.Schott M, Seissler J, Lettmann M, et al. Immunotherapy for medullary thyroid carcinoma by dendritic cell vaccination. J Clin Endocrinol Metab. 2001;86:4965–4969. doi: 10.1210/jcem.86.10.7949. [DOI] [PubMed] [Google Scholar]
- 93.Stift A, Sachet M, Yagubian R, et al. Dendritic cell vaccination in medullary thyroid carcinoma. Clin Cancer Res. 2004;10:2944–2953. doi: 10.1158/1078-0432.ccr-03-0698. [DOI] [PubMed] [Google Scholar]
- 94.Kundra P, Burman KD. Thyroid cancer molecular signaling pathways and use of targeted therapy. Endocrinol Metab Clin North Amer. 2007;36:839–853. doi: 10.1016/j.ecl.2007.06.001. viii. [DOI] [PubMed] [Google Scholar]
- 95.Barzon L, Pacenti M, Taccaliti A, et al. A pilot study of combined suicide/cytokine gene therapy in two patients with end-stage anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:2831–2834. doi: 10.1210/jc.2004-2139. [DOI] [PubMed] [Google Scholar]