Abstract
The objective of this study was to determine whether exposing rats to ozone would result in loss of antioxidants from plasma and bronchoalveolar lavage fluid (BALF). Additional goals were to compare analyses of the same antioxidant concentration between different laboratories, to investigate which methods have the sensitivity to detect decreased levels of antioxidants, and to identify a reliable measure of oxidative stress in ozone-exposed rats. Male Fisher rats were exposed to either 2.0 ppm or 5.0 ppm ozone inhalation for 2 h. Blood plasma and BALF samples were collected 2 h, 7 h, and 16 h after the exposure. It was found that ascorbic acid in plasma collected from rats after the higher dose of ozone was lower at 2h but not later. BALF concentrations of ascorbic acid were decreased at both 2 and 7 h post-exposure. Tocopherols (α-, δ-, γ-), 5-nitro-γ-tocopherol, tocol, glutathione (GSH/GSSG) and cysteine (Cys/CySS) were not further decreased, regardless of the doses and post-exposure time points used for sample collection. Uric acid was significantly increased by the low dose at 2h and the high dose at the 7 h point, probably due to the accumulation of blood plasma in the lung from ozone-increased alveolar capillary permeability. We concluded that measurements of antioxidants in plasma are not sensitive biomarkers for oxidative damage induced by the ozone and is not a useful choice for the assessment of oxidative damage by ozone in vivo.
Keywords: ozone, rat, plasma, tocopherols, ascorbic acid, glutathione, uric acid
Introduction
Oxidative stress and antioxidant status have been repeatedly investigated in animal models and in humans, but there is a considerable paucity of studies validating the use of antioxidants as biomarkers to assess oxidative stress. It has been generally agreed among scientists who investigate oxidative stress that there is a need for validating sensitive and specific biomarkers for oxidative damage resulting from multiple types of oxidative insults in rodents and humans. Protection from damage occurs through the action of multiple antioxidants, some endogenously produced, some provided through dietary intake [1]. Ideally, biomarkers of oxidative stress would be measurable in specimens that can be collected relatively easily, and the analysis procedure should be applicable to stored specimens.
Ozone is a commonly encountered environmental air pollutant, and its exposure has been reported to induce free radical generation and antioxidant depletion [2–4]. It has been well documented in animal models and human studies that exposure to ozone induces secretion of many different pro-inflammatory factors into the lung including prostaglandins, IL-1, TNF-α, IL-6, and IL-8 [5–7]. Although ozone can impair the phagocytic capacity of alveolar macrophages [8, 9], some reports suggest that accumulation of neutrophils in the airways is critical to the response to ozone [10, 11]. Others report that neutrophil depletion inhibits airway hyper-responsiveness induced by ozone exposure [11–13]. The work of Kleeberger and colleagues shows the genetic control of susceptibility to ozone-induced lung inflammation [14, 15].
The assessment of plasma antioxidants in oxidative stress-related conditions has been associated with their consumption and decline below normal ranges [16]. It is likely that the inconsistent and variable results often found in biomarkers of oxidative stress studies are due to a lack of accuracy and comparability of the available different methods for antioxidant detection. The aim of this multi-laboratory research effort is to continue to measure and evaluate a series of antioxidant biomarkers in biological fluids collected from similarly exposed rats. An important goal of the present study is to compare analysis of the same antioxidant concentration between different laboratories, to investigate which methods have the sensitivity to detect decreased levels of antioxidants, and to identify a noninvasive, reliable and repeatable antioxidant measures of oxidative stress.
We have previously reported the results on the effects of CCl4 on plasma antioxidants that were measured as biomarkers of oxidative stress [17]. Similarly, the goal of the current study is to determine if the profiles of antioxidants in plasma and broncho-alveolar lavage fluid (BALF) may be used as biomarkers of oxidative stress in an experimental model of ozone exposure. The time- (2, 7 and 16 h) and dose- (2 ppm and 5 ppm) dependent effects of ozone on blood plasma and BALF levels of ascorbic acid, tocol, α-, δ-, and γ-tocopherols, 5-nitro- γ –tocopherol, glutathione (GSH and GSSG), cysteine, cystine and uric acid were assessed to determine whether they changed compared to control values in plasma and BALF. This new Biomarkers of Oxidative Stress Study (BOSS) reports antioxidant levels in plasma using acute exposure to ozone as a model of oxidative stress to add to the original CCl4 report.
Materials and Methods
Chemicals and Reagents
All chemicals and reagents used in the study were obtained from Sigma-Aldrich Corporation (St. Louis, MO).
Animals and Treatment Protocol
Male Fisher 344 rats (260–280 g) obtained from Charles River Laboratories (Raleigh, NC) were used in all experiments. The animals were housed 3 to a cage. Autoclaved hardwood bedding was used in solid-bottom polycarbonate cages with filter tops. Animal rooms were maintained at 20–25°C with 35–70% relative humidity with alternating 12-h light and dark cycles. The rats had free access to deionized, reverse-osmosis-treated water and received autoclaved NIH 31 rodent chow (Zeigler Bros, Gardners, PA) ad libitum. For all experiments, rats were fasted overnight and then exposed to ozone for a total of 2 h. Fasting continued through the experiment. Rats were anesthetized with Nembutal (0.1 ml/100 g body weight), and 5 ml blood was removed from the dorsal aorta where it entered anterior to its distal bifurcation into the common iliac arteries. Rats were sacrificed after blood collection. The studies adhered to the National Institutes of Health and the U.S. Environmental Protection Agency Guidelines for the Care and Handling of Experimental Animals. All animal studies were approved by the National Institute of Environmental Health Sciences and the Environmental Protection Agency laboratory review boards.
Study Design
Inhalation Exposure
Rats (six animals per group) were placed in individual stainless-steel wire-mesh cages inside a 135-L exposure chamber (air flow rate of 38 liter/min) and exposed to either 2.0 ppm O3 or 5.0 ppm O3 for 2 h. Control animals were exposed to filtered room air. Chamber O3 concentration was monitored with a Dasibi model 1003AH O3 monitor (Dasibi Environmental Corp., Glendale, CA). Measurements made of chamber conditions during exposure yielded the following for 2.0 ppm and 5.0 ppm ozone exposures, respectively: range of actual chamber ozone concentration, 1.97–2.03 ppm and 4.84–5.09 ppm; range of relative humidity, 44.7 – 45.3% and 45.0–51.0; range of temperatures, 23.9–24.0°C and 24.4–25.0°C.
Specimen Collection
Animals from each of the 3 dose groups (Control, 2 ppm and 5 ppm ozone) were sacrificed at 3 time points: 2 h, 7 h, and 16 h after the exposure. Each group consisted of 6 rats for each analysis. Animal treatment and sample preparation were done at US EPA, Research Triangle Park, NC. Each sample was marked with a code number so that the investigators conducting the assays were not aware of the treatment status of the animals.
Plasma Preparation
Blood (5 ml) was drawn through single draw vacutainer needles (21 Gauge) into open vacutainer blood collection tubes containing heparin. The tubes were gently inverted 2–3 times for mixing and immediately placed on ice. Blood was centrifuged (2,000 rpm for 10 min at 4°C) no more than 30 minutes after collection.
Bronchoalveolar lavage fluid (BALF)
BALF was obtained from the lungs of rats that had been exposed to ozone for 2 h at all three time points studied. The rats were deeply anesthetized using Nembutal and killed by exsanguination of the abdominal aorta. A tracheal cannula was inserted to about 0.5 cm above the carina, and the whole lung was lavaged three times with the same volume of isotonic 0.85% NaCl (Ca2+ and Mg2+ free) that had been warmed to 38°C. A volume equal to 30 mL/kg of body weight was injected, suctioned and reinjected 3 times in succession. This saline was then withdrawn and placed on ice.
Lung preparation for histopathology
Lung specimens were stained with hematoxylin-eozin and examined at 2-, 7-, and 16 h post- exposure. Histopathology review was performed at NIEHS, RTP, NC.
Antioxidant Status
α-Tocopherol, δ-, γ-tocopherol, 5-nitro-γ–tocopherol, and α-tocopherol-quinone were measured in plasma by high-performance liquid chromatography with electrochemical detection (HPLC-ECD) [18] at OMRF, OK.
Ascorbic acid and uric acid
Plasma specimens were assayed using amperometric detection [19] at USEPA, RTP, NC.
Glutathione
GSH and GSSG were analyzed by HPLC with fluorescence detection [20, 21] at Emory University, Atlanta, GA.
Cysteine/cystine oxidation of plasma was measured by HPLC [21, 22] at Emory University, Atlanta, GA.
All measurements of antioxidants were evaluated relative to a standard curve which was created by inclusion of standards of known concentration in each sample run. This information is included in the corresponding bibliography references of the methods.
Statistical analysis
Statistical comparisons between the controls and each dose group at the same time point were done by analysis of variance at NIEHS. When appropriate, analysis was done on the log scale. Values of p < 0.05 were considered statistically significant.
Results
Histopathological findings
To understand the degree of injury caused by the ozone exposure, sections from lungs were examined from all animals. Representative photographs from control, low-ozone, and high- ozone dose animals at the 3 time points post-exposure are displayed (Figure 1A to I). No abnormalities were seen in the control animals at all three time points. Lung toxicity was observed following both high and low doses of ozone exposure as described in detail below.
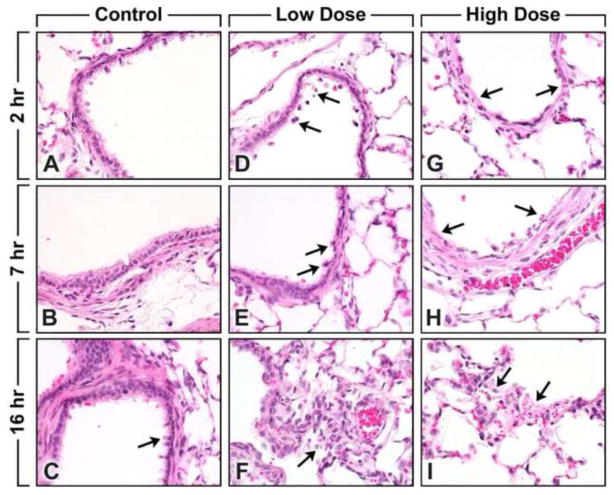
Fig. 1.
(A to I). Lung histopathology of control and ozone-exposed Fisher rats. Lung specimens were stained with hematoxylin-eozin. Original magnification, x400.
Figures A, B and C - Control animals from the 3 time points. Terminal bronchiole. Note (arrows) the normal presence of the apical blebs of the Clara cells.
Figures D, E and F - 2 ppm ozone exposure animals from the 3 time points. Terminal bronchiole. Note (arrows) the changes consist of loss of apical blebs of the Clara cells and the presence of a few exfoliated epithelial cells in the lumen.
Figures G, H and I - 5 ppm ozone exposure animals from the 3 time points. Terminal bronchiole. Note (arrows) the changes consist of loss of apical blebs of Clara cells; necrosis and denudation of epithelium lining; and accumulation of aggregates of mixed cells consisting mainly of exfoliated epithelial cells, a few polymorphonuclear cells, macrophages, and cell debris in the lumen.
Changes 2h following exposure
Changes in the lung of rats treated with the low-dose ozone exposure (2 ppm) were usually minimal and localized to bronchioles and terminal bronchioles. The changes consisted of loss of apical blebs of Clara cells and the presence of a few exfoliated epithelial cells in the lumen (Fig. 1D). Changes with the high dose ozone exposure (5 ppm) were usually moderate (Grade 3) and also localized to the bronchioles and terminal bronchioles where loss of apical blebs of Clara cells, necrosis with denudation of epithelium lining, accumulation of aggregates of exfoliated epithelial cells, polymorphonuclear (PMN) cells, macrophages, and cell debris in the lumen appear to be seen (Fig. 1G).
Changes 7h following exposure
Lungs of rats exposed to low-dose ozone exposure (2 ppm) showed loss of apical blebs of Clara cells and the presence of few exfoliated epithelial cells in the lumen of bronchioles and terminal bronchioles (Fig. 1E). Changes in the group exposed to high-dose ozone (5 ppm) were localized in the bronchioles, terminal bronchioles, alveolar ducts, and alveoli. The changes consisted of loss of apical blebs of Clara cells, necrosis and denudation of epithelium lining, and accumulation of aggregates of mixed cells consisting mainly of exfoliated epithelial cells, a few PMN cells, macrophages and cell debris in the lumen (Fig. 1H).
Changes 16 h following exposure
Rats exposed to low-dose ozone (2 ppm) primarily displayed minimal histological changes that were localized to the alveolar ducts and alveoli. The changes were characterized by the presence of macrophages and a few PMN in the lumen (Fig. 1F). Groups of high-dose (5 ppm) ozone exposed rats presented with histological changes that were usually localized to the alveolar ducts and alveoli. These changes consisted of necrosis of the epithelium lining, presence of necrotic and intact exfoliated cells, few PMN cells, macrophages and fibrin in the lumen (Fig. 1I). Lesions were localized primarily to the centriacinar region of the lungs, involving the terminal bronchioles, alveolar ducts, and proximal alveoli and sparing the larger airways.
Injury markers in BALF were used to assess immediate toxicity and inflammation in similarly exposed rats. At 2 ppm, injury was marked by significant increases in BALF total protein, N-acetylglucosaminidase, and lavageable ciliated cells, whereas infiltration of neutrophils was observed only at the higher 5 ppm concentration (data not shown).
Ascorbic Acid
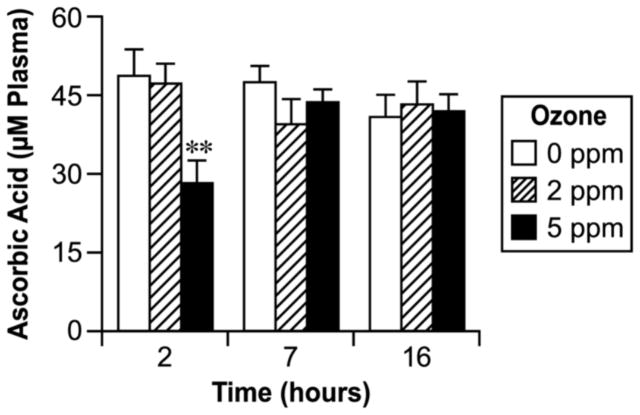
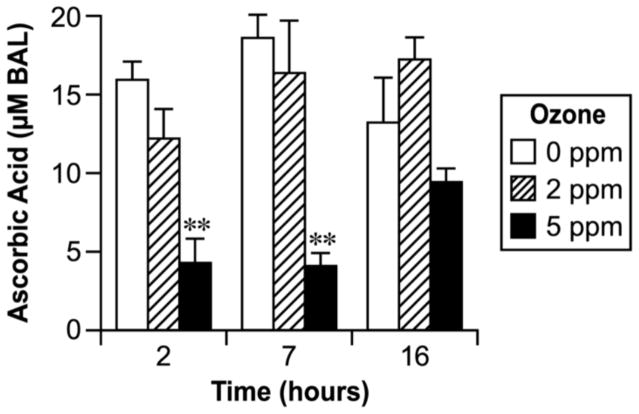
As shown in Fig. 2, the plasma level of ascorbic acid tended to be lower in the ozone- exposed group at early times, with the difference being significant at 2 h (2-fold) for the high- dose group. No significant effect of ozone exposure on ascorbic acid in plasma was found for either dose after 7 h and 16 h post-exposure (Fig. 2). Ascorbic acid in the BALF was significantly decreased in rats with the high dose of ozone at 2 h and 7 h post-exposure when compared to the controls or to those groups that were exposed to the lower dose (Fig. 3). The higher dose of ozone exposure appeared to decrease the ascorbic acid in BALF at the 16 h time point, but the differences were not statistically significant as compared to the controls (Fig. 3).
Fig. 2.
Effect of ozone inhalation exposure for 2 h on the ascorbic acid concentration in plasma. Values are means ± SE. Asterisks indicate statistically significant (p≤0.05) differences from respective controls.
Fig. 3.
Effect of ozone inhalation exposure for 2 h on the ascorbic acid concentration in BAL fluid. Values are means ± SE. Asterisks indicate statistically significant (p≤0.05) differences from respective controls.
Tocopherols: α-, δ-, and γ-tocopherol, 5-nitro-γ-tocopherol, α-tocopherol quinone and tocol
Mean plasma levels of both γ-tocopherol (γT) and tocol decreased consistently at 2 and 5 ppm ozone, at each time point (2 h, 7 h, and 16 h) after ozone exposure relative to control values from animals within the same groups (Table 1). The magnitude of the mean decrease reached −35% for γT at 5 ppm ozone 16 h after exposure and −64% for tocol at the same time point relative to 16 h control animals (Table 1). However, due to variation these changes did not reach statistical significance at any one time point, and the values for these two tocopherols tended to vary inexplicably among the three control groups (2 h, 7 h, 16 h; Table 1). α-Tocopherol showed a nonsignificant 10–20% decrease at 16 h post-ozone exposure at both 2 ppm and 5 ppm ozone, but no trends were observed with respect to either the α-tocopherol oxidation product, α-tocopherol-quinone, or the γ–tocopherol nitration product, 5-NO2-γ tocopherol (Table 1). The ratios of α-tocopherol quinone to α-tocopherol and the ratio of 5-nitro- γ-tocopherol to γ-tocopherol showed no significant change for both doses of ozone at all three time points studied (data not shown). No significant changes were found for α-tocopherol in the BALF of animals exposed to ozone as compared to the controls. Other tocols were not measured in BALF.
Table 1.
Effect of ozone inhalation exposure for 2 h on plasma antioxidants of male Fisher rats. Values are means ± SE for 5 or 6 rats.
| PLASMA | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay | 2 h | 7 h | 16 h | ||||||
| Ozone(ppm)
|
Ozone (ppm)
|
Ozone (ppm)
|
|||||||
| 0 | 2 | 5 | 0 | 1 | 5 | 0 | 2 | 5 | |
| α-Tocopnerol (μM) | 2.8 ± 0.1 | 3.1 ± 0.3 | 3.4 ± 0.3 | 2.8 ± 0.2 | 3.1 ± 0.4 | 3.4 ± 0.2 | 3.4 ± 0.2 | 2.7 ± 0.3 | 2.9 ± 0.1 |
| δ-Tocopherol (nM) | 21 ± 8 | 22 ± 9 | 29 ± 8 | 12 ± 6 | 17 ± 6 | 20 ± 6 | 25 ± 7 | 18 ± 5 | 23 ± 5 |
| γ-Tocopherol (nM) | 44 ± 11 | 40 ± 8 | 34 ± 5 | 73 ± 13 | 47 ± 19 | 58 ± 19 | 86 ± 18 | 66 ± 19 | 55 ± 11 |
| 5-nitro-γ-Tocopherol (nM] | 5.7 ± 2.1 | 5.4 ± 0.9 | 5.1 ± 1.4 | 6.1 ± 2.7 | 6.2 ± 2.3 | 5.7 ± 2.8 | 4.6 ± 1.9 | 5.9 ± 2.0 | 3.6 ± 1.2 |
| α-Tocopherol-quinone (nM) | 35 ± 15 | 18 ± 7 | 28 ± 10 | 21 ± 9 | 20 ± 10 | 12 ± 6 | 41 ± 7 | 17 ± 6 | 20 ± 7 |
| Tocol (nM) | 5 ± 3 | 3 ± 1 | 3 ± 1 | 14 ± 8 | 8 ± 5 | 7 ± 4 | 22 ± 12 | 14 ± 9 | 8 ± 4 |
| GSH (μM) | 2.8 ± 0.4 | 1.9 ± 0.2 | 2.1 ± 0.5 | 2.2 ± 0.3 | 1.9 ± 0.3 | 1.6 ± 0.2 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.3 ± 0.4 |
| GSSG (μM) | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.6 ± 0.2 |
| Cysteine (μM) | 4.6 ± 1.0 | 5.4 ± 0.8 | 5.5 ± 1.4 | 4.6 ± 0.9 | 3.4 ± 0.9 | 3.5 ± 0.6 | 4.6 ± 0.6 | 3.7 ± 1.0 | 4.8 ± 0.9 |
| Cystine (μM) | 31 ± 1 | 34 ± 3 | 31 ± 3 | 36 ± 1 | 30 ± 1 | 30 ± 1 | 31 ± 1 | 30 ± 1 | 33 ± 1 |
| Mixed disulfide Cys + GSH (μM) | 8.9 ± 1.3 | 8.0 ± 0.6 | 7.1 ± 0.5 | 7.9 ± 0.4 | 6.9 ± 0.8 | 8 ± 1 | 10.9 ± 0.9 | 9.1 ± 1.3 | 11.3 ± 1.7 |
GSH, GSSG, Cysteine and Cystine
GSH and GSSG in plasma were not affected by either dose of ozone (Table 1). The ratio of GSH to GSSG did not change with treatment (data not shown). Total GSH concentration in BALF for the controls was on average 0.30 ± 0.03 μg/ml and did not change significantly in the ozone- exposed rats (data not shown). Assessment of cysteine (Cys) and its disulfide cystine (CySS) was also conducted since they constitute the most abundant, low-molecular-weight thiol/disulfide redox couple in the plasma, and Cys homeostasis has been shown to be adversely affected during acute lung injury [21]. Ozone exposed rats exhibited no change in Cys and CySS (Table 1) or in the Cys/CySS ratio (data not shown). In addition, no apparent adverse effects of ozone were observed in the measurement of the mixed disulfides of Cys and glutathione (Table 1).
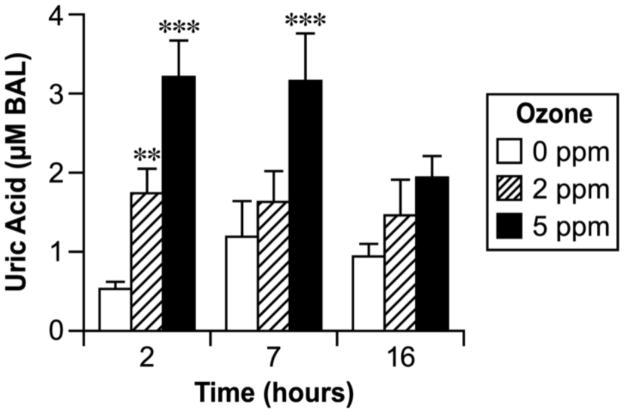
Uric acid
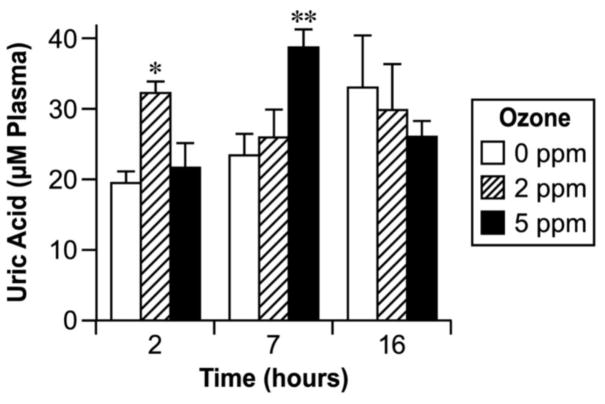
Concentrations of uric acid in plasma and BALF are shown in Figs. 4 and 5. Uric acid was significantly higher in plasma 7 h after high-dose ozone exposure and 2 h after low-dose exposure (Fig. 4). BALF contained a higher concentration of uric acid in both ozone doses at the early time point and in the high dose 7 h after exposure (Fig. 5).
Fig. 4.
Effect of ozone inhalation exposure for 2 h on the uric acid concentration in plasma. Values are means ± SE. Asterisks indicate statistically significant (p≤0.05) differences from respective controls.
Fig. 5.
Effect of ozone inhalation exposure for 2 h on the ascorbic acid concentration in BAL fluid. Values are means ± SE. Asterisks indicate statistically significant (p≤0.05) differences from respective controls.
Discussion
Oxidative stress and antioxidant status have been repeatedly investigated in animal models and in humans, but there is a considerable lack of studies validating whether antioxidants may be used as biomarkers to assess oxidative stress. This study was designed to continue to measure a series of oxidative stress biomarkers in blood and BALF collected from similarly exposed ozone rats. A strength of the design is that the experimental exposures and sample collections were all done in one laboratory under identical conditions. This sample collection design allows direct comparison of different methods using the same samples. Samples were sent in a blind manner to laboratories with extensive experience in respective analytical procedures. Thus, the design eliminates possible errors due to inexperience in sophisticated analytic techniques and also avoids any possible bias in sample analysis and reporting of data. However, in this design, there were logistical issues in collection of samples, e.g., order of collection and timing of processing, which could affect results of specific assays. These issues were addressed in the experimental design and have been previously discussed [17].
Ozone exposure was chosen as a second rodent model of oxidative stress after CCl4 [17]. It is hypothesized that highly reactive ozone molecules react with unsaturated fatty acids at the air-tissue boundary to form lipid ozonation products, which, in turn, can activate membrane lipases and lead to the generation of proinflammatory mediators and free radicals [2, 23]. Epidemiological studies implicate ozone exposure as the most prominent environmental oxidant which has been linked to exacerbation of allergic airway diseases including asthma [24].
Since antioxidants are known to scavenge free radicals [25], our aim was to evaluate major antioxidant substances in blood plasma and BALF and to determine if loss of antioxidants could be used to assess the anticipated oxidative effects of ozone.
To gain insight into ozone toxicity under our experimental conditions we compared the histopathological changes that have occurred in the lungs of rats with and without ozone exposure. Results indicated that following both doses of ozone exposure, lung tissue changes consisted of necrosis of the epithelium lining in the bronchioles and accumulation of a mixture of exfoliated epithelial cells, a few PMNs, leucocytes, macrophages, cell debris, and various amounts of fibrin. In general, lung changes described in these studies are consistent with those reported in the literature although experimental protocols of the ozone inhalation exposure vary in animal models and in humans [26–28]. Changes in the groups with the higher dose of 5 ppm were relatively more prominent in contrast to groups with the lower dose of 2ppm, where the changes were minimal. The severity of the changes induced in the lungs suggests that antioxidant concentrations in plasma and BALF might be altered in response to lung tissue damage caused by zone.
We found that ascorbic acid concentration in both plasma and BALF was significantly decreased below the control level by the higher dose of ozone 2 h after the exposure. At the higher dose of ozone, ascorbic acid in BALF was decreased at 7 h and 16 h after the inhalation exposure, but the differences were significant only at the 7 h time point. Ascorbic acid is a well studied antioxidant in plasma and tissues where it acts as a scavenger of free radicals and recycles other important antioxidant molecules [29–32]. Although evidence from in vitro studies and from some animal models has demonstrated the protective effects of ascorbic acid mediated by increases mainly in BALF, the clinical evidence is still controversial [33–36]. In this study the ozone inhalation exposure of Fisher rats for 2 h determined that the distinct pathologic effects in the lung caused by the high dose of ozone 2 h and 7 h after the exposure were complemented with the loss of ascorbic acid in both plasma and BALF. Literature data show that ascorbate concentrations in the lung can be either increased or decreased depending on the length of exposure, sampling time, or age or dietary status of the animals studied. The results of the current study are in agreement with literature that reports in vitro depletion of ascorbic acid in BALF by ozone [37, 38] and in contradiction with others that show elevation of ascorbic acid in lungs and BALF after repeated ozone inhalation or diet restrictions [34, 35].
Another potential target of ozone oxidation could be α-tocopherol since it is quantitatively the major lipid-soluble antioxidant in plasma [39], although under some circumstances α-tocopherol showed a pro- rather than anti-oxidant activity [40, 41]. Additional isomers of α-tocopherol are either present in very low concentrations or not detectable in plasma [42, 43]. Our time course measurements of α-tocopherol and its isomers in rat plasma revealed no significant changes after inhalation (2 h) of either low or high doses of ozone. In contrast, our studies showed no changes of α-tocopherol in BALF. Under different experimental conditions, other studies reported evidence of depletion of α-tocopherol in the lung and BALF following ozone exposure [26, 44]. Furthermore, ozone exposure had no significant effect on the plasma nitration product of γ-tocopherol, 5-nitro-γ-tocopherol or α-tocopheryl quinone concentrations. There are no literature data available for the effect of ozone on α-tocopheryl quinones although their role in modulating mitochondrial electron transfer and mitochondrial superoxide radical production has been reported [45].
GSH has been documented and has been recognized for decades to play many roles in biological protective mechanisms and critical physiological functions. Conjugates, disulfides, and other GSH-derived products have also been studied as biomarkers of key metabolites of toxic agents. Despite the extensive evidence implicating the depletion and/or oxidation of GSH in a wide variety of human and experimental toxicities, critical examination of such studies frequently reveals that injury is not simply related to glutathione status [46]. Indeed our studies show that neither dose of ozone exposure caused significant changes in plasma and BALF concentrations of either GSH or GSSG.
The reduction potentials (Eh) for the redox couples, GSH/GSSG and cysteine/cystine (Cys/CySS), in plasma are useful indicators of systemic oxidative stress and other medically relevant physiological states [21]. Several lines of evidence indicate that perturbations in the extracellular thiol/disulfide redox environment correlate with the progression and severity of acute lung injury. Cysteine (Cys) and its disulfide Cystine (CySS) constitute the most abundant, low-molecular-weight thiol/disulfide redox couple in the plasma, and Cys homeostasis is adversely affected during the inflammatory response to infection and injury. While much emphasis has been placed on GSH and GSSG, little is known about the regulation of the Cys/CySS couple in acute lung injury [22]. The calculated redox states of the GSH/GSSG and Cys/CySS couples showed no statistically significant differences between the experimental and control groups.
Uric acid is the metabolic degradation product of xanthine and a potent hydrophilic antioxidant that scavenges certain oxygen radicals [47]. It has also been shown to stabilize ascorbic acid in human plasma at physiological concentrations [48]. Several lines of evidence suggest that uric acid in upper airway secretions may play a significant role in removing inhaled ozone [37]. Others have concluded that the reducing and acidic properties of urate are important in the effective scavenging of peroxynitrite and that Cys and ascorbic acid enhance urate’s antioxidant effect by reducing urate-derived radicals [49]. Our findings in the present study that increases of uric acid in BALF by both doses of ozone in the early ozone post-exposure period and by the high dose 7 h after the exposure are surprising since BALF contains ascorbic acid, UA, and GSH all of which react easily with ozone [50]. Among these three antioxidants, ascorbic acid decreased, GSH remained the same and uric acid increased. A mechanism which may explain the increased uric acid in BALF is enhanced permeability of the lung to blood plasma, which contains concentrations of uric acid commensurate with the increased BALF protein observed here (data not shown). The increase in uric acid might also be attributed to increased purine nucleotide catabolism in the lung with increased degradation of xanthine. Our histopathology findings and other studies have demonstrated that ozone could induce apoptosis and massive necrosis in lung tissue [51]. Plasma uric acid concentration was increased in this study by only the high dose (5 ppm) of ozone inhalation 7 hours after the exposure and did not show any other time or dose dependence. Results from previous studies have demonstrated that ozone exposure could increase plasma uric acid concentration in humans and in guinea pigs [52].
In summary, exposure of Fisher rats to ozone inhalation did not cause a significant decrease in plasma or BALF concentrations of various antioxidant substances in a time- and dose-dependent pattern. On the basis of this finding, we suggested that antioxidants measured in plasma or BALF do not play a major role in identifying early ozone-induced oxidative lung injury. We previously found that measurements of plasma antioxidants were unsuccessful biomarkers of oxidative damage by CCl4 [17]. Again, this continuation of the Biomarkers of Oxidative Stress Study (BOSS) found that rat plasma antioxidants - ascorbic acid, α-, δ-, γ-tocopherols, 5-nitro-γ-tocopherol, tocol, glutathione (GSH/GSSG), cysteine (Cys/CySS) and uric acid - were not further decreased by the ozone exposure and therefore did not constitute selective, sensitive and specific biomarkers for oxidative damage. We concluded that measurement of antioxidants in plasma is not a useful choice for the assessment of oxidative damage by ozone in vivo.
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences Intramural Research Program, NIH.
The authors thank Jean Corbett, Ralph Wilson, John Seely, Ralph Slade, Joel Norwood, John McKee, Robert McConnaughey, Kay Crissman, Judy Richards, Linda K. Wong and Qu Feng for excellent technical support. The authors also wish to thank Dr. Ann Motten and Ms. Mary J. Mason for editorial assistance.
Footnotes
Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halliwell B. The wanderings of a free radical. Free Radic Biol Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Pryor WA. Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radic Biol Med. 1994;17:451–465. doi: 10.1016/0891-5849(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy CH, Hatch GE, Slade R, Mason RP. Application of the EPR spin-trapping technique to the detection of radicals produced in vivo during inhalation exposure of rats to ozone. Toxicol Appl Pharmacol. 1992;114:41–46. doi: 10.1016/0041-008x(92)90094-9. [DOI] [PubMed] [Google Scholar]
- 4.Vincent R, Janzen EG, Chen G, Kumarathasan P, Haire DL, Guenette J, Chen JZ, Bray TM. Spin trapping study in the lungs and liver of F344 rats after exposure to ozone. Free Radic Res. 1996;25:475–488. doi: 10.3109/10715769609149070. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proc Am Thorac Soc. 2007;4:240–246. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren HS, Devlin RB, Graham DE, Mann R, McGee MP, Horstman DH, Kozumbo WJ, Becker S, House DE, McDonnell WF, Bromberg PA. Ozone-induced inflammation in the lower airways of human subjects. Am Rev Respir Dis. 1989;139:407–415. doi: 10.1164/ajrccm/139.2.407. [DOI] [PubMed] [Google Scholar]
- 7.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol. 1994;266:L612–L619. doi: 10.1152/ajplung.1994.266.6.L612. [DOI] [PubMed] [Google Scholar]
- 8.Gilmour MI, Hmieleski RR, Stafford EA, Jakab GJ. Suppression and recovery of the alveolar macrophage phagocytic system during continuous exposure to 0.5 ppm ozone. Exp Lung Res. 1991;17:547–558. doi: 10.3109/01902149109062864. [DOI] [PubMed] [Google Scholar]
- 9.Gilmour MI, Park P, Selgrade MK. Ozone-enhanced pulmonary infection with Streptococcus zooepidemicus in mice. The role of alveolar macrophage function and capsular virulence factors. Am Rev Respir Dis. 1993;147:753–760. doi: 10.1164/ajrccm/147.3.753. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman MJ, Fabbri LM, O’Byrne PM, Gold BD, Aizawa H, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis. 1983;127:686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- 11.O’Byrne PM, Walters EH, Gold BD, Aizawa HA, Fabbri LM, Alpert SE, Nadel JA, Holtzman MJ. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis. 1984;130:214–219. doi: 10.1164/arrd.1984.130.2.214. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Taube C, Joetham A, Takeda K, Kodama T, Dakhama A, McConville G, Allen CB, Sfyroera G, Shultz LD, Lambris JD, Giclas PC, Holers VM, Gelfand EW. Complement activation is critical to airway hyperresponsiveness after acute ozone exposure. Am J Respir Crit Care Med. 2004;169:726–732. doi: 10.1164/rccm.200307-1042OC. [DOI] [PubMed] [Google Scholar]
- 13.Pino MV, Stovall MY, Levin JR, Devlin RB, Koren HS, Hyde DM. Acute ozone-induced lung injury in neutrophil-depleted rats. Toxicol Appl Pharmacol. 1992;114:268–276. doi: 10.1016/0041-008x(92)90077-6. [DOI] [PubMed] [Google Scholar]
- 14.Kleeberger SR, Bassett DJP, Jakab GJ, Levitt RC. A genetic model for evaluation of susceptibility to ozone-induced inflammation. Am J Physiol. 1990;258:L313–L320. doi: 10.1152/ajplung.1990.258.6.L313. [DOI] [PubMed] [Google Scholar]
- 15.Kleeberger SR. Genetic susceptibility to ozone exposure. Toxicol Lett. 1995;82–83:295–300. doi: 10.1016/0378-4274(95)03564-8. [DOI] [PubMed] [Google Scholar]
- 16.Polidori MC, Stahl W, Eichler O, Niestroj I, Sies H. Profiles of antioxidants in human plasma. Free Radic Biol Med. 2001;30:456–462. doi: 10.1016/s0891-5849(00)00345-2. [DOI] [PubMed] [Google Scholar]
- 17.Kadiiska MB, Gladen BC, Baird DD, Dikalova AE, Sohal RS, Hatch GE, Jones DP, Mason RP, Barrett JC. Biomarkers of oxidative stress study: are plasma antioxidants markers of CCl4 poisoning? Free Radic Biol Med. 2000;28:838–845. doi: 10.1016/s0891-5849(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 18.Hensley K, Williamson KS. HPLC-electrochemical detection of tocopherol products as indicators of reactive nitrogen intermediates. Methods Enzymol. 2005;396:171–182. doi: 10.1016/S0076-6879(05)96017-5. [DOI] [PubMed] [Google Scholar]
- 19.Kutnink MA, Hawkes WC, Schaus EE, Omaye ST. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987;166:424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- 20.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LAS. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–184. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic Biol Med. 2009;47:1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer SS, Jones DP, Brigham KL, Rojas M. Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury. Am J Respir Cell Mol Biol. 2009;40:90–98. doi: 10.1165/rcmb.2007-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryor WA, Squadrito GL, Friedman M. A new mechanism for the toxicity of ozone. Toxicol Lett. 1995;82–83:287–293. doi: 10.1016/0378-4274(95)03563-x. [DOI] [PubMed] [Google Scholar]
- 24.Balmes JR. The role of ozone exposure in the epidemiology of asthma. Environ Health Perspect. 1993;101(Suppl 4):219–224. doi: 10.1289/ehp.93101s4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 26.Valacchi G, Pagnin E, Corbacho AM, Olano E, Davis PA, Packer L, Cross CE. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic Biol Med. 2004;36:673–681. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- 28.Mustafa MG. Biochemical basis of ozone toxicity. Free Radic Biol Med. 1990;9:245–265. doi: 10.1016/0891-5849(90)90035-h. [DOI] [PubMed] [Google Scholar]
- 29.Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson JA, Hodges RE. Recommended dietary intakes (RDI) of vitamin C in humans. Am J Clin Nutr. 1987;45:693–703. doi: 10.1093/ajcn/45.4.693. [DOI] [PubMed] [Google Scholar]
- 31.May JM. Is ascorbic acid an antioxidant for the plasma membrane? FASEB J. 1999;13:995–1006. doi: 10.1096/fasebj.13.9.995. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B. Vitamin C and genomic stability. Mutat Res. 2001;475:29–35. doi: 10.1016/s0027-5107(01)00072-0. [DOI] [PubMed] [Google Scholar]
- 33.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 34.Tepper JS, Costa DL, Lehmann JR, Weber MF, Hatch GE. Unattenuated structural and biochemical alterations in the rat lung during functional adaptation to ozone. Am Rev Respir Dis. 1989;140:493–501. doi: 10.1164/ajrccm/140.2.493. [DOI] [PubMed] [Google Scholar]
- 35.Kari F, Hatch G, Slade R, Crissman K, Simeonova PP, Luster M. Dietary restriction mitigates ozone-induced lung inflammation in rats: a role for endogenous antioxidants. Am J Respir Cell Mol Biol. 1997;17:740–747. doi: 10.1165/ajrcmb.17.6.2844. [DOI] [PubMed] [Google Scholar]
- 36.Lavnikova N, Prokhorova S, Lakhotia AV, Gordon R, Laskin DL. Distinct inflammatory responses of adherent vascular lung neutrophils to pulmonary irritants. J Inflamm. 1998;48:56–66. [PubMed] [Google Scholar]
- 37.Cross CE, Motchnik PA, Bruener BA, Jones DA, Kaur H, Ames BN, Halliwell B. Oxidative damage to plasma constituents by ozone. FEBS Lett. 1992;298:269–272. doi: 10.1016/0014-5793(92)80074-q. [DOI] [PubMed] [Google Scholar]
- 38.Vincent R, Vu D, Hatch G, Poon R, Dreher K, Guenette J, Bjarnason S, Potvin M, Norwood J, McMullen E. Sensitivity of lungs of aging Fischer 344 rats to ozone: assessment by bronchoalveolar lavage. Am J Physiol. 1996;271:L555–L565. doi: 10.1152/ajplung.1996.271.4.L555. [DOI] [PubMed] [Google Scholar]
- 39.Ingold KU, Webb AC, Witter D, Burton GW, Metcalfe TA, Muller DPR. Vitamin E remains the major lipid-soluble, chain-breaking antioxidant in human plasma even in individuals suffering severe vitamin E deficiency. Arch Biochem Biophys. 1987;259:224–225. doi: 10.1016/0003-9861(87)90489-9. [DOI] [PubMed] [Google Scholar]
- 40.Ingold KU, Bowry VW, Stocker R, Walling C. Autoxidation of lipids and antioxidation by α-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc Natl Acad Sci U S A. 1993;90:45–49. doi: 10.1073/pnas.90.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowry VW, Ingold KU, Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992;288(Pt 2):341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-α-tocopherols are secreted without discrimination in human chylomicrons, but RRR-α-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res. 1990;31:675–685. [PubMed] [Google Scholar]
- 43.Traber MG, Rudel LL, Burton GW, Hughes L, Ingold KU, Kayden HJ. Nascent VLDL from liver perfusions of cynomolgus monkeys are preferentially enriched in RRR- compared with SRR-α-tocopherol: studies using deuterated tocopherols. J Lipid Res. 1990;31:687–694. [PubMed] [Google Scholar]
- 44.Kodavanti UP, Costa DL, Richards J, Crissman KM, Slade R, Hatch GE. Antioxidants in bronchoalveolar lavage fluid cells isolated from ozone--exposed normal and ascorbate-deficient guinea pigs. Exp Lung Res. 1996;22:435–448. doi: 10.3109/01902149609046034. [DOI] [PubMed] [Google Scholar]
- 45.Gille L, Staniek K, Rosenau T, Duvigneau JC, Kozlov AV. Tocopheryl quinones and mitochondria. Mol Nutr Food Res. doi: 10.1002/mnfr.200900386. [DOI] [PubMed] [Google Scholar]
- 46.Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 47.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sevanian A, Davies KJA, Hochstein P. Conservation of vitamin C by uric acid in blood. J Free Radic Biol Med. 1985;1:117–124. doi: 10.1016/0748-5514(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 49.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med. 2005;38:515–526. doi: 10.1016/j.freeradbiomed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Kirichenko A, Li L, Morandi MT, Holian A. 4-hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol Appl Pharmacol. 1996;141:416–424. doi: 10.1006/taap.1996.0307. [DOI] [PubMed] [Google Scholar]
- 52.Mudway IS, Blomberg A, Frew AJ, Holgate ST, Sandstrom T, Kelly FJ. Antioxidant consumption and repletion kinetics in nasal lavage fluid following exposure of healthy human volunteers to ozone. Eur Respir J. 1999;13:1429–1438. doi: 10.1183/09031936.99.13614399. [DOI] [PubMed] [Google Scholar]