Abstract
Gold has been used as a therapeutic agent to treat a wide variety of rheumatic diseases including psoriatic arthritis, juvenile arthritis and discoid lupus erythematosus. Although the use of gold has been largely superseded by newer drugs, gold nanoparticles are being used effectively in laboratory based clinical diagnostic methods whilst concurrently showing great promise in vivo either as a diagnostic imaging agent or a therapeutic agent. For these reasons, gold nanoparticles are therefore well placed to enter mainstream clinical practice in the near future. Hence, the present review summarizes the chemistry, pharmacokinetics, bio-distribution, metabolism and toxicity of bulk gold in humans based on decades of clinical observation and experiments in which gold was used to treat patients with rheumatoid arthritis. The beneficial attributes of gold nanoparticles, such as their ease of synthesis, functionalization and shape control are also highlighted demonstrating why gold nanoparticles are an attractive target for further development and optimization. The importance of controlling the size and shape of gold nanoparticles to minimize any potential toxic side effects is also discussed.
Keywords: Nanoparticles, Gold, Toxicity, Humans, Applications
Introduction
One of the most significant developments in recent years has been the development of new materials in the nanometre scale called nanoparticles. Nanoparticles are expected to form the basis of many of the technological and biological innovations of this century, exhibiting distinct advantageous physical, chemical and biological properties. They also have the potential to help establish specific beneficial processes and achieve selectivity within biological settings. To date, a large number of nanoparticles have been synthesized, especially those made from noble metals such as gold. Gold nanoparticles can be manufactured into a variety of shapes including gold nanospheres, nanorods, nanobelts, nanocages, nanoprisms and nanostars.1 The chemical, optical and electromagnetic properties of gold nanoparticles are strongly influenced by their size and shape. For example, in comparison to metallic gold which is golden yellow, spherical gold nanoparticles have a visible red wine color whilst gold nanorods are blue (aspect ratio 2–3) or black (aspect ratio 3) in solution.2 The ease of synthesis and the unique properties of gold nanoparticles makes them ideal candidates for translation from the laboratory setting into the clinical arena for use in humans. Additional enthusiasm for the use of gold nanoparticles in patients stems from gold’s previous clinical use in treating several diseases, most notably rheumatoid arthritis (RA), with minimal biological side effects. Although gold may have fallen out of favor as a mainstream therapeutic agent, its use in nanoparticles is set to revive its application in medical care in both patient diagnosis and treatment. We review the chemistry, biology, pharmacokinetics and toxicology of gold and consider its new use as a clinically applicable nanoparticle, thereby potentially seeing the resurgence of gold use in everyday clinical practice in the near future.
The History of Gold
The use of gold for medicinal purposes dates back to 2,500 BC to the ancient Chinese and Egyptians.3, 4 In medieval Europe, numerous recipes for gold elixirs existed and in the 17th and 19th century gold was used to treat fevers and syphilis respectively.5 The use of gold in modern medicine began in 1890 when the German bacteriologist Robert Koch discovered that gold cyanide was bacteriostatic to the tubercle bacillus in vitro.3 This subsequently led to the treatment of tuberculosis with gold in the early 20th century. As RA was initially thought to be an atypical form of tuberculosis,6 Laude used gold to treat RA in 1927. Although gold therapy proved to be ineffective for tuberculosis, a study by the Empire Rheumatism Council confirmed gold to be effective in RA, with Forestier showing beneficial results in RA patients in 1935.5, 7 Gold has since been used as a therapeutic agent to treat a wide variety of rheumatic diseases including psoriatic arthritis,8 juvenile arthritis and discoid lupus erythematosus,9, 10 however, its use has been largely superseded by newer drugs. Gold has also been used in several other areas of Medicine including prostheses in dentistry11 and ophthalmology,12 gene delivery13 and gold coated coronary14 and renal15 stents, to name a few (Table 1).
Table 1.
Examples of the use of bulk gold in clinical practice
| Disease | Gold Form | Name | Role | Mechanism | Time Period | Side Effects |
|---|---|---|---|---|---|---|
| Oral Cavity | Gold Alloy | Jensen Foil | Cavity Filling | Dental Support | 16th Century – Present | Rare Allergic Reaction |
| Rheumatoid Arthritis | Gold Sodium Thiomalate (IM) | Aurolate | Anti-inflammatory | Mitochrondrial inactivation and apoptosis | 1930s – present | Nausea, Vomiting, GI Distress |
| Rheumatoid Arthritis | Aurothioglucose (IM) | Auranofin | Anti-inflammatory | Unclear | 1930s – Present | Dermatologic (Dermatitis, pruritus, rash) |
| Psoriatic Arthritis | Gold Sodium Thiomalate (IM) | Aurolate | Anti-inflammatory | Mitochrondrial inactivation and apoptosis | 1930s – present | Nausea, Vomiting, GI Distress |
| Discoid Lupus Erythematosus | Aurothioglucose (PO) | Auranofin | Lesion Reduction | Unclear | 1980s – 1990s | GI Distress |
The Chemistry of Gold
Gold is a noble metal found in Group 1B of the periodic table with an atomic number of 197. Gold can exist in a number of oxidation states: (-I), (0), (I), (II), (III), (IV) and (V), however, only gold (0), (I) and (III) are stable in aqueous solution. Hence, in vivo, gold exists in equilibrium between its metallic ground state (gold (0)) and its oxidized states (gold (I) or gold (III)).6 Metallic gold does not oxidize or burn in air, even when heated, and has been shown to be inert to strong alkalis and acids thereby making it one of the least chemically reactive metals known to man.11 In contrast, gold (I) and (III) are unstable with respect to gold (0), with gold (III) being a strong oxidizing agent which is reduced to gold (I) by biologically occurring reductants such as thiols.5 As gold (I) preferentially reacts with S-donors, rather than O- and N- donors, it can be stabilized by thiolate ligands. These resulting gold thiol compounds then undergo biological ligand exchange reactions which account, in part, for their pharmacological activity.5
Gold in Humans
Humans contain a mean of 0.35µg of gold (0) per gram of dry tissue weight16 which, according to calculations by Merchant, equates to 2.45mg of gold in an average 70 kg man.6 Blood gold concentrations in healthy human subjects have also been reported to be around 0–0.001 ppm,17 with additional studies reporting small quantities of gold in hair (0.3 µg/g), skin (0.03 µg/g) and nails (0.17 µg/g).18–20 Up to 0.8 µg of gold per dry weight has also been measured in fingers beneath gold wedding rings of normal individuals.21 Interestingly, gold is also sometimes used in food in very minute quantities in pastries, chocolates and even alcoholic beverages.22
Gold Therapy
Based on the chemistry of gold, gold (I) is used as the main therapeutic agent as it is water soluble, less reactive than gold (III) and is easily stabilized in a complex by the addition of ligands. Gold can be delivered to patients intravenously, intramuscularly or orally with gold preparations specifically designed for each particular route of administration. Accordingly, gold taken orally needs to be lipid soluble for it to be absorbed within the gastrointestinal tract and will therefore have different physiochemical, pharmacokinetic and toxicological properties compared to water soluble gold that is injected.9, 23 This is supported by experiments demonstrating only 1% of injectable gold is absorbed when given orally compared to 100% when given intramuscularly.24 Although gold has been recently used as an anticancer and antimicrobial agent,5 most of the studies on the efficacy, toxicity and pharmacokinetics of gold preparations were previously performed in patients with RA who were treated with gold in the late 20th century. However, despite gold being used in clinical practice for several decades there is still considerable debate as to whether injectable or oral gold preparations are better for patients.25 Initially, oral gold treatments that were developed in the 1980s, such as auranofin, were thought to have improved pharmacokinetic profiles with less tissue retention, less toxicity and reduced serum gold levels that were maintained for longer.5 However, their clinical side effect profile and fear of long term immune suppression have resulted in injectable compounds, such as gold sodium thiomalate, remaining the preferred gold drugs for RA treatment.25
Gold Pharmacokinetics and Biodistribution
The bioavailability of gold in patients very much depends on the route of administration. Whilst injectable gold compounds are fully absorbed with maximum levels attained after about 2 hours,26 only 20–25% of oral gold is absorbed.23, 24, 27 Furthermore, intermittent dosing regimens of injectable gold result in fluctuating blood gold levels with high peak and low trough concentrations.28 In contrast, oral gold preparations can be taken regularly and made with prolonged blood half-life preparations, resulting in a nearly constant concentration of gold for the duration of a patient’s treatment. However, with chronic daily oral administration the serum gold concentrations reaches a plateau, and in some cases, despite constant dosing, gradually starts to decline.29 Various mechanisms have been put forward to explain this phenomenon including insufficient drug compliance, increased drug clearance or an increased distribution volume (i.e. a shift from protein-bound gold to cell-bound gold).29
Following absorption of gold, either from tissues or the gastrointestinal tract, approximately 95% is bound to albumin and/or globulin where it can remain within the plasma for several months.4, 30 Gold has also been found within the cellular compartment of blood, primarily in the erythrocyte fraction.31, 32 Here, gold has been shown to be within or attached to the membranes of red blood cells (RBCs) ,24, 33 with uptake dependent on either the amount of gold available for red cell precursors in the bone marrow or the gold binding capacity of plasma proteins.32 Indeed, it has been shown that uptake into RBCs ceases after 48 hours even though there is still considerable gold in the plasma, presumably as all the gold by this time is tightly bound to plasma proteins. As gold uptake into RBCs differs amongst people, being more pronounced in smokers,34 this could explain the large variability in gold distribution seen amongst patients. Gold is widely distributed throughout the body with organs of the reticuloendothelial system, especially the lymph nodes, having the greatest affinity for this heavy metal.16 The liver and bone marrow have each been shown to account for 25% of the total body gold burden with the skin and bone each accounting for 20%.23 Furthermore, the exact form of gold in these locations remains unknown although it appears to be inactive as it remains detectable in tissue samples taken from patients whom had been treated with gold years earlier.35 In general, gold has little affinity for keratinous tissue,18 but it can accumulate in the skin dermis36, 37 during intravenous administration, with negligible levels recorded when gold is given orally.28 At very high levels of intravenous administration, gold has also been shown to deposit in the cornea as detected by slit lamp examination.38
Gold Metabolism and Excretion
Gold is primarily excreted in the urine and feces and although the rate of excretion varies considerably from patient to patient, the basic pattern remains the same.30, 36 Following intramuscular injection of gold, it has been shown that urinary excretion was greatest during the first day post-injection whilst fecal excretion was greatest during the middle of the week.30 Although, the amount of gold excreted in the urine and feces increases as the amount of injected gold increases, the excretion rate was not directly proportional to the amount injected.30 The high binding capacity of albumin for gold may explain the slow rate of gold clearance throughout the week following gold injection. When gold is given orally, 85–95% is excreted in feces and the remaining 15–5% in urine, regardless of dose.27, 28 The majority of gold recovered in the feces represents non-absorbed gold, gold breakdown products, gold shed from mucosal cells to which it was adsorbed and a minor contribution from the biliary tract.24, 39 Once gold treatment is established, a dynamic equilibrium is set up in the body with gold moving between the blood, body stores, urine and feces.
Gold Toxicity
Any toxicity associated with gold depends on its oxidation state when given to patients. Metallic gold (gold (0)) is an extremely inert metal which is widely used throughout the world in both jewelry and prostheses. Indeed, most of the human population has had prolonged dermal contact with gold (0) in the form of jewelry, with only exceptionally rare cases of adverse reactions or allergic contact dermatitis. Furthermore, approximately half of the 1 billion people in the modern industrialized world carry dental prostheses made of gold with relatively few cases of oral lesions being reported despite the close prolonged contact of the metal with the oral mucosa.6 However, gold (0) can, in very minute amounts, be converted to gold (I) by amino acids contained in sweat and saliva which can then be absorbed through the skin or gingival mucosa and later enter phagocytic and antigen presenting cells.40 That notwithstanding, the metabolic impact of this is usually insufficient to evoke clinical symptoms.6 Moreover, as gold (0) is readily available, has a very low toxicity profile and can be made into a consistently small size and shape, it has been used as a delivery vehicle for gene therapy.41 Indeed, microprojectile bombardment of cells with DNA on gold particles has been developed as an effective method of high frequency gene transfer with minimal damage to living cells.42 Experiments injecting naked gold beads into the epidermis of pigs, whose skin is an excellent model for human skin, concluded that apart from acute impact physical effects, which resulted in mild transient dermal irritation, there were no direct toxicities or adverse effects on health, survival, clinical chemistry or hematology values related to the gold beads.6
Gold (I) is normally used as therapeutic agent in both injectable and oral preparations. The toxicities surrounding gold (I) have been primarily understood by examining patients treated with gold for RA, however frustratingly, serum and urine levels of gold have been of no value in predicting impending toxicity in patients. The most common toxicity associated with gold treatment is skin and mucous membrane hypersensitivity reactions, with non-specific pruritic erythematous, macular and papular rashes appearing first. Other rarer skin reactions include cheilitis, eosinophilia, chronic papular eruptions, contact sensitivity, erythema nodosum, allergic contact purpura, exfoliative dermatitis and pityriasis rosea.6 This diverse range of dermal reactions appears not to depend on the gold concentrations in the skin and rarely occur in patients who receive less than 250 mg of gold salts.11 In fact, it is generally regarded that these reactions represent the balance between the total body burden of gold salts and the patient’s genetic and metabolic makeup. Management involves the cessation of gold therapy with most cases resolving within 3 months of onset depending on their extent and severity. The most common form of gold-induced dermatitis is non-allergic, since following clearance of the original eruption, patients can be restarted on gold treatment without developing further dermatitis.43 In contrast, allergic contact dermatitis occurs at a lower incidence and represents an immune reactivity to gold which usually necessitates total cessation of gold therapy.44 Diarrhea is also frequently associated with administration of gold complexes, but with a greater incidence when patients use oral gold preparations.45 Less frequently, gold has been associated with nephrotoxicity as demonstrated by minor and transient proteinuria in people treated with injectable gold complexes.3, 46 Occasionally, this may progress to glomerulonephritis with nephritic syndrome although patients usually recover fully within a few months.9, 47 Hematological abnormalities can also be sometimes produced by gold complexes and include eosinophilia, thrombocytopenia9 and rarely aplastic anaemia probably as a result of a direct inhibition of myelopoiesis.48 Several other reports have also mentioned the rare consequences of using gold complexes including entercolitis with bloody diarrhea,49, 50 diffuse inflammatory lung reactions51 and neurotoxicity.52 Gold therapy is not recommended during pregnancy,53 as animal studies have shown it to be teratogenic.54 Caution is also advised in the puerperium, despite conflicting reports as to whether significant absorption occurs in the infant, since gold can also be found in breast milk.55
Gold (III) is rarely used as a primary therapeutic agent as it is a strong oxidizing agent and thus very reactive. However, gold (I) can transform into gold (III) within phagolysosomes, which may account, in part, for some of its toxic effects. In brief, gold (I) is oxidized to gold (III) via a redox system involving myeloperoxidase and other lysosomal enzymes within phagolysosomes containing gold (i.e. aurosomes). Gold (III) then diffuses away from its site of generation where it can interact with and denature “self-proteins” surrounding proteins, thereby possibly explaining why auto-immunity occurs during a few cases of gold therapy.
Gold Nanoparticle Synthesis
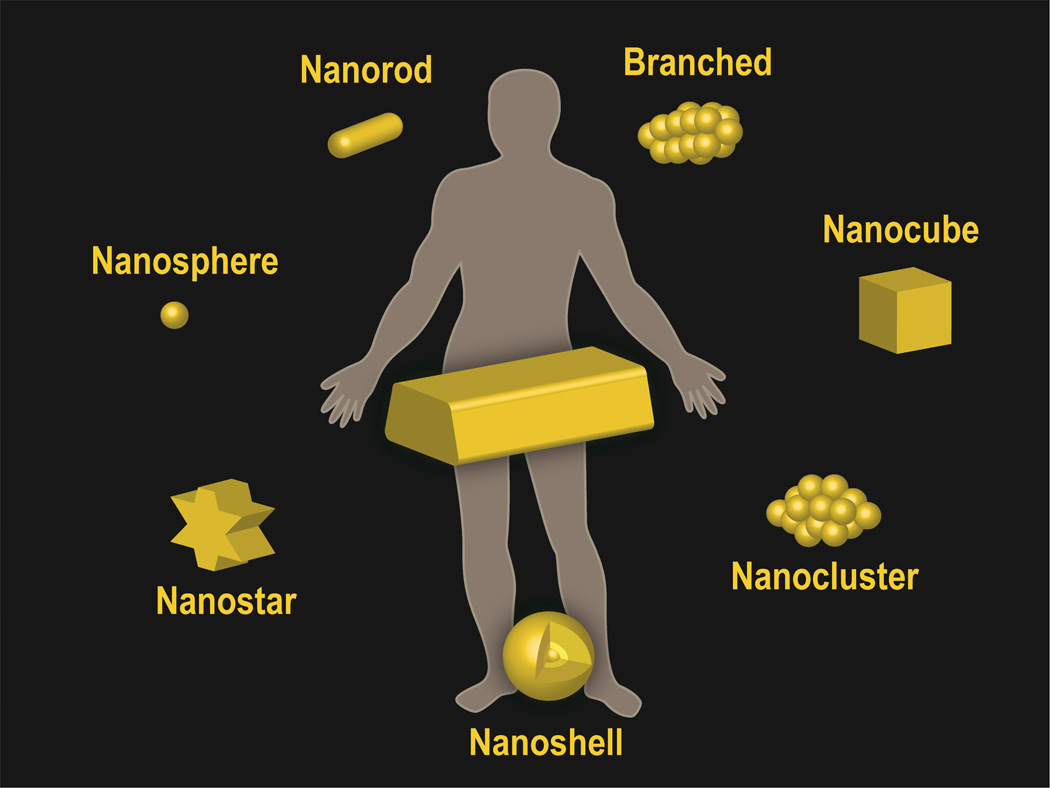
Gold nanoparticles can be synthesized into a variety of different sizes and shapes (Figure 1) by different strategies,56, 57 however, the most common method is by chemical or electrochemical reduction of a gold (III) precursor. Control over shape and size is achieved through careful experimental conditions including the specific reducing agent, reaction time, temperature, and use of a capping agent, the latter binds to select nanoparticle faces and blocks growth beyond a certain nanometer range.58 The most common method to prepare gold spherical nanoparticles is a single-phase water based reduction method using citrate reduction as described by Turkevich59 and Frens.60 By varying the concentration of citrate and gold for the thermal reduction, spherical particles of different sizes can be made.61 Newer methods, such as UV initiated particle growth, have also recently been introduced to improve the size distribution and spherical shape of larger sized gold nanoparticles (i.e. 9–120 nm).61 As spherical gold nanoparticles have absorption peaks near 540 nm, the nanoparticle size and shape can be modulated to bring this peak closer to the optical window of tissue which is between 700 and 800 nm. Furthermore, the surface plasmon resonance (SPR) peaks of gold nanoparticles can also now be optimally tuned,62 thereby allowing multiplexing of gold nanoparticles. Recently, significant progress has been made in synthesizing non-spherical gold nanoparticles using seed-mediated growth.56, 63 For gold nanorods, a gold spherical nanoparticle seed (i.e. diameter of 1–5 nm) is first synthesized. Next, a solution containing more gold ions, cetyl tetrammonium bromide (CTAB) and ascorbic acid (a mild reducing agent) is added to selectively reduce gold (III) to gold (I). A seed solution containing citrate-capped, penta-twinned gold nanoparticles then catalyses the reduction of gold (I) ions on their surface with a careful choice of experimental conditions enabling the seed to grow and elongate into a nanorod.56 The aspect ratio is controlled by both the concentration of silver nitrate in the growth solution. Branched gold nanoparticles are more difficult to synthesize reproducibly, however, their sharp edges and corresponding high localization of SPR modes makes them excellent candidates for biological applications.56 Gold nanostars with a magnetic core are able to couple polarized resonance with spatial control.64 By placing gold nanostars in an external magnetic field, the orientation of the points which make up the star shape (and hence field enhancement) are controlled. Like spheres, gold nanoprisms65 maintain an aspect ratio near unity, yet have red-shifted absorption resonance peaks. Nanoshells are created by coating a silica or polymeric core with a thin gold layer,66 the thickness of which controls the optical properties of the nanoparticle which can be subsequently tuned for efficient heating when irradiated with an near infrared (NIR) laser. A final class of gold nanoparticle are the gold nanoclusters, which contain hundreds of gold atoms (e.g. Au102) and behave as intermediates of nanoparticles and molecular gold.67 As gold nanoparticles can be easily functionalized and thus targeted, they are therefore ideal particles for in vivo gene delivery, biological imaging, diagnostics and disease treatment.
Figure 1.
Schematic representations of gold nanoparticles used in clinical practice
Gold Nanoparticle Toxicity
The toxicity of nanoparticles has also been suggested to differ dramatically from their corresponding bulk material. The small size of nanoparticles will affect their mode of endocytosis and cellular processing.68 In addition, their high surface area to volume ratio can dramatically alter their chemical and physical properties resulting in them possessing unexpected toxicities and biological interactions. Since nanoparticles will also have a greater amount of their surface in direct contact with the body, they are therefore more reactive to both themselves and their surrounding environment.69 The main molecular mechanism by which nanoparticles incur toxicity has been hypothesized to be from an increase in oxidative stress as a result of free radical formation.68 These reactive species are exceedingly toxic in vivo, especially within intracellular compartments, resulting in the oxidation and damage of lipids, proteins and DNA. Whilst the slow clearance and tissue accumulation of these free radical producing nanoparticles makes organs of the reticuloendothelial system (i.e the liver and spleen) targets for toxicity, the high blood flow through organs such as the kidney and lungs also place these organs at high risk of oxidative damage. When nanoparticles are introduced into the systemic circulation, they can also interact with blood components to cause hemolysis and thrombosis,69 and with the immune system to cause immunotoxicity.70 Furthermore, nanoparticle aggregation following systemic administration not only leads to a loss of nanoparticle function but can also cause end organ damage from capillary occlusion.
Studies by Chithrani and Chan have shown gold nanoparticles enter cells via a receptor-mediated clathrin-dependent endocytosis pathway, with 50 nm nanoparticles taken up at a faster rate and higher concentration compared to other nanoparticle sizes.71, 72 In general, they demonstrated that the rate of uptake of gold nanoparticles into cells is lower with an increasing aspect ratio. In addition, gold spherical nanoparticles have a greater efficiency of uptake compared to gold nanorods due to the thermodynamic driving forces for membrane wrapping and receptor diffusion kinetics.71 Despite this, gold nanorods have been shown to be more toxic compared to spherical particles.73 One possible explanation for this could lie in their method of synthesis with the cationic surfactant CTAB, however, reports regarding this have been conflicting.69 As the size of the gold nanoparticles decrease, their rate of exocytosis from cells dramatically and linearly increase, with 14 nm nanoparticles leaving cells twice as fast compared to particles of 100 nm size.71 Furthermore the fraction of gold nanorods exocytosed was higher than spherical-shaped nanostructures. Studies by Pan and colleagues have also shown that the cytotoxicity of gold nanoparticles primarily depends on their size, with particles 1–2 nm in diameter being toxic whereas larger 15 nm gold particles are comparatively non-toxic, irrespective of the cell type tested.74 Furthermore, particles of 1.4 nm were found to be highly toxic as they irreversibly bind to the major grooves of B-DNA; an effect not observed with larger or smaller particles due to steric reasons. Although there are only a limited number of in vivo studies investigating the systemic effects of gold nanoparticles, 13 nm PEG-coated gold nanoparticles have been shown to have long circulating times, eventually accumulating in the liver where they can induce acute inflammation and apoptosis.75 The surface charge of gold nanoparticles has also been shown to be important in determining particle toxicity, with cationic gold nanoparticles exhibiting moderate toxicity owing to the electrostatic binding of the particles to the negatively charged cell membrane. In contrast, anionic particles have no toxicity as they are repelled from the membrane.76
Taken together, the size, shape and surface charge of gold nanoparticles need to be carefully considered when designing gold nanoparticles for human use in order to optimize their therapeutic function, whilst concurrently decreasing their toxicity profile by minimizing their cellular uptake and interactions. One way to reduce any potential toxicity from gold nanoparticles is by the addition of surface polyethylene glycol (PEG). Polyethylene glycol is a coiled polymer of repeating ethylene ether units with dynamic conformations which is inexpensive, versatile, and FDA-approved.77 In both drug delivery and imaging applications, the addition of PEG to nanoparticles reduces uptake by the reticuloendothelial system and increases circulation time versus uncoated counterparts.78 Recent studies have also shown that PEGylated nanoparticles generally have lower accumulation in the liver compared to non-PEGylated nanoparticles and higher tumor accumulation versus background.79 Aggregation of nanoparticles also decrease following the addition of PEG due to passivation of the nanoparticle surface and the reduction in the coating of serum and tissue proteins, resulting in so-called “stealth” behavior. Polyethylene glycol also increases the solubility of nanoparticles in buffer and serum due to the hydrophilic ethylene glycol repeats and the enhanced permeability and retention (EPR) effect.80, 81 Alternative passivating polymers which can be added to gold nanoparticles besides PEG include chitosan, dextran, polyvinyl pyrrolidone (PVP) as well as the co-polymer polylacticcoglycolic acid (PLGA).
Clinical use of gold nanoparticles
Over the past few years, gold nanoparticles have been used effectively in laboratory based clinical diagnostic methodologies (Table 2). In particular, DNA-functionalized gold nanoparticles can detect specific DNA and RNA sequences by rapidly binding to nucleotide sequences within a sample with high sensitivity.82 Furthermore, arrays using gold nanoparticles with specific chemical functionalities are currently being developed for biomarker platforms to detect, identify and quantify protein targets used for clinical diagnosis.58, 83 However, the main excitement concerning gold nanoparticles is their potential to cross over into clinical practice for use in humans.
Table 2.
Examples of the use of gold nanoparticles in clinical practice
| Type of Gold Nanoparticle |
Nanoparticle Size (nm) |
Role | Disease State |
Sponser/Lab |
|---|---|---|---|---|
| Nanosphere | 13 | siRNA delivery | Unspecified | Mirkin |
| Nanorod | 10 × 40 | Photothermal ablation; CT contrast and thermal imaging | Unspecified | Bhatia |
| Gold-silica Nanosphere | 60/140 | Raman Imaging | Colon Cancer | Gambhir |
| Gold Nanoshell (Aurolase™) | 150 | Photothermal therapy | Head and Neck cancer | NanoSpectra NCT00848042 |
| Gold Colloidal Nanosphere (Aurimune™) | 27 | Stimulate immune response to tumor growth | Solid Tumors | NCI NCT00436410 |
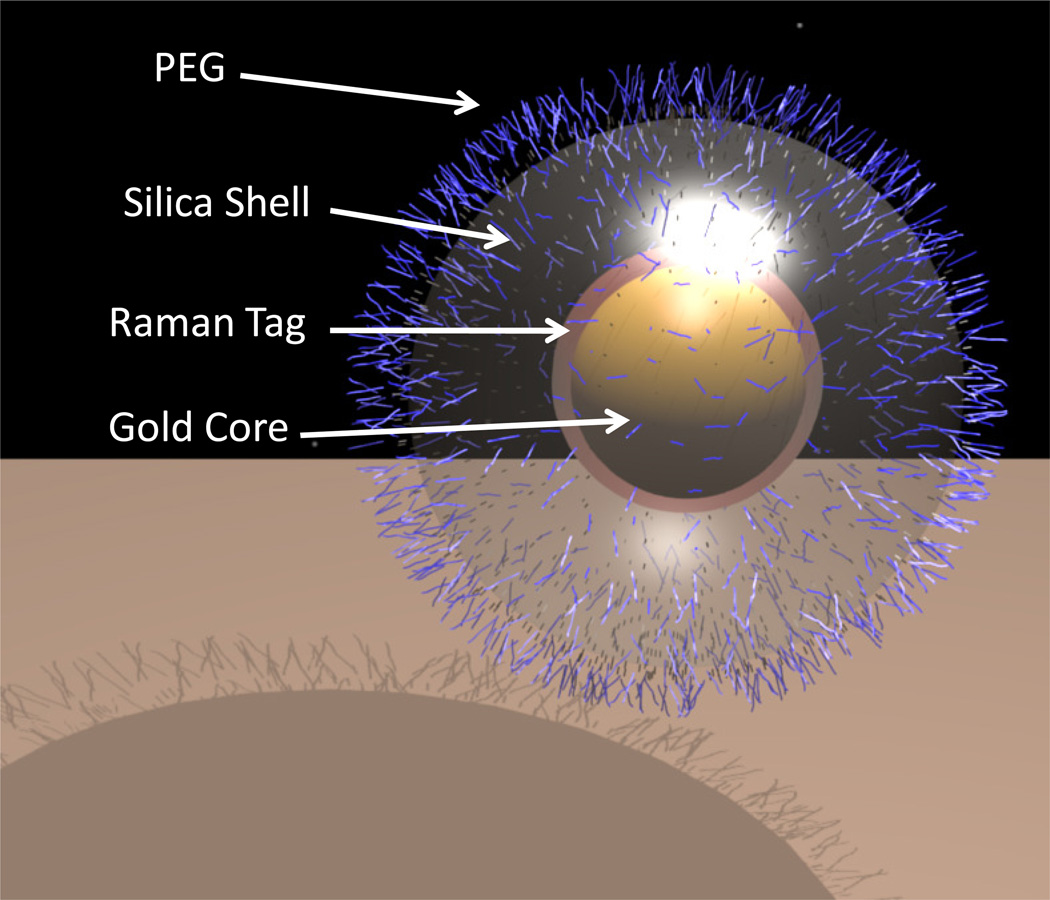
From an imaging perspective, gold nanoparticles have shown great promise for their use in computed tomography, Raman spectroscopy and photoacoustic imaging. Raman spectroscopy is an optically based technique which allows the molecular interrogation of tissues based on the inelastic scattering of light.84 However, to date this imaging modality has not crossed into mainstream clinical practice due to the limited depth penetration of the optical beam used to carry the Raman signal and the weak intrinsic signal generated by pathological tissues. The latter of these problems has recently been overcome by taking advantage of the phenomenon known as surface enhanced Raman scattering (SERS). SERS is a plasmonic effect where molecules adsorbed onto a nano-roughened noble metal surface experience a dramatic increase in the incident electromagnetic field, thereby resulting in high Raman intensities.85 Nanoparticles have therefore been created with a gold nanocore surrounded by a Raman organic molecule. This arrangement dramatically increases the incident electromagnetic field of the Raman organic molecule via SERS, thereby dramatically amplifying the intensity of the Raman signal. As the Raman organic molecules have a unique and narrow spectral signature, which can be changed between nanoparticles, this allows multiple nanoparticles to be independently detected simultaneously in vivo in a process known as multiplexing.86 The entire nanoparticle is encapsulated in a silica shell to hold the Raman organic molecule on the gold nanocore (Figure 2). This exciting discovery means that functionalized/targeted Raman gold nanoparticles may offer a non-invasive technique to detect early disease, especially in circumstances where the Raman probe can be applied closely to the target tissue. One example could be the potential use of functionalized Raman gold nanoparticles to detect early dysplastic lesions in the colon using a Raman based colonoscope. Furthermore, these Raman gold nanoparticles have been shown in human cell culture to cause negligible toxicity at low concentrations with only minimal cytotoxicty and oxidative stress was observed after prolonged exposure at high concentrations.87 Studies examining the fate of these nanoparticles in living animals have also shown that following intravenous administration, nanoparticles were removed from the circulation by marcophages in the liver and spleen with only a mild acute inflammatory response and an increase in oxidative stress in the liver.88 No evidence of significant toxicity was observed by clinical, histological, biochemical or cardiovascular parameters after 2 weeks. In addition, intrarectal administration of these nanoparticles demonstrate no significant bowel or systemic toxicity with no evidence of that these nanoparticles cross the bowel lumen. Although additional studies are required to investigate the long-term effects of these Raman gold nanoparticles, these initial results support the idea that they can be safely used in living subjects, especially when administered rectally, thereby supporting their clinical translation. Photoacoustic imaging is another imaging modality which allows deeper tissues to be imaged with high spatial resolution.89 In this technique, subjects are illuminated with short lazer pulses and as the light photons propagate through tissue, they are absorbed and converted into ultrasound waves which can be detected externally. However, as with Raman spectroscopy, many diseases do not exhibit a natural photoacoustic contrast and hence it is necessary to administer a photoacoustic contrast agent. One such agent showing great promise is the gold nanorod, which has a higher optical cross section than nanospheres in addition to a robust photoacoustic signature.90
Figure 2.
A 3D representation of the Raman-active-silica-gold nanoparticle
From a therapeutic perspective, gold nanoparticles have shown promising results in the treatment of a variety of diseases. Gold nanoparticle-oligonucleotide complexes have been used as intracellular gene regulation agents for the control of protein expression in cells41 whilst gold nanoparticles coupled to recombinant tumor necrosis factor alpha have been used with promising results in the systemic treatment of non-resectable cancers.91 Furthermore, gold nanoparticles have been shown to have intrinsic anti-angiogenic properties by inhibiting vascular endothelial growth factor induced proliferation of endothelial cells through an interaction with the heparin-binding domain.92, 93 This has led to subsequent work showing the ability of gold nanoparticles to treat cancers in which VEGF plays a major role in disease progression. Gold nanoparticles have also been shown to reduce ascites accumulation in vivo in a mouse ovarian cancer model,92 inhibit proliferation of multiple myeloma cells by up-regulating p21 and p27 which causes cell cycle arrest94 and induce apoptosis in B-chronic lymphocytic leukemia.95 In addition, as angiogenesis plays a part in the pathogenesis of RA, preliminary studies have also begun to investigate the effect of gold nanoparticles in treating RA with promising results.96 Gold nanoparticles, especially gold nanorods and nanoshells which have a resonance absorption in the NIR spectrum, can also be used for photothermal therapy.97, 98 Here, nanoparticles are first immobilized at the site of interest either via targeting ligands or by the EPR effect. Next, laser pulses heat the nanoparticle for tumor ablation. Gold nanoshells measuring ca. 120 nm, under the brand name Aurolase, are currently undergoing clinical trials in the treatment of refractory tumors of the head and neck (Clinical Trials gov. Identifier: NCT00848042). In small animal studies, these nanoshells have been used to ablate tumors by heating nanoparticles which have accumulated in tumor tissue with laser irradiation at 808 nm which causes a temperature increase of ca. 20°C.66, 99 Advantages of photoablative treatment include the ability to customize the treatment with the location and duration of the light pulse. One limitation is that deeper tissue may not receive the same thermal dose as superficial tissue and that the location of a tumor needs to identified prior to the initiation of treatment.
Finally, the diagnostic gold nanoparticle molecular imaging agents previously described can also be used for therapeutic applications, in a combined “theranostic” approach to patient care. We are currently investigating approaches that couple gold nanoparticle imaging agents with different energy pulses (i.e. radiofrequency) to heat and destroy targeted tissues. In addition, gold nanoparticles could also be coupled to different chemotherapeutic agents to deliver high concentrations of chemotherapy to specific targeted cells.
Conclusion
Although the use of gold in clinical practice has declined significantly over the past decade, parallel technological advances in the synthesis and functionalization of gold nanoparticles have generated excitement and a certain expectation that gold could be returning to use in humans, but in a different guise. Gold has been shown to be extremely biocompatible in humans on a bulk level, however, the consequences of its use as a nanoparticle may be determined by alternative chemical and biological properties. Thus, gold nanoparticles for use in humans should be designed based on data from both bulk gold treatment in humans and in vivo gold nanoparticle validation experiments. Taken together, nanoparticles made of metallic gold (gold (0)) which are spherical in shape, anionic and of a size greater than 20nm would be expected to have the least toxicity in humans. Furthermore, the gold nanoparticle preparation should be optimized depending on its method of delivery (i.e. intravenous vs. oral vs. intrarectal) to decrease systemic absorption and distribution while increasing urinary and fecal excretion. Future research will need to determine the optimal gold nanoparticles for each potential human application, and inevitably, tradeoffs will have to be made regarding some of their diagnostic and therapeutic properties vis-a-vis their associated toxicity profile. Overall, gold nanoparticles are ideally placed to make the transition from the laboratory benchtop to the clinical bedside in the very near future.
ACKNOWLEDGEMENTS
Supported by the NCI Center for Cancer Natotechnology Excellence (CCNE) U54 CA119367 (S.S.G.), NCI Network for Translational Research (NTR): Optical Imaging in Multimodality Platforms (S.S.G.), the NCI In vivo cellular and Molecular Imaging Centers (ICMIC) P50 CA114747 (S.S.G.), the Canary Foundation (S.S.G.), the American Cancer Society (A.S.T.), the European Association for Cancer Research (A.S.T.), and the PEEL Medical Research Trust (A.S.T.), and NIHR Cambridge Biomedical Research Centre (A.S.T. and T.F.M.).
REFERENCES
- 1.Yang DP, Cui DX. Chem Asian J. 2008;3(12):2010–2222. doi: 10.1002/asia.200800195. [DOI] [PubMed] [Google Scholar]
- 2.Burda C, Chen X, Narayanan R, El-Sayed MA. Chem Rev. 2005;105(4):1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 3.Antonovych TT. Ann Clin Lab Sci. 1981;11(5):386–391. [PubMed] [Google Scholar]
- 4.Lewis AJ, Walz DT. Prog Med Chem. 1982;19:1–58. doi: 10.1016/s0079-6468(08)70327-8. [DOI] [PubMed] [Google Scholar]
- 5.Fricker SP. Gold Bulletin. 1996;29(2) [Google Scholar]
- 6.Merchant B. Biologicals. 1998;26(1):49–59. doi: 10.1006/biol.1997.0123. [DOI] [PubMed] [Google Scholar]
- 7.Clark P, Tugwell P, Bennet K, Bombardier C, Shea B, Wells G, Suarez-Almazor ME. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD000520. CD000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ujfalussy I, Koo E, Sesztak M, Gergely P. Z Rheumatol. 2003;62(2):155–160. doi: 10.1007/s00393-003-0458-2. [DOI] [PubMed] [Google Scholar]
- 9.Champion GD, Graham GG, Ziegler JB. Baillieres Clin Rheumatol. 1990;4(3):491–534. doi: 10.1016/s0950-3579(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 10.Dalziel K, Going G, Cartwright PH, Marks R, Beveridge GW, Rowell NR. Br J Dermatol. 1986;115(2):211–216. doi: 10.1111/j.1365-2133.1986.tb05720.x. [DOI] [PubMed] [Google Scholar]
- 11.Belies RP. Patty's Industrial Hygeine and Toxicology. 4th ed. New York: John Wiley & Sons Inc; 1994. pp. 2021–2032. [Google Scholar]
- 12.Bair RL, Harris GJ, Lyon DB, Komorowski RA. Ophthal Plast Reconstr Surg. 1995;11(3):209–214. doi: 10.1097/00002341-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Vaccine. 1995;13(15):1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 14.Kastrati A, Schomig A, Dirschinger J, Mehilli J, von Welser N, Pache J, Schuhlen H, Schilling T, Schmitt C, Neumann FJ. Circulation. 2000;101(21):2478–2483. doi: 10.1161/01.cir.101.21.2478. [DOI] [PubMed] [Google Scholar]
- 15.Nolan BW, Schermerhorn ML, Powell RJ, Rowell E, Fillinger MF, Rzucidlo EM, Wyers MC, Whittaker D, Zwolak RM, Walsh DB, Cronenwett JL. J Vasc Surg. 2005;42(1):40–46. doi: 10.1016/j.jvs.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb NL, Smith PM, Smith EM. Arthritis Rheum. 1972;15(1):16–22. doi: 10.1002/art.1780150103. [DOI] [PubMed] [Google Scholar]
- 17.Plantin L. L'analyse par radioactivations. Paris: Presses universitaires de France; 1964. [Google Scholar]
- 18.Gottlieb NL, Smith PM, Penneys NS, Smith EM. Arthritis Rheum. 1974;17(1):56–62. doi: 10.1002/art.1780170109. [DOI] [PubMed] [Google Scholar]
- 19.Tipton IH, Cook MJ. Health Phys. 1963;9:103–145. doi: 10.1097/00004032-196302000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Petushkov AA, Linekin DM, Balcius JF, Brownell GL. J Nucl Med. 1969;10(12):730–731. [PubMed] [Google Scholar]
- 21.Brown DH, Smith WE, Fox P, Sturrock RD. Inorganica Chimica Acta. 1982;67:27–30. [Google Scholar]
- 22.Russell MA, King LE, Jr, Boyd AS. N Engl J Med. 1996;334(9):603. doi: 10.1056/NEJM199602293340917. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb NL. Scand J Rheumatol Suppl. 1983;51:10–14. doi: 10.3109/03009748309095338. [DOI] [PubMed] [Google Scholar]
- 24.Walz DT, DiMartino MJ, Griswold DE, Intoccia AP, Flanagan TL. Am J Med. 1983;75(6A):90–108. doi: 10.1016/0002-9343(83)90481-3. [DOI] [PubMed] [Google Scholar]
- 25.Kean WF, Kean IR. Inflammopharmacology. 2008;16(3):112–125. doi: 10.1007/s10787-007-0021-x. [DOI] [PubMed] [Google Scholar]
- 26.Palmer DG, Dunckley JV. Aust N Z J Med. 1973;3(5):461–466. doi: 10.1111/j.1445-5994.1973.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 27.Blocka K, Furst DE, Landaw E, Dromgoole S, Blomberg A, Paulus HE. J Rheumatol Suppl. 1982;8:110–119. [PubMed] [Google Scholar]
- 28.Gottlieb NL. J Rheumatol Suppl. 1982;8:99–109. [PubMed] [Google Scholar]
- 29.Van Riel PL, Gribnau FW, Van de Putte LB, Arts CW, Van Aernsbergen A. Clin Rheumatol. 1987;6(1):50–54. doi: 10.1007/BF02201000. [DOI] [PubMed] [Google Scholar]
- 30.Mascarenhas BR, Granda JL, Freyberg RH. Arthritis Rheum. 1972;15(4):391–402. doi: 10.1002/art.1780150410. [DOI] [PubMed] [Google Scholar]
- 31.Smith PM, Smith EM, Gottlieb NL. J Lab Clin Med. 1973;82(6):930–937. [PubMed] [Google Scholar]
- 32.Pedersen SM, Graabaek PM. Ann Rheum Dis. 1980;39(6):576–579. doi: 10.1136/ard.39.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walz DT, Griswold DE, DiMartino MJ, Bumbier EE. J Rheumatol Suppl. 1979;5:56–60. [PubMed] [Google Scholar]
- 34.Graham GG, Champion GD, Haavisto TM, McNaught PJ. Ann Rheum Dis. 1981;40(2):210. doi: 10.1136/ard.40.2.210-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grahame R, Billings R, Laurence M, Marks V, Wood PJ. Ann Rheum Dis. 1974;33(6):536–539. doi: 10.1136/ard.33.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb NL, Smith PM, Smith EM. Arthritis Rheum. 1972;15(6):582–592. doi: 10.1002/art.1780150604. [DOI] [PubMed] [Google Scholar]
- 37.Penneys NS, Kramer K, Gottlieb NL. J Invest Dermatol. 1975;65(3):331–333. doi: 10.1111/1523-1747.ep12598396. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto A, Maeda Y, Ito H, Okazaki M, Hara T. Arthritis Rheum. 1972;15(3):309–315. doi: 10.1002/art.1780150313. [DOI] [PubMed] [Google Scholar]
- 39.Weisman MH, Hardison WG, Walz DT. J Rheumatol. 1980;7(5):633–638. [PubMed] [Google Scholar]
- 40.Rapson WS. Contact Dermatitis. 1985;13(2):56–65. doi: 10.1111/j.1600-0536.1985.tb02505.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Science. 2006;312(5776):1027–1030. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 42.N S Yang PC. Particle bombardment technology for gene transfer. Oxford: Oxford University Press; 1994. [Google Scholar]
- 43.Penneys NS, Ackerman AB, Gottlieb NL. Arch Dermatol. 1974;109(3):372–376. doi: 10.1001/archderm.109.3.372. [DOI] [PubMed] [Google Scholar]
- 44.Rasanen L, Kaipiainen-Seppanen O, Myllykangas-Luosujarvi R, Kasnanen T, Pollari P, Saloranta P, Horsmanheimo M. Br J Dermatol. 1999;141(4):683–688. doi: 10.1046/j.1365-2133.1999.03107.x. [DOI] [PubMed] [Google Scholar]
- 45.Heuer MA, Pietrusko RG, Morris RW, Scheffler BJ. J Rheumatol. 1985;12(4):695–699. [PubMed] [Google Scholar]
- 46.Horton RJ. Scand J Rheumatol Suppl. 1983;51:100–110. doi: 10.3109/03009748309095361. [DOI] [PubMed] [Google Scholar]
- 47.Clark P, Tugwell P, Bennett K, Bombardier C. J Rheumatol. 1989;16(4):442–447. [PubMed] [Google Scholar]
- 48.Hamilton JA, Williams N. J Rheumatol. 1985;12(5):892–896. [PubMed] [Google Scholar]
- 49.Langer HE, Hartmann G, Heinemann G, Richter K. Ann Rheum Dis. 1987;46(10):787–792. doi: 10.1136/ard.46.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rocha MP, Burrichter PJ, Blodgett RC. Semin Arthritis Rheum. 1987;16(4):294–299. doi: 10.1016/0049-0172(87)90007-2. [DOI] [PubMed] [Google Scholar]
- 51.Evans RB, Ettensohn DB, Fawaz-Estrup F, Lally EV, Kaplan SR. Semin Arthritis Rheum. 1987;16(3):196–205. doi: 10.1016/0049-0172(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 52.Levine JD, Moskowitz MA, Basbaum AI. Neurosci Lett. 1988;87(1–2):200–202. doi: 10.1016/0304-3940(88)90170-x. [DOI] [PubMed] [Google Scholar]
- 53.Preston S, Needs C. Baillieres Clin Rheumatol. 1990;4(3):687–698. doi: 10.1016/s0950-3579(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 54.Szabo KT, Guerriero FJ, Kang YJ. Vet Pathol Suppl. 1978;15(5):89–96. [PubMed] [Google Scholar]
- 55.Jones G, Brooks PM. Br J Rheumatol. 1996;35(11):1154–1158. doi: 10.1093/rheumatology/35.11.1154. [DOI] [PubMed] [Google Scholar]
- 56.Grzelczak M, Perez-Juste J, Mulvaney P, Liz-Marzan LM. Chem Soc Rev. 2008;37(9):1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 57.Murphy CJ, Sau TK, Gole AM, Orendorff CJ, Gao J, Gou L, Hunyadi SE, Li T. J Phys Chem B. 2005;109(29):13857–13870. doi: 10.1021/jp0516846. [DOI] [PubMed] [Google Scholar]
- 58.Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Anal Bioanal Chem. 2008;391(3):943–950. doi: 10.1007/s00216-007-1768-z. [DOI] [PubMed] [Google Scholar]
- 59.Turkevich J. Discussions of the Faraday Society. 1951:55. [Google Scholar]
- 60.Frens G. Nat. Phys. Sci. 1973;241:20–22. [Google Scholar]
- 61.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. J Phys Chem B. 2006;110(32):15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 62.Hu M, Chen J, Li ZY, Au L, Hartland GV, Li X, Marquez M, Xia Y. Chem Soc Rev. 2006;35(11):1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 63.Nikoobakht B, El-Sayed MA. Chem. Mater. 2003;15(10):1957–1962. [Google Scholar]
- 64.Wei Q, Song HM, Leonov AP, Hale JA, Oh D, Ong QK, Ritchie K, Wei A. J Am Chem Soc. 2009;131(28):9728–9734. doi: 10.1021/ja901562j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin R, Cao Y, Mirkin CA, Kelly KL, Schatz GC, Zheng JG. Science. 2001;294(5548):1901–1903. doi: 10.1126/science.1066541. [DOI] [PubMed] [Google Scholar]
- 66.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Proc Natl Acad Sci U S A. 2003;100(23):13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Science. 2007;318(5849):430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 68.Lanone S, Boczkowski J. Curr Mol Med. 2006;6(6):651–663. doi: 10.2174/156652406778195026. [DOI] [PubMed] [Google Scholar]
- 69.Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Adv Drug Deliv Rev. 2009 doi: 10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dobrovolskaia MA, McNeil SE. Nat Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 71.Chithrani BD, Chan WC. Nano Lett. 2007;7(6):1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 72.Chithrani BD, Ghazani AA, Chan WC. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 73.Wang S, Lu W, Tomvmachenko O, Rai US, Yu H, Ray PC. Chem Phys Lett. 2008;463:145–149. doi: 10.1016/j.cplett.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Small. 2007;3(11):1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 75.Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, Kim SH, Kim HO, Lim YT, Chung BH. Toxicol Appl Pharmacol. 2009;236(1):16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 76.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Bioconjug Chem. 2004;15(4):897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 77.Knop K, Hoogenboom R, Fischer D, Schubert US. Angew Chem Int Ed Engl. 49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 78.van Vlerken LE, Vyas TK, Amiji MM. Pharm Res. 2007;24(8):1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 79.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 80.Kanaras AG, Kamounah FS, Schaumburg K, Kiely CJ, Brust M. Chem Commun (Camb) 2002;(20):2294–2295. doi: 10.1039/b207838b. [DOI] [PubMed] [Google Scholar]
- 81.Kwon GS. Crit Rev Ther Drug Carrier Syst. 2003;20(5):357–403. doi: 10.1615/critrevtherdrugcarriersyst.v20.i5.20. [DOI] [PubMed] [Google Scholar]
- 82.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382(6592):607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 83.You CC, Miranda OR, Gider B, Ghosh PS, Kim IB, Erdogan B, Krovi SA, Bunz UH, Rotello VM. Nat Nanotechnol. 2007;2(5):318–323. doi: 10.1038/nnano.2007.99. [DOI] [PubMed] [Google Scholar]
- 84.Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS. Phys Med Biol. 2000;45(2):R1–R59. doi: 10.1088/0031-9155/45/2/201. [DOI] [PubMed] [Google Scholar]
- 85.Banholzer MJ, Millstone JE, Qin L, Mirkin CA. Chem Soc Rev. 2008;37(5):885–897. doi: 10.1039/b710915f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS. Proc Natl Acad Sci U S A. 2009;106(32):13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thakor AS, Paulmurugan R, Kempen P, Zavaleta C, Sinclair R, Massoud TF, Gambhir SS. Small. 2011;7(1):126–136. doi: 10.1002/smll.201001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thakor AS, Luong R, Paulmurugan R, LIN FI, Kempen P, Zavaleta C, Chu P, Massoud TF, Sinclair R, Gambhir SS. Science and Translational Medicine. 2011;3(79) doi: 10.1126/scitranslmed.3001963. 79ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Nat Nanotechnol. 2008;3(9):557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen YS, Frey W, Kim S, Kruizinga P, Homan K, Emelianov S. Nano Lett. 2011;11(2):348–354. doi: 10.1021/nl1042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Clin Cancer Res. 2010;16(24):6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukherjee P, Bhattacharya R, Wang P, Wang L, Basu S, Nagy JA, Atala A, Mukhopadhyay D, Soker S. Clin Cancer Res. 2005;11(9):3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 93.Bhattacharya R, Mukherjee P. Adv Drug Deliv Rev. 2008;60(11):1289–1306. doi: 10.1016/j.addr.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Bhattacharya R, Patra CR, Verma R, Kumar PR, Greipp P, Mukherjee P. Adv Mater. 2007;19:711–716. [Google Scholar]
- 95.Mukherjee P, Bhattacharya R, Bone N, Lee YK, Patra CR, Wang S, Lu L, Secreto C, Banerjee PC, Yaszemski MJ, Kay NE, Mukhopadhyay D. J Nanobiotechnology. 2007;5:4. doi: 10.1186/1477-3155-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai CY, Shiau AL, Chen SY, Chen YH, Cheng PC, Chang MY, Chen DH, Chou CH, Wang CR, Wu CL. Arthritis Rheum. 2007;56(2):544–554. doi: 10.1002/art.22401. [DOI] [PubMed] [Google Scholar]
- 97.von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Cancer Res. 2009;69(9):3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Nano Lett. 2007;7(7):1929–1934. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Cancer Res. 2009;69(4):1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]