Abstract
Variations in the genetic code are found frequently in mitochondrial decoding systems. Four non-universal genetic codes are employed in ascidian mitochondria: AUA for Met, UGA for Trp, and AGA/AGG(AGR) for Gly. To clarify the decoding mechanism for the non-universal genetic codes, we isolated and analyzed mitochondrial tRNAs for Trp, Met, and Gly from an ascidian, Halocynthia roretzi. Mass spectrometric analysis identified 5-taurinomethyluridine (τm5U) at the anticodon wobble positions of tRNAMet(AUR), tRNATrp(UGR), and tRNAGly(AGR), suggesting that τm5U plays a critical role in the accurate deciphering of all four non-universal codes by preventing the misreading of pyrimidine-ending near-cognate codons (NNY) in their respective family boxes. Acquisition of the wobble modification appears to be a prerequisite for the genetic code alteration.
Keywords: Mitochondria, Molecular Biology, Nucleic Acid, Transfer RNA (tRNA), Translation, Animal Evolution, Codon-anticodon Pairing, Genetic Code, Modified Nucleoside
Introduction
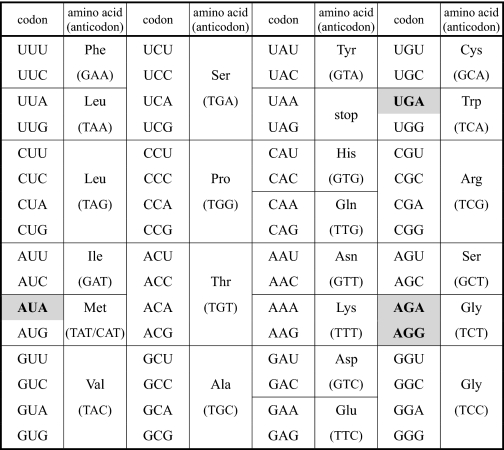
Non-universal codons are known to be used in the mitochondrial (mt)3 genetic code (1–6). DNA sequencing of protein-coding genes in various metazoan mtDNAs followed by comparison of the deduced amino acid sequences with those of other species revealed amino acid assignment deviations for the codons UGA, AUA, AGR, and AAA. The termination codon UGA in the universal genetic code specifies Trp in the mitochondria of all species investigated so far (1, 2). The AUA codon for Ile changes to specify Met in most metazoa except echinoderms, planaria, and coelenterates (2–4). AGR codons are used for Ser in most invertebrates, for Gly in urochordates, and for termination in vertebrates (7–10). The AAA codon specifies Asn in echinoderms (7) and platyhelminths (11).
Our group previously reported that the mitochondrial decoding system of ascidian Halocynthia roretzi employs four non-universal genetic codes: UGA for Trp, AUA for Met and AGR for Gly (Table 1) (9, 12). Sequence analysis of H. roretzi mtDNA identified three tDNAs responsible for these non-universal codons. Each of these tDNAs contains thymidine (T) at the first (wobble) position of their anticodons (Table 1). Because an unmodified U at the wobble position can decode any of the four bases at the third letter of the codon, according to the mitochondrial four-way wobble rule (5, 13), the uridines of these tRNAs at the wobble position must be modified in order to decipher their respective non-universal genetic codes accurately. To elucidate the molecular mechanism of the decoding system, it is necessary to determine the chemical structure of the anticodon wobble base in H. roretzi mt tRNAs. We previously reported 5-carboxymethylaminomethyluridine at the wobble position of mt tRNAMet(AUR) from H. roretzi (14). However, the nucleotide identification was inaccurate because it was analyzed by conventional two-dimensional TLC, and the modification was determined based on a comparison of its rate of flow value with that of known modified nucleotides (15). In addition, we also reported that H. roretzi mt tRNAGly(AGR) has an unidentified modified U at the wobble position (16). We describe here the chemical structures of the wobble bases in mt tRNAs for non-universal genetic codes in the H. roretzi mitochondrial decoding system and discuss evolutionary insights into the role of wobble modification in genetic code alteration.
TABLE 1.
Condon table of the ascidian mitochondrial genetic code
Non-universal genetic codes are shown in boldface on a gray background: AUA for Met, UGA for Trp, and AG(A/G) for Gly. The anticodon sequence of each tRNA gene is shown in parentheses.
EXPERIMENTAL PROCEDURES
Isolation of mt tRNAs
Two hundred grams of the commercially available edible muscle of an ascidian, H. roretzi, was minced, and the tRNA fraction was obtained by phenol extraction as described previously (17). Two thousand A260 units of crude tRNA were separated by the DEAE-cellulose column chromatography. Five hundred and eighty A260 units of tRNA were isolated from 900 A260 units of crude tRNA by removing contaminating polysaccharides with ISOGEN-LS (Wako Chemicals) followed by rinsing with chloroform. For isolating individual mt tRNAs, the following 5′-EC amino-modified DNA probes (Sigma-Aldrich) were designed using “Racess” software (18) and used for reciprocal circulating chromatography (19): 5′-TGGTGCATCAAAGGGACCAACC-CTAACTTA-3′ for tRNAGly(AGR), 5′-CCCACAAACTTTTTCATAGTTTACTGTTGT-3′ for tRNAMet(AUR), 5′-TGGCAGGAAATATATATAATCATTCTATATAACTACT-3′ for tRNATrp(UGR), 5′-TGGTACTACGTCCCTAAAGGATCCCATGAT-3′ for tRNAGly(GGN), and 5′-AAAGAAATAAACTTTCTGGTTTATATTAACCTATATTACC-3′ for tRNAMet(AUG). The tDNA sequences were obtained from H. roretzi mtDNA (accession no. AB024528) (12).
The DNA probes were covalently immobilized on NHS-activated Sepharose 4 Fast Flow (GE Healthcare) and packed in the respective tip columns for reciprocal circulating chromatography. Reciprocal circulating chromatography was performed with five-tip columns under the following conditions: the binding of tRNA to the tip columns was performed at 66 °C with binding buffer (1.2 m NaCl, 30 mm HEPES-KOH (pH 7.5), 15 mm EDTA, and 1 mm DTT). Washing was performed at 40 °C with washing buffer (20 mm NaCl, 0.5 mm HEPES-KOH (pH 7.5), 0.25 mm EDTA, and 0.5 mm DTT). The elution of individual tRNAs from tip columns was performed at 68 °C with washing buffer.
Mass Spectrometric Analysis of tRNAs
Three hundred femtomol of each purified tRNA was digested with RNase T1 and then analyzed by capillary liquid chromatography/nanoelectrospray ionization mass spectrometry as described (20). A linear ion trap-orbitrap hybrid mass spectrometer (LTQ Orbitrap XL, Thermo Fisher Scientific) equipped with a custom-made nanospray ion source and a splitless nano HPLC system (DiNa, KYA Technologies) was employed in this study. Modified bases were assigned by comparing observed m/z values of RNase T1-digested fragments to calculated ones and allocated based on their sequences by the interpretation of collision-induced dissociation spectra assisted by evolutionary conservation of each modified base in tRNA. Mono- or dinucleotide fragments passed through the LC and were not detected. The percent frequencies of modifications were calculated by the ratio of the mass chromatogram peak heights for RNA fragments containing modified or unmodified bases.
RESULTS
Five species of mt tRNAs, including three tRNAs for non-universal genetic codes and their isoacceptors, were successfully isolated homogeneously by reciprocal circulating chromatography from H. roretzi muscle (supplemental Fig. 1). The yields of tRNAGly(AGR), tRNAMet(AUR), tRNATrp(UGR), tRNAGly(GGN), and tRNAMet(AUG) were 2.15, 1.52, 3,54, 1.24, and 1.09 μg, respectively.
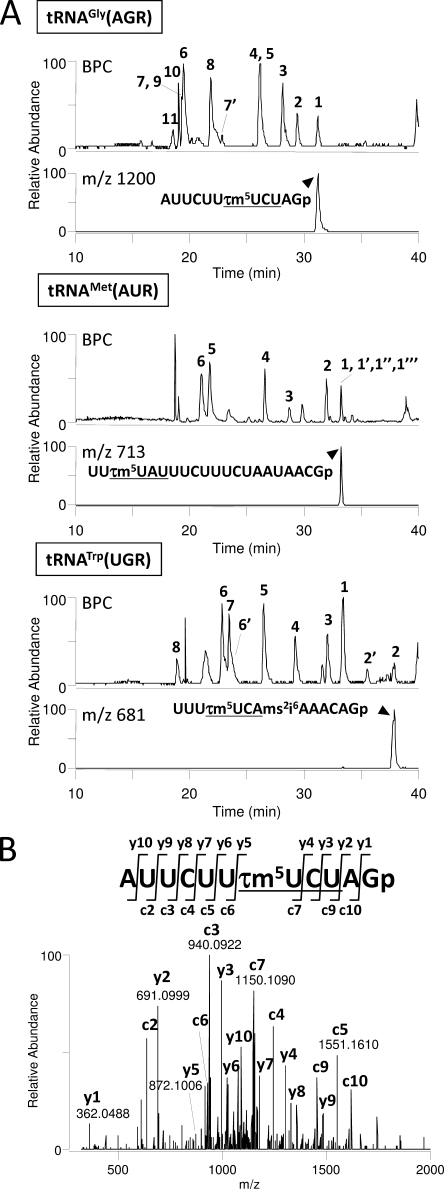
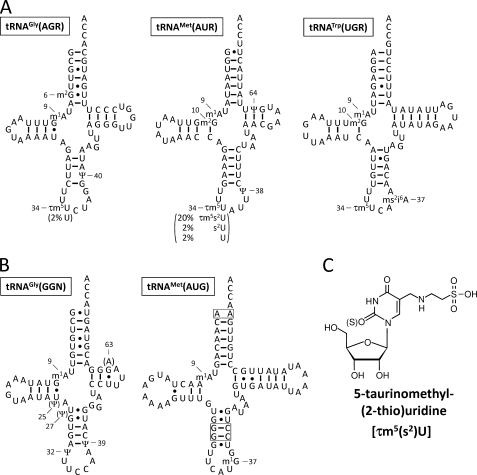
The isolated tRNAs were digested by RNase T1 and subjected to LC/MS to analyze the chemical structures of the modifications. RNA fragments from each tRNA were well separated by LC due to their length, base composition and sequence (Fig. 1A). Singly or multiply charged negative ions of RNA fragments were detected with high mass resolution (30,000). Modified bases in the fragments could be identified from the exact molecular mass of each RNA fragment, which was calculated by deconvoluting the observed m/z values from the mass spectra (supplemental Table 1). Each fragment was further analyzed by an MS/MS experiment using collision-induced dissociation to determine the positions of detected modifications within the tRNA sequence (Fig. 1B). The modifications can be unambiguously positioned in the sequence by the assignment of y- and c-series product ions (21). As shown in the mass chromatograms of the anticodon-containing fragments, the three tRNA species for AGR, AUR, and UGA commonly possess 5-taurinomethyluridine (τm5U) at their wobble positions (Figs. 1, A and B, and 2C). Nucleoside analysis was also carried out by LC/MS to confirm the presence of τm5U in tRNAGly(AGR) (22). The anticodon sequences of tRNAGly(AGR), tRNAMet(AUR), and tRNATrp(UGR) were found to be τm5UCU, τm5UAU, and τm5UCA, respectively (Fig. 2A). The wobble base of mt tRNAMet(AUR), which was previously misidentified as 5-carboxymethylaminomethyluridine (14), has therefore now been corrected. Full sequences of the tRNAs with other modifications are shown in Fig. 2A. In contrast, the anticodons of isoacceptor tRNAs for Gly(GGN) and Met(AUG) were not modified (Fig. 2B).
FIGURE 1.
Mass spectrometric analysis of H. roretzi mt tRNAs. A, base peak chromatograms (BPC) of RNase T1-digested fragments are shown for tRNAGly(AGR) (top), tRNAMet(AUR) (middle), and tRNATrp(UGR) (bottom). Mass chromatograms for the anticodon-containing fragments of each tRNA are also indicated. Sequences and molecular masses of the RNA fragments numbered on base peak chromatograms are listed in supplemental Table 1. The anticodons are underlined. B, collision-induced dissociation spectrum of the anticodon-containing fragment derived from tRNAGly(AGR). The triply charged negative ion (m/z 1200.8) was employed as a precursor ion for collision-induced dissociation. Product ions of the c- and y-series (21) are indicated on the spectrum and assigned to the sequence.
FIGURE 2.
Clover leaf structures including modifications of H. roretzi mt tRNAs. Secondary structures of H. roretzi mt tRNAs responsible for the non-universal (A) and universal (B) genetic codes. Abbreviations for modified nucleosides are as follows: m1G, 1-methylguanosine; m2G, N2-methylguanosine; m1A, 1-methyladenosine; i6A, N6-isopentenyladenosine; ms2i6A, 2-methylthio-N6-isopentenyladenosine; Ψ, pseudouridine; τm5U, 5-taurinomethyluridine; and s2U, 2-thiouridine. The numbering system of tRNAs is based on the tRNA database (tRNAdb 2009) (47). Pseudouridines in tRNAsGly and tRNAMet(AUR) were determined previously (14, 16, 22). Variations of the wobble bases are indicated in parenthesis (A). Other partial modifications were also found: N2-methylguanosine 10 (7%), ms2i6A37 (56%) and i6A37 (44%) in tRNATrp(UGR); m1A9 (88%) in tRNAGly(AGR); and 1-methylguanosine 37 (97%) in tRNAMet(AUG). At position 63 in tRNAGly(GGN), 24% of A63 is replaced with G63, probably due to heteroplasmy or a polymorphism in the mtDNA. The unpaired top bases and consecutive GCs are boxed in tRNAMet(AUG).
The relative frequency of τm5U modifications in each tRNA can be calculated based on the signal intensity of the RNA fragments. The wobble bases of tRNAGly(AGR) and tRNATrp(UGR) were almost completely modified to τm5U (Fig. 2A). However, the wobble base was modified to τm5U in 76% of tRNAMet(AUR) molecules and was modified to its 2-thio derivative, 5-taurinomethyl-2-thiouridine (τm5s2U), in 20% of tRNAMet(AUR) molecules (Fig. 2A). The remaining molecules have a 2-thiouridine or an unmodified U as the wobble base (Fig. 2A).
The two methylguanosines 1-methylguanosine and N2-methylguanosine cannot be distinguished from each other by their molecular mass. Because N2-methylguanosine is cleavable by RNase T1, whereas 1-methylguanosine is not, the methylguanosines at position 6 in tRNAGly(AGR) as well as position 10 in tRNAMet(AUR) and tRNATrp(UGR) can be identified as N2-methylguanosine (Fig. 2A and supplemental Table 1). Intriguingly, the 1-methylguanosine at position 37 in tRNAMet(AUG) was cleaved, leaving 2′,3′-cyclic phosphate at the 3′ terminus of the cleaved fragment (supplemental Table 1). Pseudouridine is a mass-silent modification that cannot be identified only by mass measurement because it has the same molecular mass as U. For tRNAGly(AGR) (16), tRNAGly(GGN) (16) and tRNAMet(AUR) (14), positions for pseudouridine were determined biochemically by the post-labeling method (15).
DISCUSSION
These experiments have identified τm5(s2)U at the wobble positions of ascidian mt tRNAs. τm5(s2)U was first identified in human and bovine mt tRNAs (23, 24), and has subsequently been found in fish.4 Thus, τm5(s2)U appears to be conserved in vertebrate mt tRNAs (23). τm5(s2)U was not found in yeast and Caenorhabditis elegans mt tRNAs (25–27), which instead used cmnm5(s2)U as the wobble modification. Due to the chemical similarity between 5-carboxymethylaminomethyluridine and τm5U, both nucleotides are thought to be synthesized by a similar biosynthetic pathway, in which glycine is replaced with taurine in the latter case (23, 27). Regarding the evolutionary aspect of xm5s2U-type modifications, the identification of τm5U in ascidian mt tRNAs in this study provides a key piece of information suggesting the phylogenetic cross-road at which 5-carboxymethylaminomethyluridine was replaced with τm5U. Specifically, τm5U seems to have emerged at least in protochordate mt tRNAs.
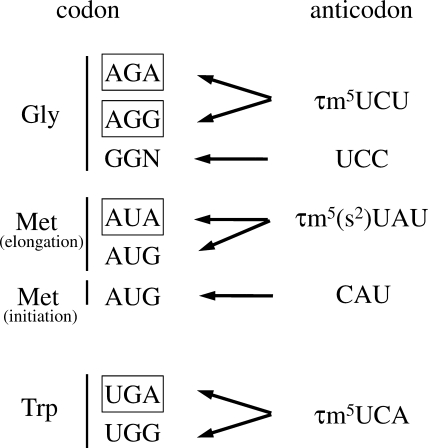
Because the non-universal genetic codes AUA, AGR, and UGA are all purine-ending codons (NNR), it is unsurprising that the three tRNAs for these codons commonly bear τm5U at the wobble positions (Fig. 3). In general, tRNAs responsible for decoding NNR codons frequently possess the xm5(s2)U-type modifications, including τm5(s2)U, at their wobble positions (13, 28), where they prevent the misreading of NNY near-cognate codons (28). Furthermore, it has been shown that the τm5U modification in human mt tRNALeu(UUR) stabilizes the τm5U:G wobble pairing at the ribosomal A site during decoding (29). A structural study of the ribosomal 30S subunit containing codon-anticodon pairing at the A-site provided a mechanistic explanation for the manner in which the 5-taurinomethyl modification enables efficient decoding of G-ending codons (30). In the human mitochondrial decoding system, τm5(s2)U is found in five tRNAs for Leu(UUR), Trp, Lys, Glu, and Gln (23, 24, 31, 32). Human homologs of the enzymes GTPBP3 and MTO1 were predicted to be responsible for synthesizing τm5U (33, 34). The substrate specificity of GTPBP3/MTO1 homologs in H. roretzi might be altered to enable them to recognize the three mt tRNA species carrying the non-universal codons. Acquisition of the wobble modification by modulating the substrate specificity of the tRNA-modifying enzyme seems to be critical for genetic code alterations in the urochordate lineage.
FIGURE 3.
Decoding system of the non-universal codons in H. roretzi mitochondria. Non-universal AGR and universal GGN codons are decoded by tRNAGly with the τm5UCU and UCC anticodons, respectively. AUR codons for Met in elongation are decoded by tRNAMet with the τm5(s2)UAU anticodon. The AUG codon for initiation can be recognized by tRNAMet with the CAU anticodon. UGR codons for Trp are recognized by tRNATrp with the τm5UCA anticodon. Non-universal codons are boxed.
Another intriguing feature in the ascidian mitochondrial decoding system is the presence of two tRNAMet species bearing τm5UAU and CAU anticodons (Fig. 2AB). Two mt tRNAMet species are also encoded in the mtDNAs of porifers (35) and bivalves (Mollusca) (36). Except for these species, however, a single tRNAMet is encoded in animal mtDNAs in general (37). Mammalian mt tRNAMet has 5-formylcytidine at the wobble position (38), which enables the decoding of AUA in addition to AUG through 5-formylcytidine: A base pairing (39–41). 5-Formylcytidine is a cytidine modification that allows the CAU anticodon to expand its decoding capability and recognize the AUA codon. This single tRNAMet can be charged with Met and then formylated by mitochondrial transformylase (42, 43). Then, the formylmethionyl(fMet)-tRNAMet is recognized by mitochondrial initiation factor 2 and used as an initiator tRNA (44, 45). As Met-tRNAMet is not fully formylated by mitochondrial transformylase in mitochondria, Met-tRNAMet is also used as an elongator tRNA. In fact, mammalian mt tRNAMet possesses characteristic structural features conserved in bacterial initiator tRNAMet, including an unpaired top base pair in the acceptor stem, which is recognized by mitochondrial transformylase, and consecutive GC pairs in the anticodon stem for targeting to the P-site of the ribosome (46). As shown in Fig. 2, A and B, H. roretzi mt tRNAMet(AUG) has an unpaired top base pair (A1:A72) in the acceptor stem, whereas tRNAMet(AUR) contains a G1:U72 wobble pair. The consecutive GC pairs are partially conserved in tRNAMet(AUG). These findings suggest that, in ascidian mitochondria, tRNAMet(AUG) and tRNAMet(AUR) act as the initiator and the elongator tRNAs, respectively (Fig. 3). Further studies will be necessary to clarify the functional and physiological significance of this issue.
This work was supported by grants-in-aid for scientific research (C) from The Japan Society for the Promotion of Science (to K. W.) and by grants-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports, and Culture of Japan (to Takeo Suzuki and Tsutomu Suzuki).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Fig. 1.
T. Suzuki, T. Yamamoto, and T. Suzuki, unpublished results.
- mt
- mitochondrial
- τm5U
- 5-taurinomethyluridine
- τm5s2U
- 5-taurinomethyl-2-thiouridine.
REFERENCES
- 1. Barrell B. G., Bankier A. T., Drouin J. (1979) Nature 282, 189–194 [DOI] [PubMed] [Google Scholar]
- 2. Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F., Schreier P. H., Smith A. J., Staden R., Young I. G. (1981) Nature 290, 457–465 [DOI] [PubMed] [Google Scholar]
- 3. Osawa S., Jukes T. H., Watanabe K., Muto A. (1992) Microbiol. Rev. 56, 229–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe K., Osawa S. (1995) in tRNA: Structure, sBiosynthesis, and Function (Soll D., Rajbhandary U. L. eds) American Society for Microbiology, Washington, D. C [Google Scholar]
- 5. Watanabe K. (2010) Proc. Jpn. Acad Ser. B. Phys. Biol. Sci. 86, 11–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe K., Yokobori S. (2011) J. Nucleic Acids, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Himeno H., Masaki H., Kawai T., Ohta T., Kumagai I., Miura K., Watanabe K. (1987) Gene 56, 219–230 [DOI] [PubMed] [Google Scholar]
- 8. Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. (1989) J. Biol. Chem. 264, 10965–10975 [PubMed] [Google Scholar]
- 9. Yokobori S., Ueda T., Watanabe K. (1993) J. Mol. Evol. 36, 1–8 [DOI] [PubMed] [Google Scholar]
- 10. Garey J. R., Wolstenholme D. R. (1989) J. Mol. Evol. 28, 374–387 [DOI] [PubMed] [Google Scholar]
- 11. Bessho Y., Ohama T., Osawa S. (1992) J. Mol. Evol. 34, 331–335 [DOI] [PubMed] [Google Scholar]
- 12. Yokobori S., Ueda T., Feldmaier-Fuchs G., Pääbo S., Ueshima R., Kondow A., Nishikawa K., Watanabe K. (1999) Genetics 153, 1851–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki T. (2005) in Fine-tuning of RNA Functions by Modification and Editing (Grosjean H. ed) pp. 23–69, Springer, Berlin [Google Scholar]
- 14. Kondow A., Yokobori S., Ueda T., Watanabe K. (1998) Nucleosides Nucleotides 17, 531–539 [PubMed] [Google Scholar]
- 15. Kuchino Y., Hanyu N., Nishimura S. (1987) Methods Enzymol. 155, 379–396 [DOI] [PubMed] [Google Scholar]
- 16. Kondow A., Suzuki T., Yokobori S., Ueda T., Watanabe K. (1999) Nucleic Acids Res. 27, 2554–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueda T., Ohta T., Watanabe K. (1985) J. Biochem. 98, 1275–1284 [DOI] [PubMed] [Google Scholar]
- 18. Kiryu H., Terai G., Imamura O., Yoneyama H., Suzuki K., Asai K. (2011) Bioinformatics 27, 1788–1797 [DOI] [PubMed] [Google Scholar]
- 19. Miyauchi K., Ohara T., Suzuki T. (2007) Nucleic Acids Res. 35, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y. (2007) Methods Enzymol. 425, 211–229 [DOI] [PubMed] [Google Scholar]
- 21. Mcluckey S. A., Vanberkel G. J., Glish G. L. (1992) J. Am. Soc. Mass Spectr 3, 60–70 [DOI] [PubMed] [Google Scholar]
- 22. Kondow A. (1998) Molecular Mechanism of Genetic Code Change in Halocynthia roretzi. Ph.D thesis, University of Tokyo [Google Scholar]
- 23. Suzuki T., Suzuki T., Wada T., Saigo K., Watanabe K. (2002) EMBO J. 21, 6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki T., Nagao T., Suzuki T. (2011) WIREs RNA 2, 376–38621957023 [Google Scholar]
- 25. Martin R. P., Sibler A. P., Gehrke C. W., Kuo K., Edmonds C. G., McCloskey J. A., Dirheimer G. (1990) Biochemistry 29, 956–959 [DOI] [PubMed] [Google Scholar]
- 26. Sakurai M., Ohtsuki T., Suzuki T., Watanabe K. (2005) FEBS Lett. 579, 2767–2772 [DOI] [PubMed] [Google Scholar]
- 27. Umeda N., Suzuki T., Yukawa M., Ohya Y., Shindo H., Watanabe K., Suzuki T. (2005) J. Biol. Chem. 280, 1613–1624 [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 4905–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirino Y., Yasukawa T., Ohta S., Akira S., Ishihara K., Watanabe K., Suzuki T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15070–15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurata S., Weixlbaumer A., Ohtsuki T., Shimazaki T., Wada T., Kirino Y., Takai K., Watanabe K., Ramakrishnan V., Suzuki T. (2008) J. Biol. Chem. 283, 18801–18811 [DOI] [PubMed] [Google Scholar]
- 31. Nagao A., Suzuki T., Katoh T., Sakaguchi Y., Suzuki T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16209–16214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suzuki T., Nagao T., Suzuki T. (2011) Annu. Rev. Genet., in press [DOI] [PubMed] [Google Scholar]
- 33. Li X., Guan M. X. (2002) Mol. Cell. Biol. 22, 7701–7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X., Li R., Lin X., Guan M. X. (2002) J. Biol. Chem. 277, 27256–27264 [DOI] [PubMed] [Google Scholar]
- 35. Lavrov D. V., Forget L., Kelly M., Lang B. F. (2005) Mol. Biol. Evol. 22, 1231–1239 [DOI] [PubMed] [Google Scholar]
- 36. Hoffmann R. J., Boore J. L., Brown W. M. (1992) Genetics 131, 397–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yokobori S., Suzuki T., Watanabe K. (2001) J. Mol. Evol. 53, 314–326 [DOI] [PubMed] [Google Scholar]
- 38. Moriya J., Yokogawa T., Wakita K., Ueda T., Nishikawa K., Crain P. F., Hashizume T., Pomerantz S. C., McCloskey J. A., Kawai G., Hayashi N., Yokoyama S., Watanabe K. (1994) Biochemistry 33, 2234–2239 [DOI] [PubMed] [Google Scholar]
- 39. Takemoto C., Spremulli L. L., Benkowski L. A., Ueda T., Yokogawa T., Watanabe K. (2009) Nucleic Acids Res. 37, 1616–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lusic H., Gustilo E. M., Vendeix F. A., Kaiser R., Delaney M. O., Graham W. D., Moye V. A., Cantara W. A., Agris P. F., Deiters A. (2008) Nucleic Acids Res. 36, 6548–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bilbille Y., Gustilo E. M., Harris K. A., Jones C. N., Lusic H., Kaiser R. J., Delaney M. O., Spremulli L. L., Deiters A., Agris P. F. (2011) J. Mol. Biol. 406, 257–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takeuchi N., Vial L., Panvert M., Schmitt E., Watanabe K., Mechulam Y., Blanquet S. (2001) J. Biol. Chem. 276, 20064–20068 [DOI] [PubMed] [Google Scholar]
- 43. Takeuchi N., Kawakami M., Omori A., Ueda T., Spremulli L. L., Watanabe K. (1998) J. Biol. Chem. 273, 15085–15090 [DOI] [PubMed] [Google Scholar]
- 44. Yassin A. S., Haque M. E., Datta P. P., Elmore K., Banavali N. K., Spremulli L. L., Agrawal R. K. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 3918–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liao H. X., Spremulli L. L. (1991) J. Biol. Chem. 266, 20714–20719 [PubMed] [Google Scholar]
- 46. RajBhandary U. L. (1994) J. Bacteriol. 176, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jühling F., Mörl M., Hartmann R. K., Sprinzl M., Stadler P. F., Pütz J. (2009) Nucleic Acids Res. 37, D159–162 [DOI] [PMC free article] [PubMed] [Google Scholar]