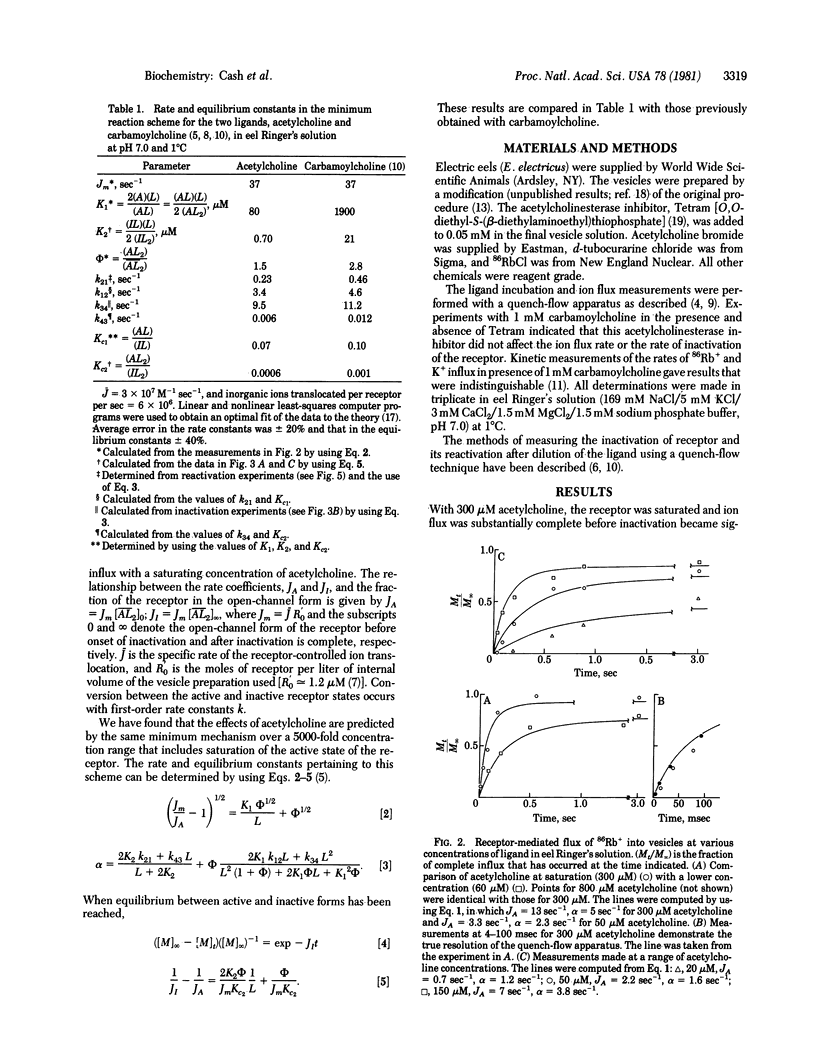
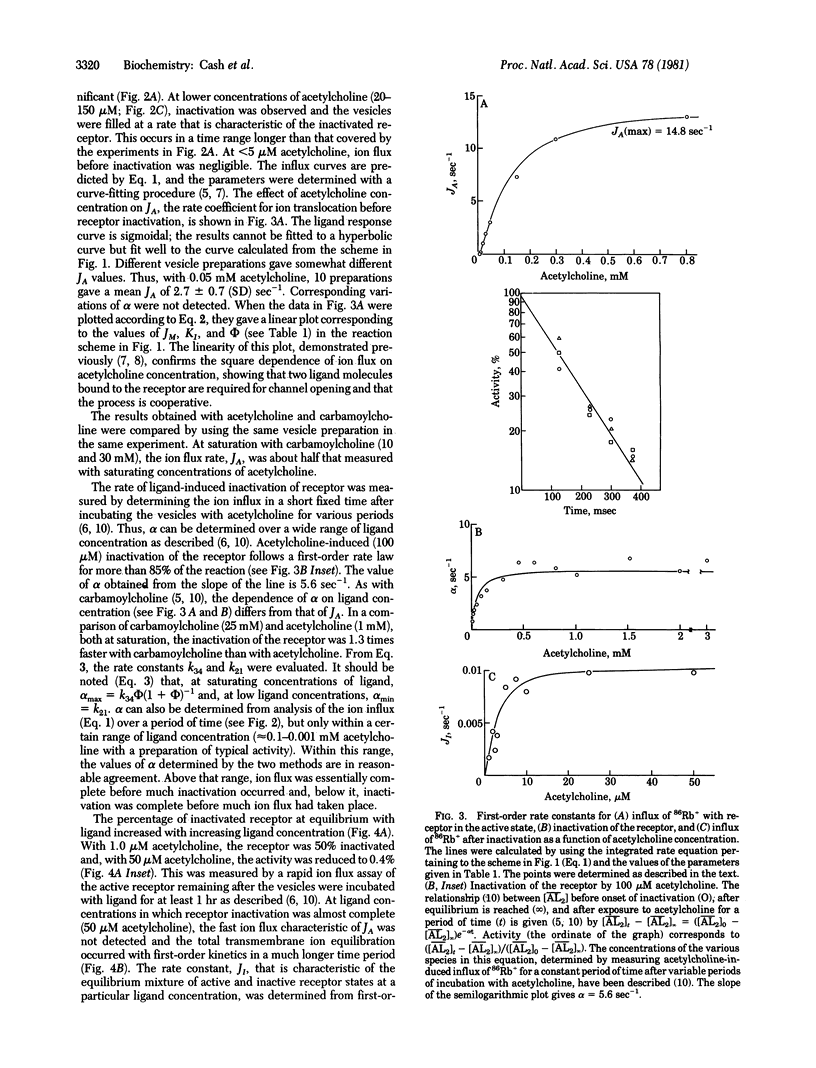
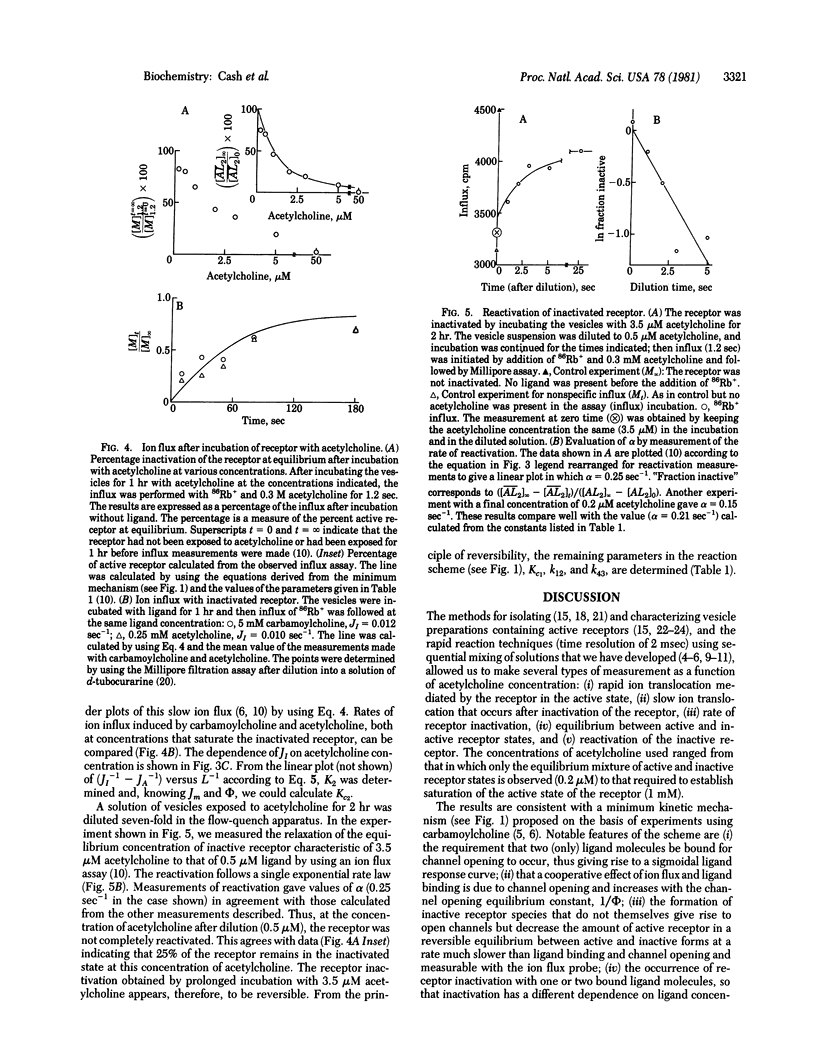
Abstract
Acetylcholine-induced flux of inorganic ions across membranes and inactivation of the acetylcholine receptor were measured at pH 7.0, 1 degrees C, over a 5000-fold concentration range of acetylcholine. Receptor-containing electroplax membrane vesicles prepared from Electrophorus electricus and a quench-flow technique were used, allowing flux to be measured in the 2-msec to 1-min time region. Five different measurements were made: (i) rate of ion translocation with the active state of the receptor, (ii) rate of the slower ion translocation after equilibration of active and inactive receptor states, (iii) rate of inactivation, (iv) equilibrium between active and inactive forms of the receptor, and (v) reactivation of inactivated receptor. The kinetics of the steps in the receptor-controlled ion flux follow single-exponential rate laws, and simple analytical expressions for their ligand concentration dependence can be used. Thus, the rate and equilibrium constants in a scheme that relates the ligand binding steps to ion translocation could be evaluated. It was found that the dependence of the receptor-controlled ion translocation over the concentration range investigated obeys the integrated rate equation based on the proposed mechanism. The flux rate before inactivation was approximately 10(7) ions sec-1 per receptor, which is comparable with that measured electrophysiologically in muscle cells. The half-time of inactivation is approximately 100 msec when the receptor is saturated with acetylcholine. The specific reaction rate of the ion translocation (J) is 3 X 10(7) M-1 sec-1. The results support a minimum reaction mechanism previously proposed on the basis of experiments in which carbamylcholine was used.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoshima H., Cash D. J., Hess G. P. Acetylcholine receptor-controlled ion flux in electroplax membrane vesicles: a minimal mechanism based on rate measurements in the millisecond to minute time region. Biochem Biophys Res Commun. 1980 Feb 12;92(3):896–904. doi: 10.1016/0006-291x(80)90787-1. [DOI] [PubMed] [Google Scholar]
- Cash D. J., Hess G. P. Molecular mechanism of acetylcholine receptor-controlled ion translocation across cell membranes. Proc Natl Acad Sci U S A. 1980 Feb;77(2):842–846. doi: 10.1073/pnas.77.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein N., Hess G. P., Kim P. S., Noble R. L. Inactivation (desensitization) of the acetylcholine receptor in Electrophorus electricus membrane vesicles by carbamylcholine: comparison between ion flux and alpha-bungarotoxin binding. J Membr Biol. 1980 Sep 30;56(2):133–137. doi: 10.1007/BF01875964. [DOI] [PubMed] [Google Scholar]
- Fu J. L., Donner D. B., Moore D. E., Hess G. P. Allosteric interactions between the membrane-bound acetylcholine receptor and chemical mediators: equilibrium measurements. Biochemistry. 1977 Feb 22;16(4):678–684. doi: 10.1021/bi00623a019. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P. Functional acetylcholine receptor--electroplax membrane microsacs (vesicles): purification and characterization. Proc Natl Acad Sci U S A. 1977 Feb;74(2):482–486. doi: 10.1073/pnas.74.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Andrews J. P., Struve G. E., Goombs S. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane preparations. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4371–4375. doi: 10.1073/pnas.72.11.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Aoshima H., Cash D. J., Lenchitz B. Specific reaction rate of acetylcholine receptor-controlled ion translocation: a comparison of measurements with membrane vesicles and with muscle cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1361–1365. doi: 10.1073/pnas.78.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G. P., Cash D. J., Aoshima H. Acetylcholine receptor-controlled ion fluxes in membrane vesicles investigated by fast reaction techniques. Nature. 1979 Nov 15;282(5736):329–331. doi: 10.1038/282329a0. [DOI] [PubMed] [Google Scholar]
- Hess G. P., Lipkowitz S., Struve G. E. Acetylcholine-receptor-mediated ion flux in electroplax membrane microsacs (vesicles): change in mechanism produced by asymmetrical distribution of sodium and potassium ions. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1703–1707. doi: 10.1073/pnas.75.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes R., Fersht A. R. Tyrosyl-tRNA synthetase from Escherichia coli. Stoichiometry of ligand binding and half-of-the-sites reactivity in aminoacylation. Biochemistry. 1975 Jul 29;14(15):3344–3350. doi: 10.1021/bi00686a009. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Walter B., Einarson B. Immunochemical similarities between subunits of acetylcholine receptors from Torpedo, Electrophorus, and mammalian muscle. Biochemistry. 1979 Oct 16;18(21):4470–4480. doi: 10.1021/bi00588a004. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Raftery M. A. Direct spectroscopic studies of cation translocation by Torpedo acetylcholine receptor on a time scale of physiological relevance. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4509–4513. doi: 10.1073/pnas.77.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry. 1980 Jun 10;19(12):2770–2779. doi: 10.1021/bi00553a036. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]



