Background: Signaling mechanisms regulating the endocrine specification of stem cell derivatives.
Results: Inhibiting Src family kinase (SFK) and focal adhesion kinase (FAK) activity promotes endocrine commitment.
Conclusion: Select inhibitors of SFK/FAK signaling increase the derivation of β-cells.
Significance: Enhanced derivation of insulin-producing β-cells for the cell-based therapy of diabetes.
Keywords: Differentiation, Embryonic Stem Cell, Pancreatic Islets, Signal Transduction, Src, Beta Cell, FAK
Abstract
Stepwise approaches for the derivation of β-cells from human embryonic stem cells have been described. However, low levels of endocrine specification limit the final yield of insulin-producing β-cells. In this study, we show that the pyrrolo-pyrimidine Src family kinase (SFK) inhibitor PP2 effectively promotes the endocrine specification of human embryonic stem cell derivatives based on its capacity to induce the expression of proendocrine transcription factors (NGN3, NEUROD1, NKX2.2, and PAX4) and to significantly increase the final yield of insulin-positive cells. We further demonstrate that PP2 inhibits the activation of focal adhesion kinase (FAK), and selective inhibition of this kinase is also sufficient to induce early endocrine commitment based on increased expression of NGN3, NEUROD1, and NKX2.2. Additional studies using dominant negative constructs and isolated human fetal pancreata suggest that c-Src is at least partially responsible for inhibiting early endocrine specification. Mechanistically, we propose that inhibition of SFK/FAK signaling can promote endocrine specification by limiting activation of the TGFβR/Smad2/3 pathway. Moreover, we show that inhibition of SFK/FAK signaling suppresses cell growth, increases the expression of the β-cell-associated cyclin-dependent kinase inhibitor p57kip2, and simultaneously suppresses the expression of Id1 and Id2. This study has important implications for the derivation of β-cells for the cell-based therapy of diabetes and sheds new light on the signaling events that regulate early endocrine specification.
Introduction
Our understanding of the complex genetic and environmental events involved in islet development has progressed significantly in recent years, and this knowledge has provided the foundation for the development of some sophisticated stage-by-stage approaches for the derivation of β-cells from human embryonic stem cell (hESCs)3 (1–6). For the most part, these protocols attempt to recreate in vitro the key developmental stages required for the development of bona fide β-cells including, in order, the derivation of mesendoderm, definitive endoderm, primitive gut tube, posterior foregut, pancreatic progenitors, and endocrine progenitors (1–6). Directed differentiation through these stages is primarily achieved by a stepwise exposure to different growth factors and differentiating agents (1–6). However, small compound inhibitors of select signaling pathways have also been used to potentiate specific developmental steps (7). For example, inhibitors of the hedgehog pathway and PI3K have been used to respectively optimize the derivation of pancreatic progenitors and definitive endoderm (1, 3, 7, 8). However, despite such improvements, the subsequent differentiation of pancreatic progenitors into insulin-producing β-cells remains limited because of suboptimal levels of endocrine specification. Unfortunately, attempts to improve levels of endocrine commitment using small compound inhibitors, including inhibitors of the Notch pathway, have been only marginally successful (1, 7, 9).
The impact of Notch, hedgehog, and PI3K signaling on β-cell derivation is now well documented (7); however, the contribution of other signaling pathways or intermediates remains unknown or ill defined. Recent studies have shown that individual members of the Src family of protein-tyrosine kinases (SFKs) have a major impact on early ESC differentiation as well as other late stage differentiation events (10–15), however, little to nothing is known about how individual SFKs influence β-cell development. SFKs are nonreceptor protein-tyrosine kinases comprising nine members that include Blk, Fgr, Fyn, Hck, Lck, Lyn, Yes, YrK, and prototypical family member c-Src (12). These kinases serve to transduce signals from various cell surface receptors including growth factor receptors, cytokine receptors, integrins, and other cell adhesion molecules (12, 16–20). In this intermediary capacity, SFKs have been shown to play an essential role in a wide range of cellular activities including growth and differentiation (12, 16–20). Importantly, PP2, a well established SFK inhibitor, has recently been shown to promote the ex vivo differentiation of several cell types including cardiomyocytes (21) and chondrocytes (13). Interestingly, PP2 appears to induce the differentiation of these cells via a common mechanism that involves the inhibition of focal adhesion kinase (FAK) (13, 14, 21). FAK is a broadly expressed cytoplasmic protein-tyrosine kinase that is activated by integrin ligation and clustering, by growth factor stimulation, and by G-protein-linked receptor activation (19, 22–24). Among other things, FAK recruits and activates various SFKs, including the prototypical c-Src (19, 22–24). The subsequent formation of FAK-SFK complexes results in further FAK phosphorylation, which then triggers the activation of other downstream signaling cascades that then influence growth and differentiation (19, 22–24). The specific mechanism(s) whereby inhibition of SFK/FAK signaling by PP2 induces differentiation remains to be fully defined. However, several studies have shown that inhibition of SFK and FAK signaling alters the association between cells and the underlying extracellular matrix, which in turn can have profound consequences for anchorage-dependent growth and differentiation (13, 25). Interestingly, anchorage-dependent SFK/FAK signaling has also been shown to induce the activation of Smad2/3, possibly as a result of cross-talk between integrins and TGFβ receptors (TGFβRs) (26, 27). This is significant because TGFβ1-dependent activation of Smad2/3 has also recently been shown to suppress endocrine specification (28, 29). Finally, it is noteworthy that PP2 and related SFK antagonists have also been shown to regulate the expression of both cyclin-dependent kinase inhibitors (CDKIs) and inhibitors of differentiation proteins (Ids), which together can have a major can impact on cell fate determination and/or differentiation (25, 30–32).
In this study, we show for the first time that the SFK inhibitor PP2 can be used to significantly increase endocrine specification and the subsequent derivation of insulin producing β-cells from hESCs. Moreover, we confirm that this increase in endocrine commitment can be attributed to the inhibition of FAK as well as the prototypical SFK c-Src. Inhibition of SFK and FAK activity is further shown to limit Smad2/3 activation, which in turn promotes endocrine specification (28, 29). Finally, we show that inhibition of SFK/FAK activity inhibits progenitor cell proliferation, induces the expression of the human β-cell-associated CDKI p57kip2, and suppresses the expression of both Id1 and Id2.
EXPERIMENTAL PROCEDURES
hESC Culture and Differentiation
Human ESC lines, H9 (WA09), CyT203, and CyT49 were maintained in DMEM/F12 medium supplemented with FGF2 (4 ng/ml; R & D Systems, Minneapolis, MN), activin A (10–20 ng/ml; R & D Systems), knockout serum replacement (20% v/v; Invitrogen), nonessential amino acids (1 mm), 2-mercaptoethanol (0.55 mm), GlutaMAXTM, and penicillin/streptomycin. Growth medium was replaced daily, and the ESC lines were grown in the presence of a sparse monolayer of mitomycin C-treated mouse embryonic fibroblasts (∼1 × 105 cells/60-mm plate). The cultures were mechanically passaged using a StemPro EZPassage passaging tool (Invitrogen) and were routinely split at a 1:6–1:9 ratio every 5–7 days.
Differentiation was carried out essentially as described (1, 2) with minor modifications. The media conditions used, and the duration of individual differentiation steps, are shown in Fig. 1A. Basal media used included RPMI (Mediatech, Manassas, VA), DMEM high glucose (Invitrogen), and CMRL 1066 (Invitrogen). These media were supplemented with GlutaMAXTM and with either FBS (0.2–2%; HyClone, Lakewood, NJ) or B27 supplement (1% v/v; Invitrogen). Human recombinant activin A (100 ng/ml), mouse recombinant Wnt3a (25 ng/ml), mouse recombinant Noggin (50 ng/ml), and human recombinant KGF (50 ng/ml) were all purchased from R & D Systems. KAAD-cyclopamine (0.25 μm) was purchased from EMD Biosciences (Gibbstown, NJ), and all-trans-retinoic acid (2 μm) was from Sigma. The cultures were variously treated with PP2 (2–20 μm; EMD Biosciences), SKI-1 (3 μm; EMD Biosciences), ALK5 inhibitor-II (0.5–5 μm; Enzo Life Sciences Inc., Farmingdale, NY), or the FAK inhibitor PF-228 (2–3 μm; Tocris, Ellisville, MI). Please note that ESC cultures were grown to a high cell density prior to initiating differentiation, and cultures were washed with PBS (Invitrogen) at the beginning of stages 1 and 2.
FIGURE 1.
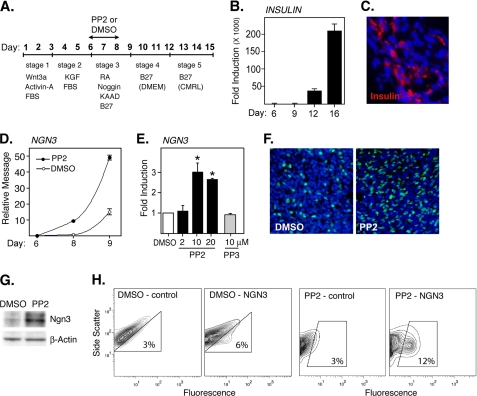
PP2 induces NGN3 expression. A, design of differentiation protocol and timing of PP2 addition and removal. B, real time PCR results showing induction of insulin message after the directed differentiation of CyT49 hESCs. The results are fold induction relative to the lowest detectable levels of insulin prior to day 12. C, photomicrographs (40×) showing differentiated CyT49 cultures (day 15) stained for insulin (red) and DNA (DAPI; blue). D, Q-PCR analysis showing induction of NGN3 after stage 3 culture in the presence or absence of PP2 (10 μm). PP2 or Me2SO (DMSO) alone was added on day 6, and cultures were evaluated 48 or 72 h later. The results show relative levels of NGN3 expression based on standard curves established using fetal ICCs. E, Q-PCR analysis showing a dose-dependent induction of NGN3 by PP2 and lack of induction by PP3. The cells were harvested on day 9, and the results show fold induction relative to Me2SO control. *, levels induced by PP2 were significantly greater than by Me2SO alone at p < 0.01 (n = 3). F, photomicrographs (20×) showing nuclear NGN3 expression in CyT49 cells differentiated in the presence of PP2 or Me2SO alone. PP2 was added on day 6 and was removed 48 h later. The cells were immunostained on day 9 using an anti-NGN3 pAb (green), and the nuclei were stained using DAPI (blue). G, Western blot showing levels of NGN3 expression in CyT49 cells differentiated in the presence or absence of PP2. PP2 or Me2SO alone was added for 48 h at the start of stage 3 culture (day 6), and the lysates were generated on day 9. The membranes were probed with an anti-NGN3 pAb, and the bands correspond to the predicted size of NGN3. H, FACS contour plots showing an increase in the frequency of NGN3-positive cells after treatment with PP2. PP2 or Me2SO alone was added on day 6 and was removed 48 h later. On day 9, cells were stained using an anti-NGN3 pAb or nonspecific IgG control.
Generation and Treatment of Fetal Pancreatic Islet-like Cell Clusters
Fetal pancreata (gestational age, 18–21 weeks) were obtained from Advanced BioResources (Alameda, CA). These pancreata were minced and digested with collagenase prior to being allowed to form islet-like cell clusters (ICCs) as described (33, 34). ICCs were subsequently washed and resuspended in RPMI 1640 supplemented with 10% FCS and were then seeded into 24-well plates precoated with C-IV (30 μg/ml). As required, ICCs were seeded in the presence of PP2 (20 μm) or Me2SO vehicle alone and were then cultured for 72 h. For Q-PCR analysis, total RNA was extracted from ICCs prior to and after culture in the presence of PP2 or Me2SO alone.
To assess the contribution of c-Src, ICC preparations were nucleofected with a dominant negative kinase-inactive mutant of c-Src (c-Src K297M) or with empty vector (pcDNA 3.1). The plasmid c-Src K297M was kindly provided by Dr. David Schlaepfer (University of California, San Diego) and has been described (35). For transfection, fetal ICCs were cultured as a monolayer on HTB-9 matrix for 3 days as and were then nucleofected essentially as described (36). In brief, cell monolayers were harvested as single cells using a 0.025% trypsin-EDTA solution, and 2 × 106 cells were resuspended in 100 μl of Amaxa nucleofector solution (VPI-1005; Amaxa GmbH, Gaithersburg, MD) containing 5 μg of plasmid and were then nucleofected using an Amaxa Nucleofector II (S-005 program). Using the same approach, additional cells were nucleofected with 10 nm of Ambion Silencer Select Validated src siRNA (identifier s13414), Silencer Select Validated lyn siRNA (identifier s8356), or scrambled siRNA (identifier 4611) from Invitrogen. After pulsing, the cells were transferred to C-IV-coated dishes and were cultured in RPMI 1640 supplemented with 10% FCS for an additional 2 days. For Q-PCR analysis, total RNA was extracted from expanded ICCs prior to and after nucleofection and subsequent culture. β-Cell transfection efficiency based on the use of a GFP construct under identical experimental conditions was 30–35%.
Real Time Q-PCR
Q-PCR analysis was performed on differentiated hESC cultures and fetal pancreatic preparations (ICCs). Total RNA was extracted using an RNeasy Plus mini kit 50 (Qiagen), and cDNA was synthesized from 100–300 ng of RNA using an iScript cDNA synthesis kit (Bio-Rad). cDNA reactions (20 μl) were further diluted 1:5. PCRs (20 μl of total volume) were then run in duplicate using 3 μl of cDNA, combined with TaqMan® Universal PCR Master Mix (4324018; Applied Biosystems) and unlabeled PCR primers and TaqMan FAMTM dye-labeled probes listed in supplemental Table S1. RT-Q-PCR was performed using an Applied Biosystems StepOnePlus real time PCR system machine. The results were analyzed by the standard curve method or by the comparative Ct (ddCt) method as indicated in the text. Standard curves were generated using cDNA prepared from pooled 11–17-week human fetal ICCs (provided by University of Washington, Seattle, WA). Quantified values for each gene of interest were normalized to cyclophilin A (stem cells) or RPS-16 (fetal ICCs). The Q-PCR results shown in this study are the average values of 2–5 repeat experiments (means ± S.D.). The statistical significance of Q-PCR data were assessed using Student's t test.
Western Blotting
Levels of NGN3 and p57kip2 expression and the phosphorylation status of FAK, SFKs, and SMAD2 were assessed by Western blot analysis. The lysates were generated using a buffer consisting of Triton X-100 (1%), sodium deoxycholate (0.5%), SDS (0.1%), Tris (50 mm), and sodium chloride (150 mm) (pH 8.0). To assess NGN3 expression, lysates were generated using a buffer containing SDS (1%), urea (6 m), and Hepes (50 mm). Both buffers were further supplemented with PMSF (1 mm), pepstatin (10 μg/ml), leupeptin (10 μg/ml), and a complete protease inhibitor mixture (Roche Applied Science). To inhibit phosphatase activity, the buffers were further supplemented with sodium orthovanadate (1 mm), β-glycerophosphate (25 mm), sodium pyrophosphate (2 mm), and sodium fluoride (10 mm). Equal quantities of protein were separated under reducing conditions using polyacrylamide gels (4–12% Bis-Tris, NuPage; Invitrogen). Proteins were transferred by electroblotting onto PVDF membranes (Immobilon-P; Millipore), and the membranes were then probed with rabbit Abs to p57kip2 (antibody 2557), phosphoFAK Tyr-397 (antibody 3283), phosphoFAK Tyr-576/577 (antibody 3281), phospho-FAK Tyr-925 (antibody 3284), phospho-SFK Tyr-416 (antibody 2101), and phospho-SMAD2 (antibody 3108). All of the rabbit Abs were from Cell Signaling (Danvers, MA) and were used at 1:1000 overnight at 4 °C. Additional membranes were probed with a sheep pAb to NGN3 (1 μg/ml antibody AF3444; R & D) for 2.5 h at room temperature. Detection was performed using donkey anti-rabbit or anti-sheep IgG-HRP conjugates (Jackson Immunoresearch, West Grove, PA) and by chemiluminescence (ECL normal RPN2109; Amersham Biosciences). To ensure equal loading, the membranes were striped and probed for total FAK (1:1000 pAb 3285; Cell Signaling), total Src (1:000 pAb 21080), total SMAD2 (1:1000 antibody 5339), or β-actin (1:5000 mouse mAb AC-15; Sigma).
Immunofluorescence
Differentiated hESC cultures, were fixed with 4% paraformaldehyde and then treated for 45 min with PBS containing 0.2% Triton X-100 (Sigma) and 5% normal donkey serum (Jackson ImmunoResearch). The cells were subsequently washed and incubated with primary mAbs or pAbs to insulin (15 μg/ml sheep pAb PC059; Binding Site), glucagon (15 μg/ml mouse mAb, Clone K7961310; Sigma), NGN3 (5 μg/ml sheep pAb AF3444; R & D Systems), phospho-SFK Y416 (1:100 rabbit pAb 2101), phospho-FAK Y925 (1:100 rabbit pAb 3284), phospho-FAK Y397 (1:100 rabbit pAb 3283), p57kip2 (1:25 rabbit pAb SC-1040 Santa Cruz), or PDX1 (1:5000 goat pAb) (kindly provided by Dr. Christopher Wright, Vanderbilt University Medical Center, Nashville, TN). Rabbit pAbs to phospho-FAK or phospho-SFK were added in combination with the goat pAb to human PDX1. The sheep pAb to NGN3 was added alone or in combination with the rabbit pAb to p57kip2. The sheep pAb to insulin was used in combination with the mouse mAb to glucagon. The cells were incubated with primary antibodies overnight at 4 °C in PBS containing 0.1% Triton X-100 and 0.5% normal donkey serum. Bound antibodies were subsequently detected using donkey anti-sheep Alexa Fluor 488 (1:500 antibody A11015; Molecular Probes-Invitrogen), donkey anti-goat Rhodamine RedTM-X (1:500; Jackson Immunoresearch), donkey anti-rabbit Alexa Fluor 488 or 568 (1:500 antibody A21206/10042; Molecular Probes-Invitrogen) or donkey anti-mouse Alexa Fluor 488 or 568 (1:500 antibody A21202/A10037; Molecular Probes-Invitrogen). Secondary immunoconjugates were used alone or in combination as required, and staining was performed for 90 min at room temperature.
Flow Cytometry
Differentiated hESC cultures were harvested as single cells using 0.25% Trypsin/EDTA (Invitrogen) and were permeablized and fixed using IntraPrep permeabilization reagent kit (PN IM2388; Beckman Coulter) according to the manufacturer's instructions. After fixation and permeabilization, the cells were blocked with 5% normal donkey serum in the presence of the permeabilization reagent, for 20 min at room temperature. The cells were subsequently stained with antibodies to NGN3 (5 μg/ml sheep pAb; pAb AF3444; R & D Systems), human C-peptide (10 μg/ml mouse mAb Clone C-PEP-01; AbB Serotec), insulin (15 μg/ml sheep pAb; pAb PC059; Binding Site), or glucagon (12 μg/ml mouse mAb; Clone K7961310; Sigma). Incubations were performed for 1–2 h at room temperature. For detection, the cells were incubated with donkey anti-sheep Alexa Fluor 488 (1:500 antibody A11015; Molecular Probes-Invitrogen) or donkey anti-mouse Alexa Fluor 488 (1:500 antibody A21202; Molecular Probes-Invitrogen) for 1 h. Washing and incubation steps were performed using PBS containing 0.5% BSA. The cells were analyzed on a Becton Dickinson FACScan using CellQuest v. (3.3) software.
RESULTS
The SFK Inhibitor PP2 Promotes Endocrine Specification and the Derivation of β-Cells from hESCs
The impact of the SFK inhibitor PP2 on the derivation of pancreatic endocrine progenitors from hESCs was assessed using a modified version of a protocol described by D'Amour et al. (1) and Kroon et al. (2) (Fig. 1A). Using this stage-by-stage approach, we were able to confirm a greater than 200,000-fold induction of insulin message and the generation of discrete clusters of insulin-positive cells (Fig. 1, B and C). Based on FACS analysis, the final yield of insulin- and C-peptide-positive cells from various ESC lines including H9, CyT49, and CyT203 was between 6 and 13% (not shown).
The initial recruitment of cells into endocrine lineages has been shown to occur during stage 3 culture or between days 6 and 9 of the differentiation protocol described by Kroon et al. (2) (Fig. 1A). The impact of PP2 on the initial endocrine commitment of CyT49 hESCs was subsequently assessed by adding the inhibitor for no more than 48 h starting on day 6 (Fig. 1A). Levels of endocrine specification were subsequently determined on day 9 based on the relative expression of the transcription factor NGN3. NGN3 is required for endocrine commitment and, based on its restricted expression, can be used to monitor the emergence of endocrine progenitors (37). Importantly, Q-PCR analysis confirmed that treatment with PP2 induces a significant, time- and dose-dependent increase in the expression of NGN3 (Fig. 1, D and E). In contrast, PP3, an inactive structural analog of PP2, was found to be ineffective (Fig. 1E). PP2 was also found to be effective in inducing NGN3 expression in CyT203 cells, H9 cells, and a cloned population of human induced pluripotent stem cells derived from human skin fibroblasts (supplemental Fig. S1). It is important to note that PP2 also induced NGN3 expression at later stages of differentiation (i.e. stages 4 and 5) (supplemental Fig. S2). However, NGN3 expression was suppressed if PP2 was added at an earlier developmental stage (i.e. stage 2) (supplemental Fig. S2) or if it was added for more than 48 h (not shown). SKI-1, another small compound SFK inhibitor, was also found to induce NGN3 expression (supplemental Fig. S3). The SFK inhibitor SU6656 induced cell death and was ineffective (supplemental Fig. S3).
The ability of PP2 to induce expression of NGN3 was further confirmed at the protein level. Thus, immunostaining revealed a marked increase in the frequency of cells expressing NGN3 (Fig. 1F), and Western blot analysis verified the induction of a band corresponding to the predicted molecular mass of NGN3 (∼29 kDa) (Fig. 1G). FACS analysis performed on day 9 cultures confirmed that PP2 treatment increased the derivation of NGN3-positive cells by 2-fold (Fig. 1H). It is important to note that NGN3 is only transiently expressed by endocrine progenitors and that prior treatment with PP2 continued to induce higher levels of NGN3 expression during subsequent stage 4 culture (not shown). Consequently the number of NGN3-positive cells detected on day 9 underestimates the final yield of hormone-positive cells induced in the presence of PP2. It should also be noted that PP2 treatment induced the retraction of confluent stage 3 cultures into cord-like structures (supplemental Fig. S4) and altered the side scatter profile of cells harvested for FACS analysis (Fig. 1H).
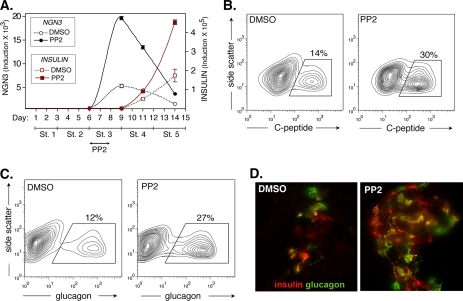
To verify that PP2 is promoting endocrine commitment, we further assessed its impact on insulin expression. Q-PCR performed over the course of the differentiation protocol confirmed that PP2 induces a marked temporal increase in NGN3 expression, and this is then followed by a further significant induction of insulin relative to the Me2SO control (Fig. 2A). Importantly, FACS analysis performed upon completion of the protocol confirmed that prior treatment with PP2 promotes an over 2-fold increase in the final yield of C-peptide-positive cells (Fig. 2B). In this regard, C-peptide is a component of proinsulin that is exclusively expressed as a result of endogenous insulin production. Indicating a role in both β- and α-cell differentiation, PP2 was also found to double the yield of glucagon-expressing cells (Fig. 2C). Consistent with the observed increase in C-peptide-positive cells, insulin release in response to IBMX treatment was also found to be significantly greater in those cultures that had previously been exposed to PP2 (supplemental Fig. S5).
FIGURE 2.
PP2 induces insulin and glucagon expression. A, Q-PCR was used to assess NGN3 and INSULIN expression during the course of CyT49 hESC differentiation. The cells were cultured in the presence or absence of PP2 (10 μm) for the first 2 days of stage 3 culture. Note that PP2 causes a large temporal increase in NGN3 expression and a subsequent increase in INSULIN expression. The results are fold induction relative to the lowest prior detectable levels of NGN3 or INSULIN. B and C, FACS contour plots showing that prior stage 3 treatment with PP2 increases the final yield of C-peptide-positive and glucagon-positive cells. CyT49 hESCs were harvested and stained on day 15. D, photomicrograph (40×) showing insulin (red) or glucagon (green) expression after and prior to stage 3 culture in the presence or absence of PP2. CyT49 cells were harvested on day 15 and were subsequently embedded in OTC for sectioning and staining. Double positive cells are yellow.
Double immunofluorescence staining confirmed an increase in the frequency of insulin- and glucagon-positive cells after treatment with PP2 (Fig. 2D). It should be noted, however, that ∼40% of the hormone-positive cells detected were found to co-express insulin and glucagon, suggesting that at least some of these cells remain immature (Fig. 2C and supplemental Fig. S6). We found no evidence that prior treatment with PP2 altered the fraction of hormone-positive cells expressing either insulin or glucagon alone (supplemental Fig. S6).
PP2 Inhibits SFK and FAK Activity, and Inhibition of FAK Also Induces Endocrine Specification
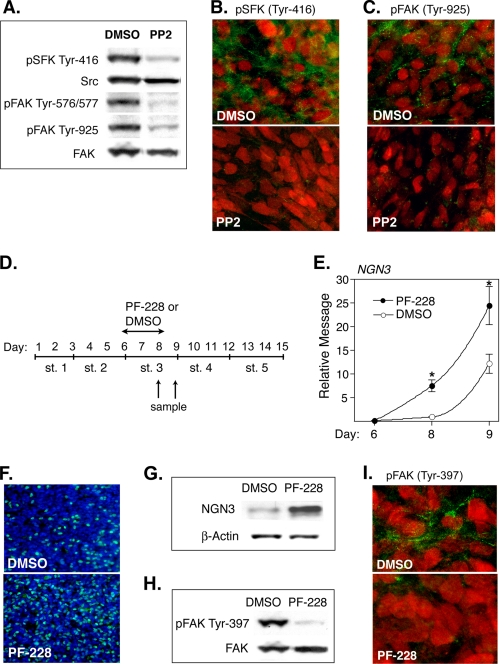
Western blot analysis of lysates derived from stage 3 cultures confirmed the presence of SFK activity (pSFK Tyr-416) and confirmed a marked inhibition of this activity by PP2 (Fig. 3A, top bands). Because PDX-1-positive progenitors are the presumptive source of committed endocrine progenitors, we further determined whether the SFK activity detected is localized to PDX-1-positive cells and whether this activity is also inhibited by PP2. Double-immunofluorescent staining confirmed the presence of active SFK (Tyr-416) within the cytoplasm of PDX-1-positive progenitors and verified that this activity is markedly inhibited by PP2 (Fig. 3B). Double staining with DAPI confirmed that the PDX-1 observed is primarily confined to the nuclei of our putative progenitor population (supplemental Fig. S7).
FIGURE 3.
PP2 inhibits SFK and FAK activity and the FAK inhibitor PF-228 also induces NGN3 expression. A, Western blots showing levels of pSFK and pFAK activity in CyT49 cells that had been differentiated in the presence or absence of PP2. PP2 was added at the start of stage 3 culture, and lysates were generated 48 h later. The membranes were probed with pAbs specific for pSFK (Tyr-416), pFAK (Tyr-576/577), or pFAK (Tyr-925). Equal loading was confirmed using pAbs to Src and FAK. B and C, photomicrographs (40×) showing CyT49 stage 3 cultures stained for PDX1 (red) and either pSFK (Tyr-416) or pFAK (Tyr-925) (green). The cells were differentiated in the presence or absence of PP2 for 48 h prior to staining. D, schematic showing the timing of PF-228 addition and removal. E, Q-PCR analysis showing induction of NGN3 after culture in the presence or absence of PF-228 (2 μm). Relative message for NGN3 is based on standard curves established using fetal ICCs. *, levels induced by PF-228 were significantly greater than by Me2SO (DMSO) alone at p < 0.01 (n = 3). F, photomicrographs (20×) showing nuclear NGN3 expression in CyT49 cells that had been differentiated in the presence or absence of PF-228. PF-228 was added on day 6 and was removed 48 h later. The cells were stained on day 9 using a pAb to NGN3 (green), and the nuclei were stained using DAPI (blue). G, Western blot showing levels of NGN3 in CyT49 cells that had been differentiated in the presence or absence of PF-228. PF-228 was added for 48 h at the start of stage 3 culture (day 6), and the lysates were generated on day 9. The membranes were probed with a pAb to NGN3, and the bands correspond to predicted size of NGN3. H, Western blot showing levels of pFAK in CyT49 hESCs that had been differentiated in the presence or absence of PF-228. PF-228 was added on day 6, and the lysates were generated 48 h later. The membranes were probed with pAbs specific for pFAK (Tyr-397) or total FAK. I, photomicrographs (40×) showing CyT49 stage 3 cultures stained for PDX-1 (red) and pFAK(Tyr-397) (green). The cells were cultured in the presence or absence of PF-228 for 48 h prior to staining.
Based on prior reports demonstrating that PP2 induces differentiation by inhibiting FAK (13, 14, 21), we determined whether PP2 also inhibits FAK activation within our stage 3 cultures. Importantly, Western blot analysis confirmed that PP2 markedly inhibited FAK phosphorylation at several sites known to be activated following SFK recruitment including pFAK Tyr-576/577 and pFAK Tyr-925 (Fig. 3A) (19). Moreover, double immunofluorescent staining confirmed the presence of active FAK (pFAK Tyr-925) within the cytoplasm of PDX-1-positive progenitors and verified that this activity is markedly inhibited by PP2 (Fig. 3C).
Given the ability of PP2 to suppress FAK activation, we further determined whether pharmacological inhibition of FAK is sufficient to promote endocrine specification. For this purpose, we assessed the impact of a novel small compound inhibitor (PF-228 or PF-573,228) that selectively inhibits FAK catalytic activity by blocking phosphorylation at Tyr-397 (38). This inhibitor is highly specific for FAK relative to other tyrosine kinases, and used at 1–5 μm, it selectively disrupts FAK-dependent processes including cell migration and focal adhesion turnover (38–40). Using the same approach adopted for PP2, PF-228 (2 μm) was added to cultures for 48 h at the start of stage 3 culture, and its impact on NGN3 expression was evaluated 2–3 days later (Fig. 3D). Importantly, Q-PCR analysis confirmed that PF-228 was also able to induce a significant time-dependent increase in the expression of NGN3 (Fig. 3E). Moreover, immunostaining confirmed a significant increase in the frequency of cells expressing nuclear NGN3 (Fig. 3F), and Western blot analysis verified that PF-228 induces a band corresponding to the predicted molecular mass of NGN3 (∼29 kDa; Fig. 3G). The ability of PF-228 to inhibit FAK phosphorylation in stage 3 cultures and within PDX-1-positive progenitors was confirmed by Western blot analysis and by immunostaining (Fig. 3, H and I). Together these data support the contention that PP2 can promote endocrine specification by inhibiting FAK activation.
Inhibition of c-Src Is Sufficient to Induce NGN3 Expression in Fetal Islet Preparations
Induction of NGN3 by both PP2 and SKI-1 supports the view that endocrine specification is potentiated by the suppression of SFK activity. However, PP2 targets multiple kinases including some that do not belong to the Src family. Additional studies were therefore performed to determine whether specific targeting of the prototypical SFK c-Src is sufficient to induce NGN3 expression. To address this issue we used human fetal pancreatic preparations (ICCs) that contain PDX1-positive progenitors and are amenable to nucleofection (36). Despite repeated attempts, using a variety of approaches, we were unable to successfully transfect stage 3 hESC cultures. This can partly be attributed to the fact that these cultures need to be maintained as intact highly confluent monolayers to promote endocrine specification. Moreover, based on results obtained using PP2, it would likely be detrimental to inhibit Src activity prior to stage-3 culture.
Fetal islet-like cell clusters (ICCs) were generated from 18-week fetal pancreata essentially as described (36) and were subsequently cultured for 3 days in the presence or absence of PP2. As observed in our stage 3 hESC cultures, PP2 induced a 2–3-fold increase in the expression of NGN3 and NEUROD1, as well as a marked increase in the expression of INSULIN (Fig. 4A). To determine whether this induction can be specifically attributed to the inhibition of c-Src, expanded ICC preparations were nucleofected with a kinase-inactive mutant of c-Src (c-Src K297M) or with empty vector (36). Importantly, Q-PCR analysis performed prior to and after the culture of transfected populations confirmed that ectopic expression of c-Src K297M also induces a significant increase in the expression of NGN3, NEUROD1, and INSULIN (Fig. 4B).
FIGURE 4.
Inhibition of c-Src promotes endocrine specification. A, fetal ICCs (18 weeks) were cultured for 3 days on collagen in the presence of PP2 or Me2SO (DMSO) vehicle alone. Q-PCR was used to assess levels of INSULIN, NGN3, and NEUROD1 message prior to and after culture. The results show message levels relative to the ICC starting material. *, levels induced by PP2 were significantly greater than by Me2SO alone at p < 0.01 (n = 3). B, ICCs were expanded on HTB-9 matrix for 3 days prior to nucleofection with a dominant negative c-Src construct (c-Src K297M) or an empty vector control (Vect., pcDNA 3.1). Transfected cells were then reseeded onto collagen for 48 h. Q-PCR was used to assess levels of INSULIN, NGN3, and NEUROD1 message prior to and after culture on collagen. The results show message levels relative to ICC starting materials. *, levels induced by dominant negative Src were significantly greater than by the empty vector at p < 0.01 (NGN3 and INSULIN) or at p < 0.05 (NEUROD1) (n = 3). C, ICCs were expanded on HTB-9 matrix for 3 days prior to nucleofection with Src or Lyn siRNA. Control cells received scrambled Src siRNA. Transfected cells were then reseeded onto collagen for 48 h, and Q-PCR was used to assess levels of NGN3 message prior to and after culture on collagen. The results show message levels relative to ICC starting material. *, levels induced by Src siRNA were significantly greater than by the scrambled Src siRNA at p < 0.01 (n = 3).
Using a second approach, we also observed a significant induction of NGN3 following nucleofection with a Src siRNA (Fig. 4C). In contrast, nucleofection with scrambled Src siRNA or with a Lyn siRNA had no significant impact (Fig. 4C). Together these data support the contention that inhibition of c-Src is sufficient to induce NGN3 expression.
SFK and FAK Inhibitors May Induce NGN3 by Inhibiting Activation of the Smad2/3 Pathway
Inhibitors of TGFβ-dependent Smad2/3 activation have recently been shown to induce the endocrine commitment of hESCs derivatives, suggesting that this signaling pathway normally acts to suppress early β-cell development (28, 29). Importantly, adhesion-dependent activation of FAK and Src has also recently been shown to promote signaling via the TGFβR/Smad2/3 axis (26, 27). Based on these observations, it is possible that PP2 and PF228 induce endocrine commitment by limiting activation of the Smad2/3 pathway.
To address this possibility, we first determined whether inhibition of TGFβR signaling induces NGN3 expression by the end of stage 3 culture. The addition of a highly specific inhibitor of TGFβ R1 kinase (ALK5 inhibitor II) did indeed significantly increase NGN3 expression by the end of stage 3 culture (Fig. 5, A and B). Inhibition of TGFβR signaling by this inhibitor was subsequently confirmed based on reduced levels of Smad2 activation (Fig. 5C). Supporting prior reports that Src and FAK contribute to activation of the TGFβR/Smad2/3 pathway, PP2, SKI-1, and PF-228 all reduced the levels of Smad2 activation in our stage 3 cultures (Fig. 5C). Based on these data, we conclude that PP2 and PF228 may induce NGN3 expression by limiting SFK/FAK-dependent activation of the Smad2/3 pathway.
FIGURE 5.
PP2 and PF-228 inhibit SMAD2 activation. A, schematic showing the timing of ALK5 inhibitor II addition and removal. B, Q-PCR analysis showing fold induction of NGN3 after culture in the presence of increasing amounts of ALK5 inhibitor II. Fold is relative to Me2SO (DMSO) treatment alone. *, levels induced by the ALK5 inhibitor II were significantly greater than by Me2SO alone at p < 0.01 (n = 3). C, Western blots showing levels of pSMAD2 activity in CyT49 cells that had been differentiated in the presence or absence of ALK5 inhibitor II (3 μm), PP2 (10 μm), PF-228 (3 μm), or SKI-1 (3 μm). Control cells were incubated in the presence of Me2SO alone. Inhibitors were added at the start of stage 3 culture, and lysates were generated 48 h later. The membranes were probed with a pAb specific for pSMAD2, and equal loading was confirmed using a pAbs to total SMAD2.
PP2 and PF-228 Induce Expression of p57kip2 but Suppress the Expression of Id1 and Id2
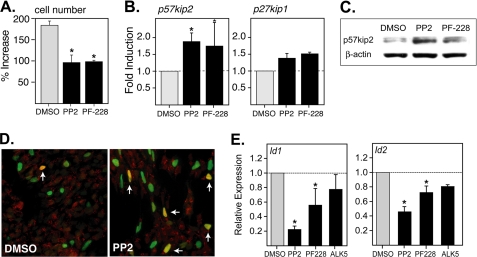
Endocrine commitment requires that pancreatic progenitors exit the cell cycle. Experiments were therefore performed to determine whether inhibition of SFK or FAK signaling suppresses the growth of stage 3 cultures that are highly enriched for PDX-1-positive pancreatic progenitors (2). Importantly, both PP2 and PF-228 significantly inhibited the expansion of stage 3 cultures without inducing a significant increase in cell death or apoptosis (Fig. 6A and supplementary Fig. S8). In other cell types, SFK and FAK inhibitors have been shown to inhibit proliferation and promote differentiation by increasing the expression or activity of p57kip2 and p27kip1 (25, 30, 31, 41). In this regard, exposure of stage 3 cultures to both PP2 and PF-228 was found to significantly increase the transcription p57kip2 and had a more modest impact on the expression p27kip1 (Fig. 6B). Given a report showing that p57kip2 is expressed almost exclusively by post-mitotic β-cells in the human pancreas (42), we determined whether this CDKI is also up-regulated at the protein level and whether it is induced in committed endocrine progenitors. Importantly, Western blot analysis confirmed that p57kip2 is induced by PP2 and PF-228 (Fig. 6C). Moreover, double-immunofluorescent staining confirmed that p57kip2 expression is induced in at least a subset of committed NGN3-positive progenitors following treatment with PP2 (Fig. 6D). Together these findings are consistent with the notion that inhibition of SFK activity can promote cell cycle exit and subsequent β-cell differentiation by inducing the expression of p57kip2.
FIGURE 6.
PP2 and PF-228 inhibit the growth of stage 3 cultures and modulate the expression of p57kip2, Id1, and Id2. A, PP2 (10 μm) or PF-228 (2 μm) was added at the start of stage 3 culture (day6) for 48 h, and the percentage of increase in cell number was determined on day 9. *, the percentage of increase was significantly lower in the presence of PP2 and PF-228 at p < 0.01 (n = 2). B, Q-PCR analysis showing the induction of p57kip2 and p27kip1 expression after culture in the presence of PP2 or PF-228. Inhibitors were added on day 6 for 48 h, and cultures were harvested on day 9. The results show fold induction relative to Me2SO (DMSO) control. *, levels induced by PP2 and PF-228 were significantly greater than by Me2SO alone at p < 0.05 (n = 3). C, Western blot showing levels of p57kip2 in CyT49 cells that had been differentiated in the presence or absence of PP2 or PF-228. Compounds were added for 48 h at the start of stage 3 culture (day 6), and the lysates were generated on day 9. The membranes were probed with a pAb specific for p57kip2, and equal loading was confirmed using a mAb to β-actin. D, stage 3 CyT49 cells that had been differentiated in the presence or absence of PP2 were stained for NGN3 (green) and p57kip2 (red). PP2 was added for 48 h at the start of stage 3 culture (day 6), and the cells were stained on day 9. E, Q-PCR analysis showing levels Id1 and Id2 expression after culture in the presence of PP2, PF-228, or ALK5 inhibitor II. Inhibitors were added on day 6 for 48 h, and cultures were harvested on day 9. The results show levels relative to Me2SO control. *, levels seen in the presence of PP2 and PF-228 were significantly lower than that seen in the presence of Me2SO alone at p < 0.05 (n = 3).
The inhibitors of differentiation proteins (Ids) are a family of proteins that suppress the activity of basic HLH transcription factors and in this capacity regulate cell fate determination and inhibit endocrine differentiation (43). Based on this we determined whether PP2 and PF228 might also potentiate endocrine development by suppressing Id expression. In this regard, exposure of stage 3 cultures to PP2, and to a lesser degree to PF-228, was indeed found to suppress the expression of Id1 and Id2 (Fig. 6E). PP2 was found to suppress Id1 and Id2 expression more effectively than ALK5 inhibitor II, suggesting a mechanism largely independent of the TGFβR/Smad2/3 pathway (Fig. 6E).
PP2 and PF-228 Differentially Impact the Expression of the Pancreatic Hormones and the Proendocrine Transcription Factors ARX and PAX4
Further experiments were performed to compare the impact of PP2 and PF-228 on the derivation of the different endocrine subtypes. In addition to inducing insulin and glucagon, PP2 also promoted the expression of somatostatin, and to a lesser degree, pancreatic polypeptide (PP) and ghrelin (Fig. 7A). However, contrary to expectation, prior treatment with PF-228 only effectively induced the expression of somatostatin (Fig. 7A). These data suggest that inhibition of FAK alone is not sufficient to promote the derivation of every endocrine subtype. For its part, PP2 may preferentially induce other transcription factors that direct NGN3-positive progenitors into the α- or β-cell lineage. To test this hypothesis, we determined whether PP2 and PF-228 differentially regulate the expression of lineage-associated transcription factors downstream of NGN3 (Fig. 7B).
FIGURE 7.
PP2 and PF-228 have a differential impact on the expression of pancreatic hormones and proendocrine transcription factors. A, results show the expression of pancreatic hormones by CyT49 cells previously exposed to PP2 or PF-228. Inhibitors were added at the start of stage 3 culture for 48 h, and the cells were subsequently harvested for Q-PCR analysis on day 15. Results show fold induction relative to Me2SO (DMSO) control. *, levels induced by PP2 or PF-228 were significantly greater than by Me2SO alone at p < 0.01 (n = 3–5). B, schematic showing the putative involvement of different transcription factors in the generation of the different endocrine subtypes. C, results show the expression of various proendocrine transcription factors after CyT49 cells were differentiated in the presence or absence of PP2 or PF-228. Inhibitors were added at the start of stage 3 culture (day 6) for 48 h, and the cells were harvested for Q-PCR analysis on day 9. *, levels induced by PP2 or PF-228 were significantly greater than by Me2SO alone at p < 0.01 (n = 3–4).
Proendocrine factors immediately downstream of NGN3, namely NEUROD1 and NKX2.2, were significantly induced by both PP2 and PF-228 (Fig. 7, B and C). However, the transcription factor PAX4, which is essential for normal β-cell development (44) was found to be markedly and preferentially induced by PP2 (Fig. 7C). Based on this finding, it is conceivable that PF-228 (unlike PP2) is unable to induce insulin expression because it fails to induce the requisite levels of PAX4. The observation that PP2 is more effective in suppressing Id2 expression than PF-228 (Fig. 6E) might explain why this inhibitor is more effective in inducing PAX4 expression (43).
Interestingly, PF-228 was much more effective than PP2 in inducing the expression of ARX, which would normally be expected to favor the development of ϵ- and α-cells (Fig. 7, B and C) (45). However, the observation that PP2 treatment induces lower levels of ARX expression is consistent with the fact that ARX and PAX4 are part of antagonistic cross-regulatory circuit (45). The transcription factor BRN4, which has been linked to the development of α-cells, was induced by both PP2 and PF-228 (Fig. 7, B and C). Given the ability of PF-228 to induce both ARX and BRN4, it remains to be determined why this inhibitor ultimately fails to induce glucagon expression. Additional transcription factors involved in α-cell or β-cell maturation including PAX6, MAFA and MAFB were not significantly induced by PP2 or PF-228 during the course of stage 3 culture (not shown). However, pretreatment with PP2 did significantly increase both MAFB and MAFA expression by the end of stage 5 (supplemental Fig. S9). This is consistent with PP2 increasing overall levels of endocrine commitment, which then allows for the subsequent emergence of MAFA and MAFB positive cells. Finally, it should be noted that under the conditions used, PP2 and PF-228 were not found to induce transcription factors involved in the development of the exocrine pancreas, including MIST1 or PTF1A (Fig. 7C and not shown).
DISCUSSION
We have demonstrated that the SFK inhibitor PP2 promotes endocrine specification and the subsequent derivation of insulin-producing cells. We propose that PP2 induces endocrine specification, at least in part, by inhibiting the activation of FAK. These findings are consistent with prior reports showing that inhibition of FAK by PP2 also promotes the derivation of cardiomyocytes and chondrocytes (13, 21, 25). Because SFKs, including c-Src, promote the activation and catalytic activity of FAK, it is not surprising that PP2 can function as a potent inhibitor of FAK activation (19). However, whether inhibition of dual SFK/FAK signaling ultimately serves to induce differentiation is likely to be dependent on the cell type targeted. Thus, PP2 has been shown to actually inhibit the differentiation of certain cell types, including neuroectodermal cells (21) and myofibroblasts (46), and in this study we found no evidence that PP2 induces the emergence of exocrine cells.
Several lines of evidence suggest that inhibition of SFK/FAK signaling by PP2 potentiates endocrine differentiation by inhibiting the TGFβR/Smad2/3 pathway. Recent studies have shown that pharmacological inhibitors that target the TGFβ type I receptor ALK5 (ALK5 inhibitor II) or ALK5 and its relatives ALK4 and ALK7 (SB431542) promote the endocrine specification of hESC derivatives (29) and ultimately increase β-cell yields (28). Using ALK5 inhibitor II, we have been able to confirm that this is also true using our own differentiation method. Importantly, we can confirm that PP2 and PF228 also inhibit the activation of Smad2. This is consistent with prior reports demonstrating that anchorage-dependent activation of SFK/FAK results in the activation of Smad2/3, possibly because of cross-talk between integrins and TGFβRs (26, 27). In this regard it has been shown that TGFβ1 ligation results in enhanced FAK activation (27). Moreover, it has also been shown that integrin/TGFβR cross-talk can occur even in the absence of TGFβ ligation (26).
Several studies have shown that SFK inhibitors can promote cell cycle exit and terminal differentiation by inducing the expression of CDKIs including p57kip2 (25, 30, 31). In this regard, we can confirm that PP2 also induces p57kip2 expression in a subset of NGN3-positive endocrine progenitors. Interestingly, inactivation of the transcription factor HES1 in mice has been shown to induce p57kip2 expression, and this in turn drives pancreatic progenitors out of the cell cycle, forcing them to undergo precocious differentiation (47). In this mouse model, however, p57kip2 failed to co-localize with endocrine progenitors, and p57kip2 is not expressed by β-cells in the rat (47, 48). However, it should be noted that p57kip2 is expressed by post-mitotic β-cells in human islets (42, 49). Based on this observation and our own findings, p57kip2 could have an instructive role in the specification and differentiation of human β-cells, and repressing SFK/FAK signaling should serve to promote this process.
Based on the induction of NGN3, NKX2.2, NEUROD1, and somatostatin by PF-228, we propose that inhibition of FAK is sufficient to induce endocrine commitment. However, in contrast to PP2, this inhibitor failed to induce the expression of either insulin or glucagon, suggesting that other specification factors associated with the α- and β-cell lineage need to be induced. In this regard, a prior report has shown that ectopic expression of NGN3 in ductal epithelial cells effectively induces markers of early islet cell differentiation (e.g. NEUROD1 and NKX2.2), but subsequent differentiation tended to favor the development of somatostatin-positive cells (50). The authors suggest that this may be due to an intrinsic deficit in the basal expression of other transcription factors such as Pax4, Pax6, or Nkx6.1 (50). This said, it is noteworthy that PP2, which did promote insulin expression, was more effective than PF-228 in inducing the expression of PAX4. Accordingly, FAK inhibition alone may not induce the threshold of PAX4 expression required for β-cell development. One explanation based on our own observations could be that PF228 is less effective in suppressing the expression of Id2. Higher levels of Id2 may then suppress the activity of basic HLH transcription factors required for β-cell development. In this regard, it is noteworthy that Id2 has been shown to inhibit the activity of NeuroD1 and subsequently inhibits PAX6 expression and endocrine differentiation (43). In addition to inhibiting FAK, PP2 will broadly inhibit all SFK activity, and this may also be important for the optimal induction of PAX4. Moreover, it should be noted that the retraction of cell monolayers into cord-like structures observed in the presence of PP2 was not observed after the addition of PF-228. Thus, it is conceivable that a shift from cell-matrix to cell-cell association is required for the optimal emergence of insulin- and glucagon-positive cells.
Our work with isolated fetal pancreata indicates that selective inhibition of c-Src is sufficient to induce NGN3, NEUROD1, and insulin expression. Such a role is consistent with prior reports showing that selective inhibition of c-Src inhibits cell growth (31), whereas active Src effectively prevents differentiation (51). Moreover, c-Src has been shown to activate FAK and to inhibit the expression or stability of both p57kip2 and p27kip1 (31).
The observation that the SFK inhibitor PP2 can be used to significantly enhance the derivation of β-cells from hESCs has significant translational implications. Islet transplantation into type I diabetics has been shown to provide clear benefits in terms of glucose control, but a critical shortage of donor pancreata means that few can benefit from this approach. Any small compound inhibitors that can be used to significantly enhance the ex vivo derivation of β-cells prior to transplantation should help to ameliorate this problem and thus bring us closer to a universal cell-based therapy for type I diabetes.
Supplementary Material
This work was supported by the American Diabetes Association (Innovation Award), by the Juvenile Diabetes Research Foundation (Regular Research Grant), by the California Institute of Regenerative Medicine (Early Translation Grant), and by the Larry L. Hillblom Foundation (network grant).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S9.
- hESC
- human embryonic stem cell
- SFK
- Src family kinase
- FAK
- focal adhesion kinase
- CDKI
- cyclin-dependent kinase inhibitor
- ICC
- islet-like cell cluster
- pAb
- polyclonal antibody
- TGFβR
- TGFβ receptors
- Q-PCR
- quantitative PCR
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. D'Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K., Baetge E. E. (2006) Nat. Biotechnol. 24, 1392–1401 [DOI] [PubMed] [Google Scholar]
- 2. Kroon E., Martinson L. A., Kadoya K., Bang A. G., Kelly O. G., Eliazer S., Young H., Richardson M., Smart N. G., Cunningham J., Agulnick A. D., D'Amour K. A., Carpenter M. K., Baetge E. E. (2008) Nat. Biotechnol. 26, 443–452 [DOI] [PubMed] [Google Scholar]
- 3. Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y., Deng H. (2009) Cell Res. 19, 429–438 [DOI] [PubMed] [Google Scholar]
- 4. Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., Leibel R. L., Melton D. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen S., Borowiak M., Fox J. L., Maehr R., Osafune K., Davidow L., Lam K., Peng L. F., Schreiber S. L., Rubin L. L., Melton D. (2009) Nat. Chem. Biol. 5, 258–265 [DOI] [PubMed] [Google Scholar]
- 6. Jiang W., Shi Y., Zhao D., Chen S., Yong J., Zhang J., Qing T., Sun X., Zhang P., Ding M., Li D., Deng H. (2007) Cell Res. 17, 333–344 [DOI] [PubMed] [Google Scholar]
- 7. Champeris Tsaniras S., Jones P. M. (2010) J. Endocrinol. 206, 13–26 [DOI] [PubMed] [Google Scholar]
- 8. McLean A. B., D'Amour K. A., Jones K. L., Krishnamoorthy M., Kulik M. J., Reynolds D. M., Sheppard A. M., Liu H., Xu Y., Baetge E. E., Dalton S. (2007) Stem Cells 25, 29–38 [DOI] [PubMed] [Google Scholar]
- 9. Phillips B. W., Hentze H., Rust W. L., Chen Q. P., Chipperfield H., Tan E. K., Abraham S., Sadasivam A., Soong P. L., Wang S. T., Lim R., Sun W., Colman A., Dunn N. R. (2007) Stem Cells Dev. 16, 561–578 [DOI] [PubMed] [Google Scholar]
- 10. Annerén C., Cowan C. A., Melton D. A. (2004) J. Biol. Chem. 279, 31590–31598 [DOI] [PubMed] [Google Scholar]
- 11. Meyn M. A., 3rd, Schreiner S. J., Dumitrescu T. P., Nau G. J., Smithgall T. E. (2005) Mol. Pharmacol. 68, 1320–1330 [DOI] [PubMed] [Google Scholar]
- 12. Tatosyan A. G., Mizenina O. A. (2000) Biochemistry 65, 49–58 [PubMed] [Google Scholar]
- 13. Pala D., Kapoor M., Woods A., Kennedy L., Liu S., Chen S., Bursell L., Lyons K. M., Carter D. E., Beier F., Leask A. (2008) J. Biol. Chem. 283, 9239–9247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daoud G., Rassart E., Masse A., Lafond J. (2006) J. Physiol. 571, 537–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theus M. H., Wei L., Francis K., Yu S. P. (2006) Exp. Cell Res. 312, 3096–3107 [DOI] [PubMed] [Google Scholar]
- 16. Frame M. C. (2004) J. Cell Sci. 117, 989–998 [DOI] [PubMed] [Google Scholar]
- 17. Bromann P. A., Korkaya H., Courtneidge S. A. (2004) Oncogene 23, 7957–7968 [DOI] [PubMed] [Google Scholar]
- 18. Fizazi K. (2007) Ann. Oncol. 18, 1765–1773 [DOI] [PubMed] [Google Scholar]
- 19. Mitra S. K., Schlaepfer D. D. (2006) Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 20. Avizienyte E., Brunton V. G., Fincham V. J., Frame M. C. (2005) Cells Tissues Organs 179, 73–80 [DOI] [PubMed] [Google Scholar]
- 21. Hakuno D., Takahashi T., Lammerding J., Lee R. T. (2005) J. Biol. Chem. 280, 39534–39544 [DOI] [PubMed] [Google Scholar]
- 22. Lim S. T., Mikolon D., Stupack D. G., Schlaepfer D. D. (2008) Cell Cycle 7, 2306–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schlaepfer D. D., Mitra S. K. (2004) Curr. Opin. Genet. Dev. 14, 92–101 [DOI] [PubMed] [Google Scholar]
- 24. Vadali K., Cai X., Schaller M. D. (2007) IUBMB Life 59, 709–716 [DOI] [PubMed] [Google Scholar]
- 25. Bursell L., Woods A., James C. G., Pala D., Leask A., Beier F. (2007) Arthritis Res. Ther. 9, R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garamszegi N., Garamszegi S. P., Samavarchi-Tehrani P., Walford E., Schneiderbauer M. M., Wrana J. L., Scully S. P. (2010) Oncogene 29, 2368–2380 [DOI] [PubMed] [Google Scholar]
- 27. Park M. S., Kim Y. H., Lee J. W. (2010) Matrix Biol. 29, 135–142 [DOI] [PubMed] [Google Scholar]
- 28. Nostro M. C., Sarangi F., Ogawa S., Holtzinger A., Corneo B., Li X., Micallef S. J., Park I. H., Basford C., Wheeler M. B., Daley G. Q., Elefanty A. G., Stanley E. G., Keller G. (2011) Development 138, 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rezania A., Riedel M. J., Wideman R. D., Karanu F., Ao Z., Warnock G. L., Kieffer T. J. (2011) Diabetes 60, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker J. L., Wolff I. M., Zhang L., Menko A. S. (2007) Invest. Ophthalmol. Vis. Sci. 48, 2214–2223 [DOI] [PubMed] [Google Scholar]
- 31. Xing J., Zhang Z., Mao H., Schnellmann R. G., Zhuang S. (2008) Am. J. Physiol. Renal Physiol. 295, F145–F152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gautschi O., Tepper C. G., Purnell P. R., Izumiya Y., Evans C. P., Green T. P., Desprez P. Y., Lara P. N., Gandara D. R., Mack P. C., Kung H. J. (2008) Cancer Res. 68, 2250–2258 [DOI] [PubMed] [Google Scholar]
- 33. Beattie G. M., Cirulli V., Lopez A. D., Hayek A. (1997) J. Clin. Endocrinol. Metab. 82, 1852–1856 [DOI] [PubMed] [Google Scholar]
- 34. Beattie G. M., Levine F., Mally M. I., Otonkoski T., O'Brien J. S., Salomon D. R., Hayek A. (1994) J. Clin. Endocrinol. Metab. 78, 1232–1240 [DOI] [PubMed] [Google Scholar]
- 35. Broome M. A., Hunter T. (1996) J. Biol. Chem. 271, 16798–16806 [DOI] [PubMed] [Google Scholar]
- 36. Kaido T. J., Yebra M., Kaneto H., Cirulli V., Hayek A., Montgomery A. M. (2010) J. Cell. Physiol. 224, 101–111 [DOI] [PubMed] [Google Scholar]
- 37. Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzio M. J., Cooper B., Kath J. C., Roberts W. G., Parsons J. T. (2007) J. Biol. Chem. 282, 14845–14852 [DOI] [PubMed] [Google Scholar]
- 39. Huanwen W., Zhiyong L., Xiaohua S., Xinyu R., Kai W., Tonghua L. (2009) Mol. Cancer 8, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bailey K. M., Liu J. (2008) J. Biol. Chem. 283, 13714–13724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bryant P., Zheng Q., Pumiglia K. (2006) Mol. Cell. Biol. 26, 4201–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kassem S. A., Ariel I., Thornton P. S., Hussain K., Smith V., Lindley K. J., Aynsley-Green A., Glaser B. (2001) Diabetes 50, 2763–2769 [DOI] [PubMed] [Google Scholar]
- 43. Hua H., Zhang Y. Q., Dabernat S., Kritzik M., Dietz D., Sterling L., Sarvetnick N. (2006) J. Biol. Chem. 281, 13574–13580 [DOI] [PubMed] [Google Scholar]
- 44. Sosa-Pineda B. (2004) Mol. Cells 18, 289–294 [PubMed] [Google Scholar]
- 45. Collombat P., Mansouri A. (2009) Cell Cycle 8, 3450–3451 [DOI] [PubMed] [Google Scholar]
- 46. Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. (2003) J. Biol. Chem. 278, 12384–12389 [DOI] [PubMed] [Google Scholar]
- 47. Georgia S., Soliz R., Li M., Zhang P., Bhushan A. (2006) Dev. Biol. 298, 22–31 [DOI] [PubMed] [Google Scholar]
- 48. Potikha T., Kassem S., Haber E. P., Ariel I., Glaser B. (2005) Lab. Invest. 85, 364–375 [DOI] [PubMed] [Google Scholar]
- 49. Bar Y., Russ H. A., Knoller S., Ouziel-Yahalom L., Efrat S. (2008) Diabetes 57, 2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boretti M. I., Gooch K. J. (2007) Tissue Eng. 13, 775–788 [DOI] [PubMed] [Google Scholar]
- 51. Vardimon L., Fox L. E., Cohen-Kupiec R., Degenstein L., Moscona A. A. (1991) Mol. Cell. Biol. 11, 5275–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.