Background: Intestinal epithelial cells (IECs) express low levels of TLR4 and are hyporesponsive to commensal bacteria.
Results: TLR4 gene is methylated in IECs, and this process is dependent on commensal bacteria in the large intestine.
Conclusion: Commensal bacteria control epigenetic modification of the host gene.
Significance: This study shows a novel mechanism underlying the maintenance of intestinal symbiosis.
Keywords: Gene Regulation, Intestinal Epithelium, Mucosal Immunology, Symbiosis, Toll-like Receptors (TLR), Intestinal Bacteria
Abstract
Intestinal epithelial cells (IECs) are continuously exposed to large numbers of commensal bacteria but are relatively insensitive to them, thereby averting an excessive inflammatory reaction. We have previously reported that the hyporesponsiveness of a human IEC line to LPS was primarily the result of a down-regulation of TLR4 gene transcription through epigenetic mechanisms. In the present study we show that DNA methylation in the 5′ region of the TLR4 gene is significantly higher in IECs than in splenic cells in vivo. The methylation was shown to be dependent on the differentiation state of the IECs, as the differentiated IEC population that expressed higher levels of intestinal alkaline phosphatase (IAP) also displayed greater methylation and lower expression of the TLR4 gene than the undifferentiated population. The IAPhigh, differentiated population also showed less abundant expression of CDX2, the transcription factor required for the development of the intestine, than the IAPlow, undifferentiated population. Overexpression of CDX2 in an IEC line decreased the methylation level of the TLR4 gene, increased transcriptional promoter activity of the gene, and increased responsiveness to the TLR4 ligand. Furthermore, the methylation level of the TLR4 gene was significantly lower in IECs of the large intestine of germ-free mice than in those of conventional mice, whereas the level in IECs of the small intestine was almost equal between these mice, indicating that commensal bacteria contribute to the maintenance of intestinal symbiosis by controlling epigenetic modification of the host gene in the large intestine.
Introduction
A huge number of commensal bacteria inhabit the intestinal tract where they contribute to the regulation of various physiological functions. The significance of these bacteria to the maintenance of health has recently attracted considerable attention. Although the commensal bacteria are not immunological “self” to the host, the intestinal immune system, the largest immune system in the body, does not exclude them completely, resulting in a symbiotic relationship. Maintenance of the symbiosis between the intestinal immune system and the commensals is required for intestinal homeostasis, with disorders in this system leading to increased risk of onset or aggravated symptoms of various diseases, including allergy, inflammatory bowel disease, autoimmune disease, and metabolic syndrome (1–7). Many of these conditions involve excessive inflammation, indicating that regulation of inflammation is indispensable for the maintenance of the intestinal symbiotic system.
When the immune system recognizes invading pathogenic bacteria, it attacks and excludes them. During this protective response, transient induction of an inflammatory reaction is required to activate immune responses, but this rapidly disappears after successful exclusion of the pathogenic bacteria. If the intestinal immune system recognizes commensal bacteria like pathogenic bacteria, excessive inflammation occurs, leading to intestinal disorder. However, under normal conditions only a base-line physiological level of inflammatory reactivity is observed despite the continuous exposure to a heavy load of commensal bacteria, indicating that inflammatory reactions are regulated by specific mechanisms in the healthy intestine.
The vast area of intestinal epithelium covering the luminal surface is continually exposed to commensal bacteria. Intestinal epithelial cells (IECs)2 not only physically separate the luminal contents from the internal milieu but also actively participate in immune reactions as a frontline defense in the mucosal immune system. IECs have been shown to receive stimulation from commensals through Toll-like receptors (TLRs), pattern recognition receptors for microbial components, thereby maintaining their homeostasis (8). However, to prevent the triggering of an excessive inflammatory reaction, they are not highly sensitive to commensals. Specifically, IECs contribute to the maintenance of the intestinal symbiotic system by partially tolerating commensals and regulating mucosal inflammation. Decreased expression of specific TLRs in IECs has been reported to be one mechanism controlling this hyporesponsiveness (9–11).
TLR4 mainly recognizes LPS, which constitutes the cell wall of Gram-negative bacteria. Increased expression of TLR4 and an elevated response to commensal organisms are often observed in patients with inflammatory bowel disease (12, 13). We have previously reported using human IEC lines that low responsiveness of IECs to LPS is mainly brought about by down-regulation of the TLR4 gene and that transcriptional repression of this gene in IECs is mediated by epigenetic mechanisms including DNA methylation and histone deacetylation (14). In this study we analyzed DNA methylation of the TLR4 gene in IECs in vivo, determining the involvement of intestinal commensal bacteria in this process. This has led us to propose that commensal bacteria play a novel role in the regulation of intestinal inflammation through their effect on epigenetic modification of the host gene, thereby providing a mechanism for the maintenance of the intestinal symbiotic system.
EXPERIMENTAL PROCEDURES
Mice
Wild-type (WT) and MyD88−/− mice on a BALB/c background were purchased from CLEA Japan (Tokyo, Japan) and from Oriental BioService (Kyoto, Japan), respectively, and bred under conventional (CV) conditions. Germ-free (GF)-WT BALB/c mice were kept in a sterile vinyl isolator. Female mice were studied when 9–11 weeks of age. All experiments were carried out in accordance with guidelines for the care and use of laboratory animals of Nihon University.
Preparation of IECs
Single cell suspensions of IECs were prepared as follows. Small intestines (jejuna and ilea) and large intestines (colon and rectum not including the cecum) were surgically excised from mice. After removing Peyer's patches from the small intestines, the intestines were washed with Ca2+- and Mg2+-free Hanks' balanced salt solution (HBSS(−); Sigma) containing 5% FCS, cut open, and minced into 2–3-mm pieces. The tissues were incubated in HBSS(−) supplemented with 5% FCS, 1 mm DTT, and 0.5 mm EDTA with shaking at 37 °C for 20 min. After washing, the tissues were treated with 2.4 units/ml dispase (BD Biosciences) in DMEM containing 5% FCS at 37 °C for 30 min with gentle stirring. EDTA was then added to give a final concentration of 5 mm, and the tissues were incubated for an additional 5 min with gentle stirring. Cell suspensions derived from the tissues were washed sequentially with DMEM containing 5% FCS and MACS buffer (PBS containing 2% FCS and 2 mm EDTA) before being subjected to MACS separation using CD45 MicroBeads (Miltenyi Biotec, Inc., Auburn, CA) to remove the lymphocytes. IEC purity was checked by flow cytometry staining with phosphatidylethanolamine-Cy-7-labeled anti-CD45 antibody (eBioscience, San Diego, CA) and propidium iodide (PI); CD45+ cells were <1% of the total number of PI− live cells. In addition, cells were stained with FITC-conjugated anti-pan cytokeratin antibody (clone PCK-26, Sigma) and observed under a confocal fluorescence laser microscope FLUOVIEW FV1000 (Olympus, Tokyo, Japan) to confirm the expression of cytokeratin, a marker of epithelial cells.
Preparation of Differentiated and Undifferentiated IEC Fractions
Small intestinal tissues were prepared and minced as described above. The tissues were incubated in HBSS(−) supplemented with 5% FCS, 1 mm DTT, and 0.5 mm EDTA with shaking at 37 °C for 10 min. Cells derived from the tissues during this process were collected and designated as Fr1. The remaining tissues were resuspended in the same buffer and incubated with shaking at 37 °C for an additional 10 min. After washing, the tissues were treated with 2.4 units/ml dispase (BD Biosciences) in DMEM containing 5% FCS at 37 °C for 10 min with gentle stirring. The tissues were then washed and retreated with dispase for an additional 20 min. After adding EDTA at a final concentration of 5 mm and incubating for 5 min with gentle stirring, the cells in the medium were collected and designated as Fr2. Fr1 and Fr2 were purified by depletion of CD45+ lymphocytes with MACS as described above.
Bisulfite Conversion Reaction
Genomic DNA was prepared from cells using a PureLink Genomic DNA Mini Kit (Invitrogen) according to the manufacturer's instructions. To analyze methylation of CpG motifs, 1 μg of genomic DNA was denatured with 6 n NaOH, modified by bisulfite conversion reaction, and purified using a BisulFastTM DNA Modification Kit (TOYOBO, Osaka, Japan). The 5′ region of the TLR4 gene was amplified by PCR from the modified genomic DNA. The sequences of the synthetic oligonucleotides used as PCR primers are as follows: for analysis of the TLR4 gene in murine cells, forward (5′-GGTTAGATGATTTTTTGGGATGAAAG-3′) and reverse (5′-CATTTTCTACAACCAATAAAAACC-3′); for analysis of the TLR4 gene in a human cell line, forward (5′-GGTAGAGGTTAGATGATTAATTGGG-3′) and reverse (5′-CCCCAATAACTACCTCTAATACCCT-3′). After purification, the PCR products were cloned into the pCR2.1 vector for sequencing. Nucleotide sequences of 15–27 independent clones were analyzed.
Quantitative RT-PCR (qRT-PCR)
Total RNA was prepared using a High Pure RNA Isolation kit (Roche Diagnostics), and first-strand cDNA synthesis was then carried out using SuperScript II Reverse Transcriptase (Invitrogen). Messenger RNA expression was quantified by real-time PCR with a LightCycler 480 (Roche Diagnostics) using SYBR Green I master reagent (Roche Diagnostics). The sequences of the synthetic oligonucleotides used as primers are shown in Table 1.
TABLE 1.
Nucleotide sequences of the primers used for quantitative PCR
| Gene | Forward | Reverse |
|---|---|---|
| IAP | 5′-CTGCTCGATTAGACCAGTGCAACA-3′ | 5′-TTGAGTGGCGATGTCCTTGCAG-3′ |
| TLR4 | 5′-TTATTCCTGGTGTAGCCATT-3′ | 5′-TAAGAAGGCGATACAATTCC-3′ |
| CDX2 | 5′-CACGAACAGCATCTACTGATGGA-3′ | 5′-GCTGATAGCTTCATGTCGGTAGT-3′ |
| SOX9 | 5′-CCACGGAACAGACTCACATCTCT-3′ | 5′-AGATCAACTTTGCCAGCTTGCAC-3′ |
| GAPDH | 5′-TGAACGGGAAGCTCACTGG-3′ | 5′-TCCACCACCCTGTTGCTGTA-3′ |
Cell Culture
The human epithelial colonic adenocarcinoma cell line Caco-2, the human monocyte line THP-1, and the human epithelial colonic carcinoma cell line T84 were purchased from DS Pharma Biomedical (Osaka, Japan) and cultured in minimum Eagle's medium (Nissui, Tokyo, Japan) supplemented with non-essential amino acids (Invitrogen), in RPMI 1640 (Nissui), respectively, and in a 1:1 mixture of Ham's F-12 (Nissui) and DMEM (Nissui). All media were supplemented with 10% (v/v) FCS, 100 units/ml of penicillin, 100 μg/ml of streptomycin, and 5 × 10−5 m 2-mercaptoethacol. Cells were cultured at 37 °C in a humidified incubator with 5% CO2.
Plasmid Construction
Full-length CDX2 and SOX9 cDNA was reverse-transcribed and amplified from total RNA prepared from the human IEC lines T84 and Caco-2, respectively. The following synthetic oligonucleotide primers were used for PCR amplification: CDX2, 5′-CACCATGTACGTGAGCTACCTCCTG-3′ and 5′-GTCACTGGGTGACGGTGGGGTTTAGC-3′; SOX9, 5′-CGGGATCCGTATGAATCTCCTGGACCCCTTC-3′ and 5′-GCTCTAGATCAAGGTCGAGTGAGCTGTGTGTAG-3′.
To construct expression plasmids for human CDX2 and SOX9, the amplified products were cloned into the pcDNA3.1 vector (Invitrogen), and the nucleotide sequences were verified. The resulting plasmids were named pcDNA3.1-CDX2 and pcDNA3.1-SOX9, respectively. The control empty vector pcDNA3.1-empty was generated by self-ligation of BstXI-digested pcDNA3.1.
Reporter Gene Assay
Cells were cotransfected with the reporter plasmid pGL-TLR4−1013/+118 (14) and the CDX2 expression plasmid or an empty control vector using FuGENE HD Transfection Reagent (Roche Diagnostics). The reporter plasmid pGL-TLR4−1013/+118 carries the 5′ region of the human TLR4 gene (nucleotides −1013/+113), which contains cis-acting elements to activate transcription, upstream of the luciferase gene. The plasmid phRL-CMV (Promega, Madison, WI) carrying the Renilla luciferase gene under the control of the CMV promoter was introduced to normalize the transfection and cell lysis efficiencies in each experiment. After 20–24 h of culture, cells were harvested and washed with PBS. Cell lysis and determination of luciferase activity were carried out using a Dual-luciferase assay kit (Promega) according to the manufacturer's instructions. Luminescence was measured with a Tristar LB941 luminometer (Berthold, Postfach, Germany).
Measurement of IL-8 Production
Cells were transfected with pcDNA3.1-CDX2 or pcDNA3.1-empty, selected with G418, and then stimulated for 18 h with 0.1–1000 ng/ml Ultra Pure Escherichia coli K12 LPS (Invivogen, San Diego, CA), which is guaranteed to only activate the TLR4 pathway. Alternatively, cells were cotransfected with pcDNA3.1-CDX2 or pcDNA3.1-empty and the TLR4 siRNA expression plasmid pBAsi-TLR4 or the control plasmid pBAsi-cont (14). Forty-eight hours later, cells were stimulated with Ultra Pure LPS (Invivogen) for 20–22 h. Secreted IL-8 in the culture supernatant was concentrated by Amicon Ultra centrifugal filters (nominal molecular weight limit, 3000) (Millipore, Billerica, MA) and then quantified with a Quantikine Human CXCL/IL-8 system (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The original concentration of IL-8 in the culture supernatant before concentration was calculated from each concentration rate.
RESULTS
TLR4 Gene Is Highly Methylated in IECs in Vivo
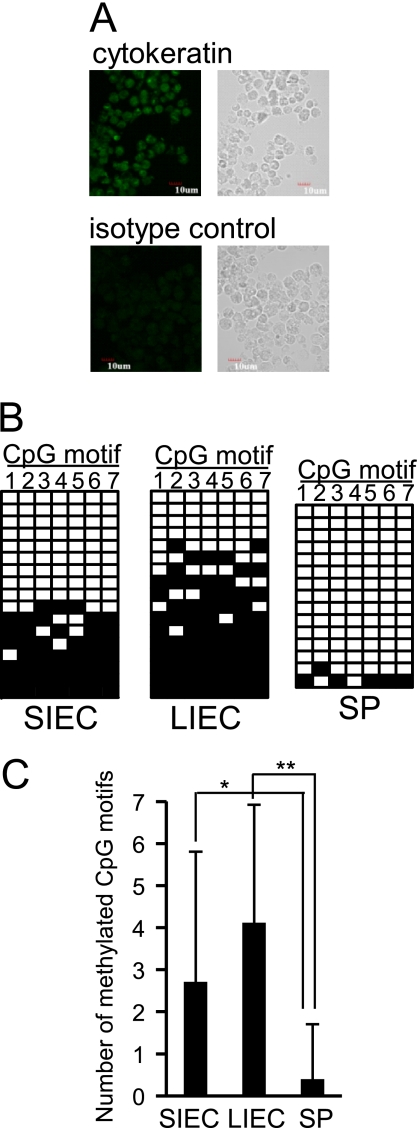
Using human cell lines, we have previously reported that expression of the TLR4 gene is repressed by epigenetic mechanisms in IECs (14). To confirm that this also occurs in vivo, the methylation frequencies of seven CpG motifs existing in the 5′ region of TLR4 gene, nucleotides −102/+202 (nucleotide numbers are counted from the major transcription start site as +1), were compared among CD45− epithelial cells of small and large intestines (SIECs and LIECs, respectively) and CD45+ splenic cells prepared from mice. Obtained IECs were stained with anti-pan cytokeratin antibody to confirm the expression of cytokeratin, a marker of epithelial cells (Fig. 1A). Similar to our in vitro observation, significantly higher methylation frequencies of CpG motifs were detected in SIECs and LIECs than in splenic cells, suggesting that TLR4 gene expression is repressed by epigenetic mechanisms in IECs in vivo (Fig. 1, B and C).
FIGURE 1.
The 5′ region of the TLR4 gene is highly methylated in IECs in vivo. A, expression of cytokeratin, a marker of epithelial cells, in SIECs and LIECs prepared from mice was confirmed by immunostaining. Cells were stained with FITC-conjugated anti-pan cytokeratin antibody (upper panels) or FITC-conjugated isotype control antibody (lower panels). Left panels show fluorescent images under confocal laser scanning microscopy, and right panels show transmission images under phase-contrast microscopy. A representative staining pattern of SIECs is shown. Similar staining patterns were obtained for LIECs. B, methylation frequencies of CpG motifs existing in the 5′ region of the TLR4 gene were compared among mouse SIECs, LIECs, and splenic cells (SP). DNA methylation of 7 CpG motifs in the 5′ region (nucleotides −102/+202) was analyzed by the bisulfite conversion reaction. The modified DNA from 6 mice was amplified by PCR, cloned, and sequenced for 15–17 independent clones. A methylation pattern of 7 CpG motifs (1–7) for each clone is shown in the order of methylation frequency. Filled squares indicate methylated CpG motifs, whereas open squares indicate unmethylated CpG motifs. C, mean numbers of methylated CpG motifs ± S.D. are represented in the graph. * and **, significantly different (*, p < 0.05; **, p < 0.0001).
TLR4 Gene Expression Is Repressed by Epigenetic Mechanisms during the Differentiation of IECs
Although IECs showed a significantly high level of the TLR4 gene methylation on the average, a wide variety of methylation frequencies was detected (Fig. 1B). This observation indicates that the TLR4 gene is highly methylated in some IEC population but not in another IEC population. Immunohistochemical and mRNA analyses have shown that TLR4 is expressed predominantly in the crypt IECs and expressed less abundantly in the villus IECs (15–18), suggesting the possibility that the status of TLR4 gene methylation is different between crypt and villus IECs. To compare DNA methylation in the 5′ region of the TLR4 gene between differentiated IECs at the villi and undifferentiated IECs at the crypts, SIECs were fractionated into two populations (Fr1 and Fr2) depending on their differentiation stage. The differentiation status of cells in Fr1 and Fr2 was confirmed based on the expression of intestinal alkaline phosphatase (IAP), a differentiation marker of IECs. As expected, greater expression of IAP was observed in Fr1 than in Fr2 (Fig. 2A). The methylation frequency of CpG motifs in the 5′ region of the TLR4 gene was higher in the IAPhigh, differentiated cells of Fr1 than in the IAPlow, undifferentiated cells of Fr2 (Fig. 2B). Consistent with this, TLR4 mRNA expression was lower in Fr1 cells than in Fr2 cells (Fig. 2C). These results indicate that regulation of TLR4 gene expression by epigenetic modification of the gene in IECs is linked to cell differentiation, with TLR4 gene expression being repressed by epigenetic mechanisms during IEC differentiation.
FIGURE 2.
TLR4 gene expression is repressed by epigenetic mechanisms during the differentiation of IECs. Differentiated (Fr1) and undifferentiated (Fr2) mouse SIECs were separately prepared. A, expression of IAP, a differentiation marker of IECs, was quantified by qRT-PCR in each SIEC population. After normalizing IAP expression using GAPDH mRNA levels, the relative expression level of IAP in Fr2 to that in Fr1 was calculated. Results are expressed as the mean ± S.D. of four independent experiments. B, methylation of CpG motifs in the 5′ region of the TLR4 gene in Fr1 and Fr2 SIECs was analyzed by a bisulfite conversion reaction. Nucleotide sequences after the conversion reaction were determined for 22 and 25 independent clones, respectively. Mean numbers of methylated CpG motifs ± S.D. are shown. C, TLR4 mRNA expression in each SIEC population was determined by qRT-PCR. Relative values normalized using GAPDH mRNA levels are shown. Results are expressed as the mean ± S.D. of four independent experiments. *, **, and ***, significantly different (*, p < 0.05; **, p < 0.01; ***, p < 0.0001).
CDX2 Decreases TLR4 Gene Methylation and Increases LPS Sensitivity in Undifferentiated IECs
Caudal type homeobox 2 (CDX2), the transcription factor that is required for the development of intestinal tissue, is specifically expressed in the intestine. CDX2 levels were higher in IAPlow, undifferentiated IECs (Fr2) than in IAPhigh, differentiated IECs (Fr1) (Fig. 3A). Luciferase assays revealed that overexpression of CDX2 increased the transcriptional promoter activity of the TLR4 gene in the human IEC line Caco-2 but decreased it in the human monocyte line THP-1 (Fig. 3B). This effect was specific for CDX2, as overexpression of sex determining region Y-box 9 (SOX9), another transcription factor that was also more highly expressed in IAPlow, undifferentiated IECs than in IAPhigh, differentiated IECs (Fig. 3C), had no effect on the transcriptional promoter activity of the TLR4 gene (Fig. 3D). In Caco-2 cells, overexpression of CDX2 significantly reduced the methylation frequency of 11 CpG motifs, existing in the 5′ region of the human TLR4 gene, nucleotides −69/+277 (Fig. 3E). Consistent with this, overexpression of CDX2 in Caco-2 cells increased sensitivity to LPS (Fig. 3F). The effect of CDX2 was inhibited when endogenous TLR4 gene expression was suppressed by RNAi (Fig. 3G), indicating that the enhanced IL-8 production by CDX2 was mediated by TLR4. Collectively, these results indicate that CDX2 increases the inflammatory response of IECs by suppressing TLR4 gene methylation in undifferentiated IECs.
FIGURE 3.
CDX2 decreases methylation of the TLR4 gene and increases LPS sensitivity in undifferentiated IECs. A, expression of CDX2 in differentiated (Fr1) and undifferentiated (Fr2) SIECs prepared from mice was determined by qRT-PCR. After normalizing CDX2 expression using GAPDH mRNA levels, relative expression levels of CDX2 in Fr2 to that in Fr1 is calculated. Results are expressed as the mean ± S.D. of four independent experiments. *, significantly different (p < 0.05). B, the IEC line Caco-2 and the monocyte line THP-1 were cotransfected with the CDX2 expression plasmid (pcDNA3.1-CDX2) or empty control vector (pcDNA3.1-empty) and a reporter plasmid carrying the 5′ region of the TLR4 gene (pGL-TLR4−1013/+118) for transient expression assays. The -fold increase in luciferase activity relative to that of cells transfected with the reporter plasmid alone is shown. Results are expressed as mean ± S.D. of three independent experiments. ** and ***, significantly different from control transfected with an empty vector (**, p < 0.001; ***, p < 0.0001). C, expression of the transcription factor SOX9 in differentiated (Fr1) and undifferentiated (Fr2) SIECs was determined by qRT-PCR. After normalizing SOX9 expression using GAPDH mRNA levels, the relative expression level of SOX9 in Fr2 to that in Fr1 was calculated. Results are expressed as the mean ± S.D. of two independent experiments. D, Caco-2 cells were cotransfected with the SOX9 expression plasmid (pcDNA3.1-SOX9) or empty control vector and a reporter plasmid carrying the 5′ region of the TLR4 gene for transient expression assays. The -fold increase in luciferase activity relative to that of cells transfected with the reporter plasmid alone is shown. Results are expressed as mean ± S.D. of two independent experiments. E, the CDX2 expression plasmid or empty control vector was introduced into Caco-2 cells. After selecting the transfected cells with G418, methylation of CpG motifs in the 5′ region of the TLR4 gene was analyzed by bisulfite conversion reaction. Mean numbers ± S.D. of methylated CpG motifs of 16–19 independent clones are shown. *, significantly different (p < 0.05). F and G, Caco-2 cells were transfected with the CDX2 expression plasmid or the empty vector (F). For RNAi experiments, the TLR4 siRNA expression plasmid (pBAsi-TLR4) or the control plasmid (pBAsi-cont) was additionally introduced into cells (G). Cells were then stimulated with 0.1–1000 ng/ml LPS for 18–22 h. Secretion of IL-8 into the culture supernatant was measured by ELISA. Results are expressed as mean ± S.D. of three independent experiments. N.D., not detected.
Commensal Bacteria Are Essential for TLR4 Gene Methylation in the Large Intestine but Not in the Small Intestine
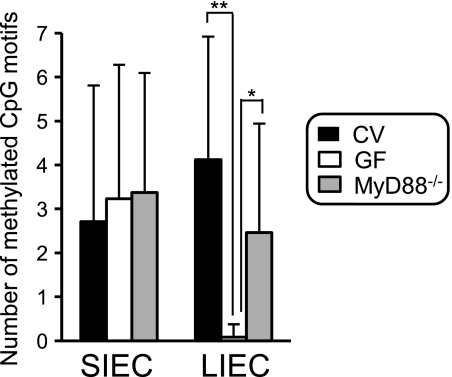
To elucidate the role of commensal bacteria in the epigenetic repression of TLR4 gene expression, the methylation level of this gene was compared in IECs prepared from CV and GF mice, the latter of which do not have intestinal commensal bacteria. Interestingly, the methylation frequency of CpG motifs in the 5′ region of the TLR4 gene was significantly lower in LIECs of GF mice than in those of CV mice, whereas the level in SIECs was almost the same in GF and CV mice (Fig. 4). These results indicate that intestinal commensal bacteria are essential for induction or maintenance of TLR4 gene methylation in LIECs but not in SIECs. Furthermore, although the frequency of methylated CpG motifs in the 5′ region of the TLR4 gene tended to be lower in mice deficient in MyD88, an adaptor molecule which plays an important role in TLR signaling, than in CV mice (p = 0.0592), it was apparently higher in GF mice, indicating that the contribution of commensal bacteria to the induction or maintenance of TLR4 gene methylation in LIECs is only partly mediated by MyD88-dependent pathways. It is speculated that fermentation-derived metabolites by commensal bacteria, in addition to bacterial components, possibly play a role in induction of TLR4 gene methylation in LIECs.
FIGURE 4.
Commensal bacteria are essential for TLR4 gene methylation in LIECs. Methylation frequencies of the TLR4 gene in SIECs and LIECs were compared between wild-type mice under CV conditions (black bars), wild-type mice under GF conditions (white bars), and MyD88−/− mice under conventional conditions (MyD88−/−, gray bars). DNA methylation was analyzed by the bisulfite conversion reaction using SIECs and LIECs prepared from 6–9 mice. Nucleotide sequences of 17–27 independent clones were determined. Mean numbers ± S.D. are represented. * and **, significantly different (*, p < 0.0005; **, p < 0.00005).
Two Axes Regulate TLR4 Gene Expression in IECs
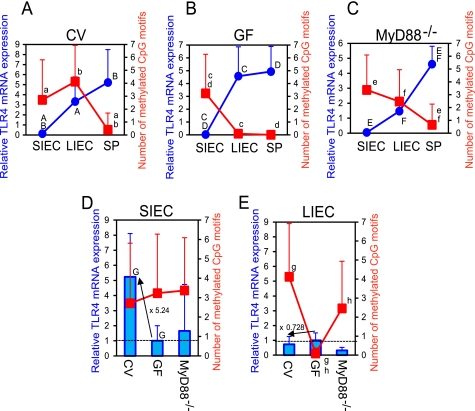
Next, the effect of commensal bacteria on TLR4 gene expression was evaluated in detail. In the absence of commensal bacteria (i.e. in GF mice) or under conditions in which bacterial stimulation through TLR was limited (i.e. in MyD88−/− mice), TLR4 mRNA expression was inversely related to the methylation level of the gene (Fig. 5, B and C), revealing that TLR4 gene expression is suppressed by DNA methylation in IECs. In contrast, a more complicated regulation pattern was observed in CV mice (Fig. 5A). Although TLR4 mRNA expression was significantly low in SIECs of both CV and GF mice because of the high DNA methylation (Fig. 5, A and B), the expression was 5.24-fold higher in CV mice than in GF mice as shown in Fig. 5D in which TLR4 mRNA expression relative to that of GF mice is expressed. This indicates the presence of a mechanism by which commensal bacteria induce TLR4 gene expression independently of methylation given that the methylation level of the gene was constant between CV and GF SIECs. Furthermore, this effect of commensal bacteria was mainly dependent on the MyD88 pathway, as TLR4 mRNA expression was lower in SIECs of MyD88−/− mice than in those of CV mice (Fig. 5D). In contract, the increase in TLR4 gene expression in LIECs of CV mice compared with GF mice was prevented (0.728-fold) by elevated TLR4 gene methylation in CV LIECs (Fig. 5E). However, on the other hand, TLR4 gene expression was enhanced by a large amount of commensal bacteria independently of DNA methylation in CV LIECs, resulting in a relatively high but permissible level of TLR4 gene expression (Fig. 5A). Collectively, these findings indicate that there are two axes for the regulation of TLR4 gene expression, a differentiation-dependent vertical axis (crypt-villus axis) and a bacterial load-dependent horizontal axis (small intestine-large intestine axis), the former of which is mediated by epigenetic mechanisms, whereas the latter is not. These results suggest that TLR4 gene expression is suppressed due to a block of transcription by epigenetic mechanisms in differentiated cells, whereas it can be up-regulated by commensal bacteria in undifferentiated cells where the block of transcription of the TLR4 gene by methylation is absent (Fig. 6). Commensal bacteria were shown to be strongly involved in both mechanisms in the large intestine.
FIGURE 5.
Two axes regulate TLR4 gene expression. A–C, TLR4 mRNA expression in SIECs, LIECs, and splenic cells (SP) of CV (A), GF (B), and MyD88−/− (C) mice was quantified by qRT-PCR. Relative values normalized using GAPDH mRNA levels are shown (blue circles). Results are expressed as the mean ± S.D. of 3–6 independent experiments. The frequency of methylated CpG motifs in SIECs, LIECs and splenic cells of CV, GF, and MyD88−/− mice is also represented in the graphs (red squares). D and E, TLR4 mRNA expression levels in SIECs (D) and LIECs (E) of CV and MyD88−/− mice relative to that of GF mice (blue bars) and the frequency of methylated CpG motifs (red squares) are shown as the mean ± S.D. a–h, significantly different (a, p < 0.05; f, p < 0.005; h, p < 0.0005; b–d, p < 0.0001; g, p < 0.00005; e, p < 0.00001). A–G, significantly different (A, B, C, D, F, and G, p < 0.05; E, p < 0.005).
FIGURE 6.
Mechanisms underlying the regulation of TLR4 gene expression in IECs. Mechanisms underlying the regulation of TLR4 gene expression in IECs are schematically drawn. TLR4 gene expression is repressed due to block of transcription by epigenetic mechanisms in differentiated villus IECs to prevent triggering an excessive inflammatory reaction against commensal bacteria ([A]). However, when invading microbes reach the crypt, undifferentiated IECs in this region expressing TLR4 at a higher level may respond to them. TLR4 expression is beyond control of epigenetic repression and is increased by stimulation with commensal bacteria in undifferentiated crypt IECs ([B]). Commensal bacteria contribute to epigenetic repression of TLR4 gene expression in IECs of the large intestine.
DISCUSSION
This study indicates that commensal bacteria can affect the epigenetic modification of host genes. This means that commensal bacteria contribute to the maintenance of intestinal symbiosis by regulating host gene expression via epigenetic modification. Our findings reveal the presence of a novel mechanism underlying the maintenance of intestinal symbiosis as well as a novel role of intestinal commensal bacteria in this process.
An overwhelming 100 trillion commensal bacteria live within the human gastrointestinal tract, constantly exposing the surface of the intestinal mucosa to the stimulatory effects of this resident microbiota. Experiments comparing germ-free and colonized animals have shown that various host systems are shaped by the presence of the microbiota (19–21). The host cells sense these microorganisms by TLRs and nucleotide binding oligomerization domain (NOD)-like receptors, which recognize conserved microbial-associated molecular patterns such as LPS, lipoproteins, and CpG DNA; however, the proinflammatory pathways associated with TLRs and NOD-like receptors are suppressed by various mechanisms in healthy hosts to prevent mounting an excessive response to commensals. It has been reported that repetitive TLR stimulation due to commensal bacterial exposure induces down-regulation of the NF-κB pathway (22) and that chronic NOD-2 stimulation leads to down-regulation of proinflammatory cytokines such as TNF-α, IL-8, and IL-1β in primary human monocyte-derived macrophages (23). Many inhibitory molecules of the host including Toll interacting protein, COX-2 inhibitors, A20, peroxisome proliferator-activated receptor-γ, NF-κB inhibitor IκB-α, IL-10, and TGF-β have been shown to suppress excessive inflammatory reactions against commensals, thereby maintaining the intestinal symbiotic system.
A single layer of IECs creates a barrier between the lumen of the intestine and the internal milieu of the body. IECs influence the function of APCs and lymphocytes by sensing the microbial-associated molecular patterns of commensals through TLRs. This helps to regulate DC functions through the secretion of immunomodulatory molecules, such as thymic stromal lymphopoietin, retinoic acid, and TGF-β, and can drive regulatory cell differentiation (24, 25). Although stimulation through TLR is required to maintain intestinal homeostasis, only limited stimulation is necessary. Negative regulation of TLR signaling in IECs plays an essential role in preventing a breakdown of intestinal homeostasis. The present study focused on regulation of TLR4 expression, which is considered to be an important mechanism for preventing an excessive inflammatory reaction against commensal bacteria and, thus, a key factor in supporting the intestinal homeostasis. IECs express low levels of TLR4 and are hyporesponsive to LPS, which explains why they can tolerate exposure to large quantities of luminal bacteria.
Based on our analyses of methylation status and mRNA expression of TLR4 gene (Fig. 2), it is suggested that TLR4 gene expression is repressed by epigenetic mechanisms in differentiated villus IECs to prevent triggering an excessive inflammatory response. This regulation is, at least in part, mediated by the transcription factor CDX2, which is highly expressed in undifferentiated IECs compared with differentiated IECs (Fig. 3). On the other hand, when invading pathogenic microbes reach the crypt, undifferentiated crypt IECs expressing TLR4 at a higher level are able to react them (Fig. 6). This would make it possible to induce different responses to commensals and to pathogenic bacteria. Our results have shown that commensal bacteria contribute to the epigenetic repression of TLR4 gene expression in LIECs but not in SIECs (Fig. 4). This difference may reflect the much larger load of commensal bacteria in the large intestine than in the small intestine, diversity in the microbiota populating these regions, and frequent exposure to undigested or partially digested food antigens occurring specifically in the small intestine. The expression of CDX2 is higher in LIECs than in SIECs but is not influenced by colonization of commensal bacteria (see supplemental Fig. 1), suggesting that the effect of commensal bacteria on induction TLR4 gene methylation in LIECs is not mediated by down-regulation of CDX2. It is thought that decreased CDX2 expression is required but not sufficient to induce TLR4 gene methylation and that additional stimuli, e.g. commensal bacteria for LIECs, are required. Other stimuli such as those derived from foods may fill the role in SIECs. Commensal bacteria increase the IEC sensitivity to microbes by enhancing TLR4 gene expression but, on the other hand, keep the expression at a permissible level by inducing or maintaining DNA methylation of the gene and thereby prevent excessive inflammation in the large intestine.
DNA methylation is an important epigenetic mark involved in diverse biological processes. Epigenetics refers to chromatin-based pathways important in the regulation of gene expression mediated mainly by mechanisms involving DNA methylation, posttranslational modifications of histones, and small non-coding RNA. DNA methylation status is dynamically regulated by DNA methylation and demethylation reactions. Given that enzymes mediating these reactions, such as DNA methyltransferases, are common for any target gene, the presence of some factor that binds to a specific sequence on the gene and recruits these enzymes to the target gene has been proposed. Commensal bacteria may regulate expression or modification of such molecules to enhance or suppress the recruitment of enzymes mediating methylation and demethylation reactions. Recently, it has been reported that epigenetic modifications occur in response to environmental changes and play a fundamental role in gene expression after environmental stimulation (26–29). In addition to diet, pollution, and infections, our study highlights the intestinal microbiota as another factor affecting epigenetic gene modification. Short-chain fatty acids such as butyrate, which are produced by intestinal microbiota, are known to act as a histone deacetylase inhibitor (30, 31), also suggesting epigenetic regulation by commensal bacteria, although the effect on transcription is opposite to that observed in our study.
The 5′ region of the human and mouse TLR4 genes share about 70% homology in their nucleotide sequences. Many transcription factor binding motifs such as those of PU.1 and AP1 in this region are conserved between human and mouse genes (32, 33). CpG motifs in the 5′ region of the TLR4 gene are present at a similar density in both human and mouse but are not conserved between them. Our past and present findings using human cell lines and mouse primary cells show that the TLR4 gene is methylated at a higher level in IECs than in other types of cells, suggesting that a common molecule binding to an element in the 5′ regions of the human and mouse genes recruits the enzyme mediating induction or maintenance of DNA methylation to modify the methylation status of CpG motifs existing in the region. Moreover, it is possible that this common molecule also binds to other symbiosis-associated genes in IECs, thereby regulating mucosal inflammation triggered by IECs and, as a result, maintaining the intestinal commensal system. Further study will help to elucidate the mechanism underlying the regulation of symbiosis-associated gene expression in IECs and will determine the contribution of the gut microbiota and their metabolic end-products to this process.
Supplementary Material
Acknowledgments
We thank the members of Food and Physiological Functions Laboratory for helpful discussions.
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and a grant-in-aid for Scientific Research from Japan Society for the Promotion of Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- IEC
- intestinal epithelial cell
- SIEC
- small IEC
- LIEC
- large IEC
- IAP
- intestinal alkaline phosphatase
- TLR
- Toll-like receptor
- CV
- conventional
- GF
- germ-free
- HBSS
- Hanks' balanced salt solution
- CDX
- Caudal type homeobox
- SOX
- sex determining region Y-box
- NOD
- nucleotide-binding oligomerization domain
- qRT
- quantitative RT.
REFERENCES
- 1. Martinez-Medina M., Aldeguer X., Gonzalez-Huix F., Acero D., Garcia-Gil L. J. (2006) Inflamm. Bowel Dis. 12, 1136–1145 [DOI] [PubMed] [Google Scholar]
- 2. Manichanh C., Rigottier-Gois L., Bonnaud E., Gloux K., Pelletier E., Frangeul L., Nalin R., Jarrin C., Chardon P., Marteau P., Roca J., Dore J. (2006) Gut 55, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frank D. N., St Amand A. L., Feldman R. A., Boedeker E. C., Harpaz N., Pace N. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13780–13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu H. J., Ivanov I. I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D. R., Benoist C., Mathis D. (2010) Immunity 32, 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tlaskalová-Hogenová H., Stěpánková R., Kozáková H., Hudcovic T., Vannucci L., Tučková L., Rossmann P., Hrnčíř T., Kverka M., Zákostelská Z., Klimešová K., Přibylová J., Bártová J., Sanchez D., Fundová P., Borovská D., Srůtková D., Zídek Z., Schwarzer M., Drastich P., Funda D. P. (2011) Cell. Mol. Immunol. 8, 110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLoughlin R. M., Mills K. H. (2011) J. Allergy Clin. Immunol. 127, 1097–1107 [DOI] [PubMed] [Google Scholar]
- 7. Ly N. P., Litonjua A., Gold D. R., Celedón J. C. (2011) J. Allergy Clin. Immunol. 127, 1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. (2004) Cell 118, 229–241 [DOI] [PubMed] [Google Scholar]
- 9. Abreu M. T., Vora P., Faure E., Thomas L. S., Arnold E. T., Arditi M. (2001) J. Immunol. 167, 1609–1616 [DOI] [PubMed] [Google Scholar]
- 10. Melmed G., Thomas L. S., Lee N., Tesfay S. Y., Lukasek K., Michelsen K. S., Zhou Y., Hu B., Arditi M., Abreu M. T. (2003) J. Immunol. 170, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 11. Abreu M. T., Thomas L. S., Arnold E. T., Lukasek K., Michelsen K. S., Arditi M. (2003) J. Endotoxin Res. 9, 322–330 [DOI] [PubMed] [Google Scholar]
- 12. Cario E., Podolsky D. K. (2000) Infect. Immun. 68, 7010–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vamadevan A. S., Fukata M., Arnold E. T., Thomas L. S., Hsu D., Abreu M. T. (2010) Innate Immun. 16, 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi K., Sugi Y., Hosono A., Kaminogawa S. (2009) J. Immunol. 183, 6522–6529 [DOI] [PubMed] [Google Scholar]
- 15. Ortega-Cava C. F., Ishihara S., Rumi M. A., Kawashima K., Ishimura N., Kazumori H., Udagawa J., Kadowaki Y., Kinoshita Y. (2003) J. Immunol. 170, 3977–3985 [DOI] [PubMed] [Google Scholar]
- 16. Furrie E., Macfarlane S., Thomson G., Macfarlane G. T. (2005) Immunology 115, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bogunovic M., Davé S. H., Tilstra J. S., Chang D. T., Harpaz N., Xiong H., Mayer L. F., Plevy S. E. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1770–G1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolfs T. G., Derikx J. P., Hodin C. M., Vanderlocht J., Driessen A., de Bruïne A. P., Bevins C. L., Lasitschka F., Gassler N., van Gemert W. G., Buurman W. A. (2010) Inflamm. Bowel Dis. 16, 68–75 [DOI] [PubMed] [Google Scholar]
- 19. Cebra J. J., Periwal S. B., Lee G., Lee F., Shroff K. E. (1998) Dev. Immunol. 6, 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Umesaki Y., Setoyama H., Matsumoto S., Imaoka A., Itoh K. (1999) Infect. Immun. 67, 3504–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talham G. L., Jiang H. Q., Bos N. A., Cebra J. J. (1999) Infect. Immun. 67, 1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng H., Wu H., Sloane V., Jones R., Yu Y., Lin P., Gewirtz A. T., Neish A. S. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G96–G108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedl M., Li J., Cho J. H., Abraham C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19440–19445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeuthen L. H., Fink L. N., Frokiaer H. (2008) Immunology 123, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iliev I. D., Mileti E., Matteoli G., Chieppa M., Rescigno M. (2009) Mucosal Immunol. 2, 340–350 [DOI] [PubMed] [Google Scholar]
- 26. Mathers J. C., Strathdee G., Relton C. L. (2010) Adv. Genet. 71, 3–39 [DOI] [PubMed] [Google Scholar]
- 27. Vucetic Z., Kimmel J., Reyes T. M. (2011) Neuropsychopharmacology 36, 1199–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Breton C. V., Byun H. M., Wenten M., Pan F., Yang A., Gilliland F. D. (2009) Am. J. Respir. Crit. Care Med. 180, 462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCall C. E., Yoza B., Liu T., El Gazzar M. (2010) J. Innate Immun. 2, 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davie J. R. (2003) J. Nutr. 133, 2485S–2493S [DOI] [PubMed] [Google Scholar]
- 31. Waldecker M., Kautenburger T., Daumann H., Busch C., Schrenk D. (2008) J. Nutr. Biochem. 19, 587–593 [DOI] [PubMed] [Google Scholar]
- 32. Rehli M., Poltorak A., Schwarzfischer L., Krause S. W., Andreesen R., Beutler B. (2000) J. Biol. Chem. 275, 9773–9781 [DOI] [PubMed] [Google Scholar]
- 33. Roger T., Miconnet I., Schiesser A. L., Kai H., Miyake K., Calandra T. (2005) Biochem. J. 387, 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.