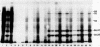Abstract
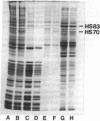
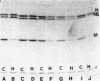
Cell-free protein-synthesizing systems made from Drosophila melanogaster cells were used to study the translational control induced in these cells by heat shock. Lysates of normally growing cells, termed 25 degrees C cells, translate both normal and heat shock mRNAs. Lysates of cells heat shocked at 36 degrees C for 1 hr, termed 36 degrees C cells, translate preferentially heat-shock mRNAs and a few 25 degrees C cell mRNAs. Thus, both lysates appear to reproduce the control displayed in vivo. Both lysates are optimally active at 28 degrees C, and all translations are done at that temperature, demonstrating that, once established, the discrimination system does not require heat-shock temperature for its activity. Addition of crude ribosome fractions from 25 degrees C cell lysates to lysates from heat-shocked cells "rescues" translation of 25 degrees C cell mRNA, which suggests that the discriminating elements are associated with ribosomes. Neither the heat-shock crude ribosome supplements nor the soluble fractions have any effect on either lysate. The experiments also show that RNA selection is determined by some feature of the RNA structure that is insensitive to protease digestion and phenol/chloroform extraction. The essential structural feature may not be unique to Drosophila mRNAs because the Drosophila lysate is capable of discriminating among mRNAs from other organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell. 1977 Sep;12(1):301–310. doi: 10.1016/0092-8674(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Translational efficiency of heat-induced messages in Drosophila melanogaster cells. J Mol Biol. 1980 Feb 25;137(2):151–158. doi: 10.1016/0022-2836(80)90322-8. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Storti R. V., Pardue M. L., Rich A. Cell-free protein synthesis in lysates of Drosophila melanogaster cells. Biochemistry. 1979 Apr 17;18(8):1588–1594. doi: 10.1021/bi00575a032. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Horovitch S. J., Scott M. P., Rich A., Pardue M. L. Myogenesis in primary cell cultures from Drosophila melanogaster: protein synthesis and actin heterogeneity during development. Cell. 1978 Apr;13(4):589–598. doi: 10.1016/0092-8674(78)90210-6. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]