Abstract
Background
Increased free radical production, decreased antioxidant capacity and excessive inflammation are well-known features in the pathogenesis of inflammatory bowel disease. Vitamin E is a powerful antioxidant and a scavenger of hydroxyl radicals, and it has been shown to have anti-inflammatory activities in tissues. We investigated the effects of vitamin E on inflammatory activities using an acetic acid (AA)–induced ulcerative colitis model in rats.
Methods
Wistar rats were divided into 4 groups. Acetic acid was given to 2 groups of animals to induce colitis while the other 2 groups received saline intrarectally. One AA-induced colitis group and 1 control group received vitamin E (30 U/kg/d) intraperitoneally and the pair groups received saline. After 4 days, we evaluated colonic changes biochemically by measuring proinflammatory cytokine levels in tissue homogenates and by histopathologic examination.
Results
Acetic acid caused colonic mucosal injury, whereas vitamin E administration suppressed these changes in the AA-induced colitis group (p < 0.001). Administration of AA resulted in increased levels of tumour necrosis factor-α, interleukin-1β, interleukin-6, myeloperoxidase and malondialdehyde, and decreased levels of glutathione and superoxide dismutase; vitamin E reversed these effects (all p < 0.001).
Conclusion
Our study proposes that vitamin E is an effective anti-inflammatory and antioxidant and may be a promising therapeutic option for ulcerative colitis.
Abstract
Contexte
La production accrue de radicaux libres, la baisse de la capacité antioxydante et l’inflammation excessive sont des caractéristiques bien connues de la pathogenèse de la maladie entérique inflammatoire. La vitamine E est un puissant antioxydant qui récupère aussi les radicaux hydroxyles et il a été démontré qu’elle a des propriétés anti-inflammatoires dans les tissus. Nous avons étudié les effets de la vitamine E sur les activités inflammatoires au moyen d’un modèle de colite ulcéreuse provoquée par l’acide acétique (AA) chez des rats.
Méthodes
Des rats Wistar ont été divisés en 4 groupes. On a administré de l’AA à 2 groupes d’animaux pour provoquer une colite tandis que les 2 autres ont reçu une solution physiologique par voie rectale. Les sujets d’un groupe chez lesquels la colite a été provoquée par l’AA et ceux d’un groupe témoin ont reçu de la vitamine E (30 U/kg/j) par voie intrapéritoniale, et les groupes pairs ont reçu de la solution physiologique. Après 4 jours, nous avons évalué les changements biochimiques du côlon en mesurant les concentrations de cytokine pro-inflammatoire dans des homogénats de tissus et par analyse histopathologique.
Résultats
L’AA a causé des lésions de la muqueuse, tandis que l’administration de vitamine E a fait disparaître ces changements chez les sujets du groupe où la colite a été provoquée par l’AA (p < 0,001). L’administration d’AA a provoqué une élévation des concentrations du facteur α de nécrose tumorale, d’interleukine-1β, d’interleukine-6, de myéloperoxydase et de malondialdéhyde et réduit les concentrations de glutathion et de superoxyde dismutase; la vitamine E a inversé ces effets (tous p < 0,001).
Conclusion
Notre étude indique que la vitamine E constitue un anti-inflammatoire et un antioxydant efficaces et peut constituer un traitement possible prometteur contre la colite ulcéreuse.
Ulcerative colitis is a chronic inflammatory disease of the large intestine. Although the etiology of ulcerative colitis is not fully understood, increased free radical production and decreased antioxidant capacity are well-known characteristics of inflammatory bowel disease (IBD).1 Reactive oxygen species and subsequent lipid peroxidation decrease cellular antioxidant capacity, resulting in prominent colonic inflammation. Excessive inflammation and oxidative stress have pivotal roles in ulcerative colitis disease pathogenesis.2–5 Inflammatory mediators are known to be secreted from migrated granulocytes in the inflamed mucosa in this disease.2,3 Inhibition of lipid peroxidation or scavenging of oxygen free radicals would provide an important protective and therapeutic treatment for ulcerative colitis.6
It is well established that vitamin E is a major antioxidant in cellular membranes7 and protects membrane lipids from peroxidation6,8 by scavenging peroxyl, oxygen and superoxide anion radicals.7 Vitamin E has also been shown to have prominent anti-inflammatory effects.7,9,10 With its high antioxidant capacity and anti-inflammatory activity, vitamin E would be expected to reduce injury and/or improve tissue after injury from ulcerative colitis. We investigated the effects of vitamin E on tissue inflammatory activities in an acetic acid (AA)–induced ulcerative colitis model in rats.
Methods
Animals
Wistar albino rats of either sex (240–280 g) were kept in a room at a constant temperature (22°C ± 1°C) on a 12-hour light/dark cycle, and they were fed standard pellet chow and water ad libitum. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and approval from the ethical committee of Istanbul University. All rats were weighed, were randomly assigned by computer into 4 groups (n = 8 per group) and fasted for 24 hours before the experimental procedure.
Acute colitis model induction and treatment protocols
We simulated colitis in the rats with AA-induced colonic inflammation under light ether anesthesia, administering 1 mL of 4% (v/v) AA in 0.9% NaCl intrarectally with a soft 6-Fr pediatric catheter. The catheter was inserted into the anus up to a length of 6 cm, and we then administered the AA. Before removing the catheter, 2 mL of air was injected to spread the AA completely in the colon.
The rats in the control group were subjected to the same procedure, except that the AA was substituted with isotonic saline. In the treatment groups, the rats received either vitamin E (d-α tocopherol; 30 IU/kg/d) or saline (1 mL/kg) intraperitoneally.
We administered vitamin E or saline 5 minutes after induction of colitis and continued the treatment for 3 consecutive days. On the fourth day after induction, all rats were sacrificed by cervical decapitation. The last 8 cm of the colon was excised, opened longitudinally and rinsed with saline solution. Then we weighed the distal colon and scored the mucosal lesions macroscopically. Tissue samples were taken for histologic evaluation of the lesions by light microscopy.
Histologic evaluation
For each animal, 4 randomly taken tissue sections (5 mm) were stained with hematoxylin and eosin and examined by 2 experienced pathologists (G.D. and V.T.) who were unaware of the treatment groups. After washing the mucosa with saline solution, mucosal injury was assessed macroscopically using the grading scale by Morris and colleagues:11 a score of 0 = no damage, 1 = localized hyperemia but no ulcers, 2 = linear ulcers with no significant inflammation, 3 = linear ulcer with inflammation at 1 site, 4 = ulceration and inflammation at 2 or more sites, and 5 = ulceration and inflammation at 2 or more sites or 1 major site of inflammation and ulceration extending more than 1 cm along the length of the colon. Additional samples were preserved in 10% formalin for histologic examination according to the method of Appleyard and Wallace.12 Formalin-fixed colonic samples were embedded in paraffin, and sections were stained with hematoxylin and eosin. The degree of inflammation of the colon was graded semiquantitatively from 0 to 11 according to described criteria.12 The semiquantitative scores (and scores on the scale of Morris and colleagues11) were as follows: 1 (0–3) referred to loss of mucosal architecture, 2 (0–3) cellular infiltration, 3 (0–3) muscle thickening, 4 (0–1) crypt abscess formation and 5 (0–1) referred to goblet cell depletion.12
After scoring, colonic tissue samples were homogenized with 10 volumes of ice-cold 0.25-M sucrose and then centrifuged at 10 000g to measure the biochemical parameters in the resulting supernatant.
Colonic tissue myeloperoxidase activity
Myeloperoxidase (MPO) activity was determined by a modification of the O-dianisidine method.13 The assay mixture was placed in a 1-cm path length cuvette that contained 0.3 mL of 0.1-M phosphate buffer (pH 6.0), 0.3 mL of 0.01-M H2O2, 0.5 mL of 0.02-M O-dianisidine (freshly prepared) in deionized water and 10 μL of colonic supernatant in a final volume of 3.0 mL. We added the supernatant last, and the change in absorbance at 460 nm was followed for 10 minutes. All measurements were carried out in duplicate. One unit of MPO was defined as that giving an increase in absorbance of 0.001 nm per minute, and specific activity is reported as U/mg protein.
Malondialdehyde measurements
Malondialdehyde (MDA) can be detected in quantifiable amounts with the thiobarbituric acid reactive species (TBARS) assay. We obtained measurements of TBARS according to Yagi’s method.14 Colonic tissues were homogenized in ice-cold 10% trichloroacetic acid (TCA) solution and then centrifuged. The supernatant portion was mixed with equal volume of TBARS (0.67%) and heated at 90°C for 15 minutes. The TBARS were measured in nmol/mg protein according to absorbance at 532 nm.
Colonic tissue antioxidant levels
We measured glutathione (GSH) levels spectrophotometrically using Boyne and Ellman’s reagent and method.15 Colonic tissue GSH levels were calculated as μmol/mg protein. We measured superoxide dismutase (SOD) activity according to the method described by Fridovich.16 This method uses xanthine and xanthine oxidase to generate superoxide radicals that react with p-iodonitrotetrazlium violet (INT) to form a red formazan dye, which we measured at 505 nm. Assay medium consisted of 0.01-M phosphate buffer, 3-cyclohexilamino-1-propanesulfonicacid (CAPS) buffer solution (50 mM CAPS, 0.94 mM ethyl-enediaminetetraacetic acid, saturated NaOH), pH 10.2, substrate solution (0.05 mM xanthine, 0.025 mM INT) and 80 UL xanthine oxidase. Superoxide dismutase activity is expressed as U/mg protein.
We measured the protein concentration of tissue samples with a Spectronic-UV 120 spectrophotometer according to the method of Lowry and colleagues.17
Cytokine tests
We analyzed the levels of rat colonic tissue tumour necrosis factor (TNF)–α, interleukin (IL)-1β and IL-6 with commercially available enzyme immunoassay kits (enzyme-linked immunosorbent assay [ELISA], rat TNF-α and IL-1β from R&D Systems Europe; ELISA, rat IL-6 from Diaclone Research). Analyses of all samples, standards and controls were run in duplicate. Cytokine activities were expressed as pg/mg protein.
Statistical analysis
All results are expressed as means and standard errors (SEs). We analyzed numerical data using the Kruskal–Wallis test. Significant differences revealed by multigroup comparisons were further analyzed using the Mann–Whitney U test. We considered results to be significant at p < 0.05.
Results
Intrarectal administration of 4% AA caused extensive macroscopic damage of the colon. The colonic mucosa appeared hemorrhagic and ulcerated.
Colonic wet weight
The colonic wet weight (g per 100 g body weight) of the colitis group (mean 1.74 [SE 0.12] g) was significantly higher than that of the control group (mean 0.65 [SE 0.01] g; p < 0.001). In the colitis group treated with vitamin E, the colonic weight (1.01 [SE 0.07] g) was significantly lower than that of the untreated colitis group (1.74 [SE 0.12] g; p = 0.032) and was not statistically different from saline (0.65 [SE 0.01] g) and vitamine E (0.64 [SE 0.02] g) control groups.
Macroscopic and microscopic evaluation of the colonic lesions
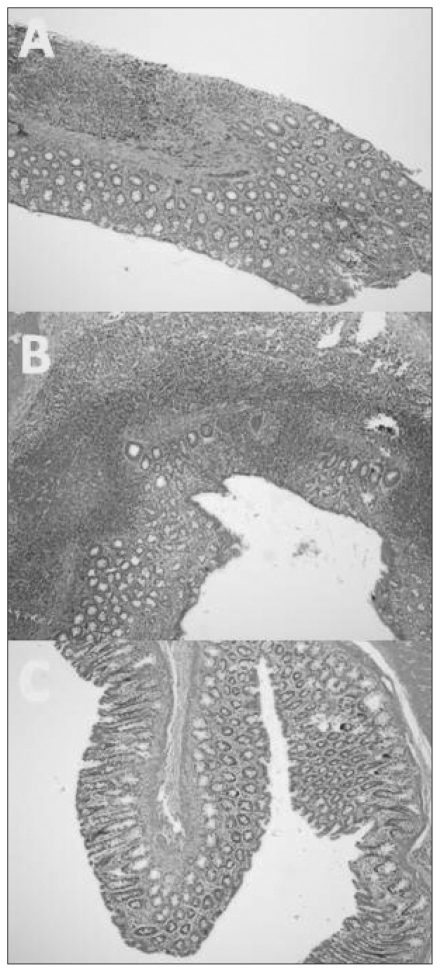
The microscopy images revealed regular colonic mucosa with epithelium, crypts and submucosa in the control group (Fig. 1A). The AA-induced colitis group demonstrated wide areas of epithelial and goblet cell loss and severe neutrophil infiltration, ulcers, distortion of crypt architecture and crypt abscesses (Fig. 1B). The colitis group treated with vitamin E showed regular epithelium and crypts, mild inflammatory cell infiltration and submucosal edema. The macroscopic and microscopic lesion scores of the colitis group were reduced by treatment with vitamin E (Table 1, Fig. 1C; p < 0.001).
Fig. 1.
(A) Normal colonic tissue architecture in the saline and vitamin E groups. (B) Acetic acid (AA)–induced mucosal damage in the colon. The distortion of crypt architecture and crypt abscess, loss of epithelial cells with ulceration, and goblet cell depletion with neutrophil infiltration were observed in AA rats. (C) Vitamin E significantly improved AA-induced mucosal damage (hematoxylin and eosin stain, magnification ×40).
Table 1.
Effects of vitamin E on histopathologic and antioxidant parameters in an acetic acid–induced colitis model
| Parameter | Group; mean (SE) | p value | |||
|---|---|---|---|---|---|
| Saline, n = 8 | Vitamin E, n = 8 | AA, n = 8 | AA + vitamin E, n = 8 | ||
| Macroscopic score | 0 (0) | 0 (0) | 4.7 (0.34) | 2.8 (1.06) | < 0.001 |
| Microscopic score | 0.1 (0.28) | 0.1 (0.24) | 7.6 (1.28) | 3.2 (0.97) | < 0.001 |
| GSH, μmol/mg protein | 1.9 (1.1) | 2.1 (1) | 0.2 (0.6) | 2.0 (1.2) | < 0.001 |
| SOD, U/mg protein | 8.7 (2.3) | 9.5 (3.8) | 1.2 (2) | 9.8 (4.2) | < 0.001 |
AA = acetic acid; GSH = glutathione peroxidase; SE = standard error; SOD = superoxide dismutase.
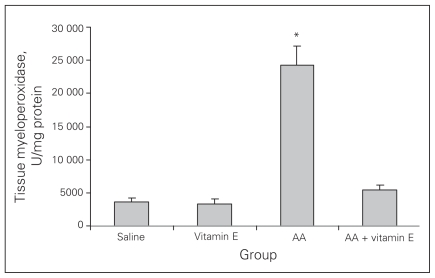
Colonic tissue–associated MPO activity
Colonic MPO activity in the colitis group was significantly higher than that in the control group (p < 0.001). Treatment with vitamin E reversed this damage (Fig. 2; p < 0.001).
Fig. 2.
Tissue myeloperoxidase activities. Results are means and standard errors. *p < 0.001 versus the other groups. AA = acetic acid.
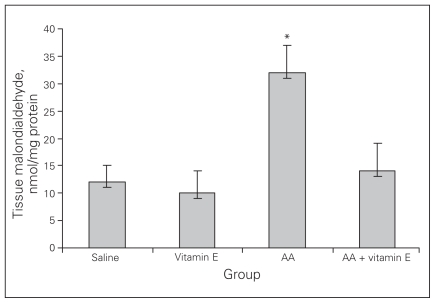
Colonic tissue MDA and antioxidant enzyme levels
As expected, the colitis group was characterized by a significant increase in the colonic tissue MDA level along with a concomitant decrease in GSH and SOD contents. The increase in colonic MDA in the colitis group was prevented by vitamin E (p < 0.001; Fig. 3).
Fig. 3.
Tissue malondialdehyde levels. Results are means and standard errors. *p < 0.001 versus the other groups. AA = acetic acid.
The tissue antioxidant enzyme (GSH and SOD) levels were lower in the colitis group than the control group and were restored by treatment with vitamin E (p = 0.006; Table 1).
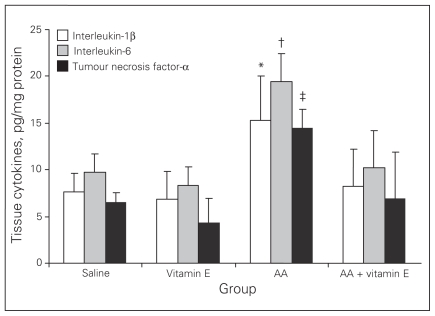
Colonic tissue cytokine levels
Colonic tissue inflammatory cytokine levels of IL-1β, IL-6 and TNF-α were significantly higher in the AA group than the other groups. Vitamin E therapy improved cytokine production to control group levels (p < 0.001; Fig. 4).
Fig. 4.
Tissue cytokine levels. Results are means and standard errors. *p < 0.001 versus the other groups; †p < 0.001 versus the other groups; ‡p < 0.001 versus the other groups. AA = acetic acid.
Discussion
Although the etiology of IBD has not been fully clarified, some possible etiologic factors are genetic influences, immunologic abnormalities and environmental agents causing colonic inflammation.2–4 Free radicals play an important role in the pathogenesis of mucosal injuries.8,18,19 The AA-induced colitis model resembles ulcerative colitis in humans in terms of histopathologic appearance by causing overproduction of oxidant radicals that have pivotal roles in its pathophysiology20 and by producing an excess of reactive oxygen species that cause damage to biologic molecules in any of the cellular components.20,21 In previous studies, free radicals and reactive oxygen species in colonic mucosa were described in colorectal specimens of ulcerative colitis.20,22 Neutrophils and macrophages were deemed responsible, disrupting epithelial integrity and causing colon injury in ulcerative colitis.23
Although it has been suggested that accumulation of reactive oxidant radicals and cytokines is responsible for the disease,5,16,21 the current literature lacks evidence for this dual effect in the pathogenesis. Ademoglu and colleagues24 studied the antioxidant effects of 30 mg/kg of vitamin E therapy on trinitrobenzenesulfonic acid–induced experimental colitis in rats. Vitamin E therapy was reported to be effective in reducing the oxidant stress in the model. Vitamin E and selenium combination therapy reduced the severity of the colonic damage. However, the study was not concerned about anti-inflammatory effects. To the best of our knowledge, the present study is the first to demonstrate the dual effects of vitamin E on oxidative damage and proinflammatory cytokine (IL-1β, IL-6 and TNF-α) production, resulting in an effective anti-inflammatory response and improvement in AA-induced ulcerative colitis pathogenesis. We have also demonstrated that vitamin E has great potential to reverse ulcerative colitis.
Inflammatory bowel disease is mainly characterized by overproduction of proinflammatory mediators, including reactive oxygen and nitrogen metabolites, eicosanoids, platelet-activating factor and cytokines. These factors initiate and prolong the inflammatory response of the gut.7 Thus, the treatment strategy for IBD focuses on eliminating these causal inflammatory triggers and mediators.
Increasing attention has been given to the role of free radicals in IBD. There have been numerous studies stating the potent role of vitamin E as a major free radical scavenger and antioxidant that protects cellular membrane lipids from peroxidation.7–10,20,22 Recent studies suggested that vitamin E also has an anti-inflammatory effect that is related to inhibition of neutrophil function or cytokine production in colon mucosa.7,8,11
In the present study, free radical production and subsequent macro- and microscopic damages were significantly increased (p < 0.001) by intrarectal AA injection and improved (p < 0.001) after treatment with vitamin E. These findings are in agreement with those of previous similar studies.20,25,26 In addition, our study established that vitamin E decreased the production of IL-1β, IL-6 and TNF-α in the colonic tissue. Thus, vitamin E produced a potent anti-inflammatory effect by scavenging radicals and eliminating cytokines causing colonic inflammation in this model.
Conclusion
Our results showed that vitamin E inhibited not only oxidant damage but also inflammatory cytokines and improved the colonic inflammation induced by AA in rats. This study suggests that vitamin E is an effective anti-inflammatory and antioxidant and that it may be a promising therapeutic option for ulcerative colitis.
Acknowledgment
We thank Kenneth Dorko from the University of Pittsburgh for his suggestions and critical review of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: Each author participated adequately in the study for substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. All authors contributed to the scientific acquisition of data, revising the content and the final version. Beyond these critical activities, additional contributions of the authors were as follows: Dr. G. Tahan was mainly responsible for the management of the study and performed the design and statistical analysis and interpretation of data. Drs. Aytac and Aytekin organized the design and feeding of animals and performed acquisition of data and the drug administrations. Drs. G. Tahan and Aytac performed surgery and euthanized animals. Dr. Dogusoy performed histopathologic examinations of the biopsies and took pictures of the tissues. Dr. V. Tahan followed up the animals and is a contributor in writing the manuscript. Drs. Aydin and Uzun collected and stored the samples and interpreted the data regarding the disease and performed biochemical, free radical and cytokine tests. All authors approved the final version of manuscript.
References
- 1.Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 2.Cetinkaya A, Bulbuloglu E, Kantarceken B, et al. Effects of L-carnitine on oxidant/antioxidant status in acetic acid-induced colitis. Dig Dis Sci. 2006;51:488–94. doi: 10.1007/s10620-006-3160-9. [DOI] [PubMed] [Google Scholar]
- 3.Buffinton GD, Doe WF. Depleted mucosal antioxidant defenses in inflammatory bowel disease. Free Radic Biol Med. 1995;19:911–8. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- 4.Koch TR, Yuan LX, Stryker SJ, et al. Total antioxidant capacity of colon in patients with chronic ulcerative colitis. Dig Dis Sci. 2000;45:1814–9. doi: 10.1023/a:1005517824877. [DOI] [PubMed] [Google Scholar]
- 5.Cetinkaya A, Bulbuloglu E, Kurutas EB, et al. Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med. 2005;206:131–9. doi: 10.1620/tjem.206.131. [DOI] [PubMed] [Google Scholar]
- 6.Burton GW, Joyce A, Ingold KU. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- 7.Mirbagheri SA, Nezami BG, Assa S, et al. Rectal administration of d-alpha tocopherol for active ulcerative colitis: a preliminary report. World J Gastroenterol. 2008;14:5990–5. doi: 10.3748/wjg.14.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isozaki Y, Yoshida N, Kuroda M, et al. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 9.Fox ES, Brower JS, Bellezzo JM, et al. N-acetylcysteine and alpha-tocopherol reverse the inflammatory response in activated rat Kupffer cells. J Immunol. 1997;158:5418–23. [PubMed] [Google Scholar]
- 10.Yoshida N, Yoshikawa T, Manabe H, et al. Vitamin E protects against polymorphonuclear leukocyte-dependent adhesion to endothelial cells. J Leukoc Biol. 1999;65:757–63. doi: 10.1002/jlb.65.6.757. [DOI] [PubMed] [Google Scholar]
- 11.Morris GP, Beck PL, Herridge MS, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 12.Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995;269:G119–25. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- 13.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 14.Yagi K. Assay for blood plasma or serum. Methods Enzymol. 1984;105:328–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 15.Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–53. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- 16.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–57. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Yoshikawa T, Yoshida N, Miyagawa H, et al. Role of lipid peroxidation, in gastric mucosal lesions induced by burn shock in rats. J Clin Biochem Nutr. 1987;2:163–70. [Google Scholar]
- 19.Yoshikawa T, Ueda S, Naito Y, et al. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun. 1989;7:285–91. doi: 10.3109/10715768909087953. [DOI] [PubMed] [Google Scholar]
- 20.Bitiren M, Karakilcik AZ, Zerin M, et al. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 21.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–84. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ademoglu E, Erbil Y, Tam B, et al. Do vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–8. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 23.Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. Ann N Y Acad Sci. 1992;664:103–15. doi: 10.1111/j.1749-6632.1992.tb39753.x. [DOI] [PubMed] [Google Scholar]
- 24.Ademoglu E, Erbil Y, Tam B, et al. Do vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–8. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 25.Blackburn AC, Doe WF, Buffinton GD. Colonic antioxidant status in dextran sulfate-induced colitis in mice. Inflamm Bowel Dis. 1997;3:198–203. [PubMed] [Google Scholar]
- 26.Camuesco D, Galvez J, Nieto A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–94. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]