Abstract
Biochemical characterization of the haemostatic system has advanced significantly in the past decades. Sub-systems, such as coagulation, fibrinolysis, blood cells and platelets and the vessel wall have been studied by specialists, mostly separately and independently. The time has come to integrate the approaches, and, in particular, to develop tests to document the state of the whole system and to have available adequate pharmacodynamic tests to evaluate treatments. Many examples are available to show that traditional general methods of clotting and lysis do not provide the information that is desired. The present tendency is to use specific methods for specific factors or effects which are very limited in pharmacological information. There is also increasing awareness of the occurrence of rather broad interindividual variability in the haemostatic system. This suggests that individually tailored treatments are required. This is the more relevant since haemostasis is a balance and treatment should be positioned between efficacy and safety. The conclusion is reached that there is a need for integrated or global methods or sets of methods that reflect the complexity and individual status appropriately and allow the practitioner to judge the effects of interventions and their individual aspects.
Keywords: coagulation, endothelium, fibrinolysis, pharmacodynamics, platelets
Introduction
Maintenance of blood fluidity within the vascular system is an important physiological theme. At the same time, the local formation of a clot serves to assist in preventing excessive blood leakages, and in aiding tissue repair. In addition clot formation is involved in host defence.
A balance with, on the one hand, a deviation in excessive clot formation and, on the other hand, defective clot formation was proposed originally by Astrup in 1958 [1]. The system requires delicate biochemical regulation to maintain this balance and to serve the various functions adequately.
Deviations in the haemostasis of excessive clot formation or thrombosis are involved in arterial diseases such as myocardial infarction and ischaemic stroke, in venous diseases such as deep vein thrombosis and pulmonary embolism, and in organ failure during trauma and infections. This is a major cause of death and disabilities. Another deviation concerns defects in clot formation and subsequent bleeding, known from congenital deficiencies in clotting factors such as factor VIII and IX, but also potentially life threatening during trauma and a major issue in critical care medicine.
Many treatment options to reinforce the system or to inhibit parts of it have been developed and are still under active development. These treatments inevitably interfere with the balance mentioned earlier and besides the intended effect, the opposite effect (bleeding or thrombosis) is always associated with the treatments as a safety issue.
The present introduction evaluates recent developments in concepts in haemostasis, the status of biomarker analysis and the desired development of pharmacodynamic tests.
Development of concepts
Biochemical concepts
Haemostasis is regulated by a complex set of mechanisms including blood cells, notably platelets, the vessel wall and the coagulation and fibrinolytic cascades.
The organization of the different contributions to the overall effects varies at specific sites and in specific tissues and organs in the body and adds to the complexity of regulation, and to differences in the clinical expression of specific abnormalities in different or specific tissues/organs.
The mechanisms are highly intertwined and interactive as indicated by the overlapping boxes in Figure 1.
Figure 1.
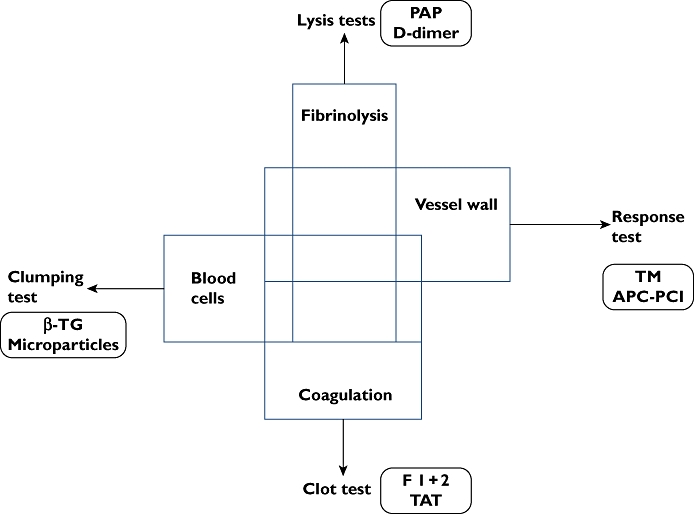
Schematic representation showing the intertwined systems of coagulation, fibrinolysis, vessel wall and blood cells. These systems are usually studied separately by sets of tests referred to as lysis, clot, clumping and response tests in the figure. Some molecular markers originating from each system are indicated
We will not provide details of the biochemical mechanisms represented by the blocks in Figure 1 for which we refer to recent reviews on blood cell [2] and platelet mechanisms[3, 4], vessel wall mechanisms [5], coagulation [6, 7] and fibrinolysis [8].
In recent decades the individual biochemical factors and their actions have been elucidated to a major extent. This has also resulted in the possibility of determining by specific methods the concentrations and activity of most of these factors. However, it should be realized that the study of individual factors and subsystems is reductionistic. Obviously, situations where only one subsystem is relevant do exist, but more frequently haemostasis should be considered by an integrated approach.
The reductionistic approach is also a feature of current treatments in haemostasis and a tendency has been to develop even more specific treatments. This can be illustrated by the transition from treatment with unfractionated heparin, antagonizing both thrombin and factor Xa, to the use of low molecular weight heparins and finally pentasaccharides that specifically target only coagulation factor Xa. Also, new oral anticoagulants are meant to target specifically factor Xa or thrombin, and the emerging antisense compounds also target specific clotting factors [9, 10]
In platelet treatments specific inhibitors have also been developed. However, there seems to be a tendency to use combinations of drugs suggesting that the reductionistic approach is losing popularity.
The ability to assess most individual factors was followed by the recognition that the synthesis or integration of this specific information for understanding the potency and action of the system was not easily possible. Attempts are now on-going to develop computer models [11] and a revival of global methods involving many factors instead of a set of data on individual factors can be noted [12]. Also molecular markers of action (Figure 1), rather than levels of components have received increased interest because of the apparent advantage that these markers report from the intertwined system in vivo.
Pharmacological concepts
Several haemostasis variables and markers show a large inter-individual variability, which has been suggested to represent the opposing evolutionary advantages of haemostasis and host defence [13]. For instance, a relatively high percentage (5%) in the Caucasian population [14, 15] carry a mutation in the gene coding for factor V (factor V Leiden). Such high prevalence suggests an advantage of this mutation (possibly protection against infections) while it simultaneously predisposes to thrombosis. Analogous findings have been reported for the mutation in the gene coding for prothrombin that results in the thrombosis related variant G20210A [15]. This heterogeneity may be advantageous for the population at large, but may be a disadvantage for individuals. We propose that given the broad ranges of ‘normal’ values, individuals deserve individual characterization, treatment and monitoring with adequate methods. This is in contrast to the current trend, which suggests abandoning monitoring [16] or introducing new methods that are very reductionistic such as, for example, monitoring anti-Xa activity.
Biochemical developments of recent date
Thrombin-thrombomodulin
An important insight in the link between coagulation and fibrinolysis is the intimate relationship via the formation of the thrombin-thrombomodulin complex [17]. Specifically, this complex down-regulates thrombin formation and increases inhibition of fibrinolysis. In these mechanisms the complex activates protein C, which inactivates the cofactors of coagulation factors Va and VIIIa and thus dampens the cascade activation. The complex is also involved in the activation of the fibrinolysis inhibitor TAFI, which is temporary due to its intrinsic instability.
These effects of thrombin can result in the following abnormalities: (i) increased thrombin formation results in increased clotting and increased stability of the thrombus against fibrinolysis and (ii) decreased thrombin formation results in reduced clotting activity, but also an increased susceptibility of the clot to lysis. This notion has resulted in the development of methods which couple coagulation and fibrinolysis to allow their interaction and detect abnormalities and the effects of anticoagulants on fibrinolysis.
Factor XII pathway
The role of the pathway starting with factor XII, formerly known as the intrinsic coagulation pathway, has been receiving new attention [18–21]. Animal experiments have shown that inhibition or defects in this pathway lead to reduced thrombus propagation and a reduced thrombotic load. These data show the benefits of deleting factor XII, prekallikrein or factor XI or of the use of aprotinin (kallikrein inhibitor). In vitro these effects can be shown in the thrombin generation test as a reduction in post-clotting thrombin, providing a potential diagnostic tool. However, the concepts are not completely congruent with human data yet. Human data do indeed show a moderate increased bleeding tendency in factor XI deficiency [22] and a relationship between high-factor XI and VTE [23]. However, the role of factor XII should be further unravelled as individuals with a genetically low factor XII show increased risk of VTE, and high-factor XII seems beneficial for reaching a high age.
Presently, several new treatments targeting the intrinsic pathway factors such as factor XII and factor XI are being actively pursued.
Microparticles
It has become increasingly clear that microparticles play a role in the haemostatic system, but there are also significant black holes in our understanding of their contribution to clinical events [24]. It has been demonstrated that an association exists between microparticles and venous thrombosis in several populations such as patients with type II diabetes mellitus and patients with certain malignancies [24, 25]. However, it is biologically implausible that in these conditions identical microparticles with regard to size and composition are involved. Also, in vitro testing shows that the contribution of microparticles is incompletely understood. For example: in some variants of the thrombin generation test the contribution of microparticles is clearly expressed, while other methods appear to be insensitive [26].
The role of microparticles in coagulation is thought to be related to various aspects such as the lipid components [27], which can be studied now by specific methods, and to the possible presence of tissue factor, which particularly in relation to malignancies is an issue of focused research [25]. More recently also the contribution to fibrinolysis has been highlighted. The origin of t-PA-bearing particles from the endothelium, and of u-PA-bearing particles from leucocytes may be employed for differential diagnostic approaches [28].
Brief critical remarks on global tests
The prothrombin time test (INR)
The Quick variant of prothrombin time is used worldwide to monitor oral anticoagulation with vitamin K antagonists. There are more than 20 marketed reagents with different sensitivity, but harmonization has been achieved by using the INR conversion. The finding that novel agents have no (fondaparinux) or marginal (rivaroxaban) effects on the PT [29, 30], clearly indicates that the test does not provide all the information available from the altered coagulation status and should be considered as an approach to coagulation testing with clear limits.
The APTT test
The APTT test (and its point-of-care variant ACT) is widely used to monitor anticoagulant therapy, but this is mainly successful for unfractionated heparin. LMW heparins show smaller effects in this test and the pentasaccharides are practically devoid of inhibitory action. Thus, the APTT also does not provide all the required information about coagulation and should not be used as a generally applicable read-out for effects on the coagulation cascade.
TGT tests
The thrombin generation test (TGT) has been added recently to the battery of coagulation tests. The TGT is valuable as it provides information on variables of clotting such as the rate of thrombin formation and the amount of thrombin formed after clotting in addition to the clotting time [31]. Thrombin formed after clotting is important for the programming of clot stability and considered to be related to fibrinolysis. A problem with this test concerns the existence of several variants with different sensitivities to factors such as microparticles and contact activation and factor VIII [26, 32]. It is also important that a clear distinction is made between TGT variants with low and high TF concentrations. It is too early yet to decide how each method variant adds to diagnosis.
Thrombo-elastographic methods
Thrombo-elastographic methods have a long history as global methods and can use whole blood. Recent adaptations to modern laboratory procedures have resulted in two current methods in use, the ROTEM (Rotation thromboelastometry) and the TEG (Thrombelastograph). Both are used as point-of-care methods in critical care medicine, because they provide information rapidly and do not require elaborate sample preparations. Broader application of these methods is theoretically appealing as the tests use whole blood and provide information on clot strength. The current variants mainly use activation procedures resembling PT and APTT, but for research purposes variants resembling TGTs with low tissue factor concentrations have been evaluated [33, 34]. Similarly to the TGT tests the various variants require validation for their relevance as PD methods and addition to diagnosis [35, 36]
Lupus anticoagulant tests
As is well known, lupus testing relies on the determination of prolongation of clotting tests, while ‘by contrast’ the feared complication is thrombotic. This indicates that the clotting tests utilized are pharmacokinetic rather than functional. More recently Rand et al. [37] proposed other tests which seem to reflect the (patho-)physiology better as they study increased coagulation in relation to thrombosis. The value of this approach will become a topic of future research and a possible guide to treatment.
Bleeding tests
Bleeding tests, such as the Ivy bleeding test, detect the immediate potential to arrest bleeding (primary haemostasis). The test can be supplemented with biochemical tests on the shed blood to assess the effects in coagulation and platelet subsystems [38]. It is likely that the further information on clot quality and clot lyzability will increase our understanding of less immediate aspects such as ‘oozing’ after initial haemostasis, and risks of re-bleeding such as typically observed in antiplasmin-deficient cases [39]. It should be realized that no data are available that relate the bleeding time to clinical events.
Platelet aggregometry
Platelet function is frequently evaluated by measurement of platelet aggregation in platelet-rich plasma (PRP). However, PRP as a matrix for the assessment of platelet aggregation has several inherent short comings: handling the sample for platelet isolation to obtain PRP modulates platelet behaviour, PRP does not contain certain specific hyper- and hypo-active platelet subsets as these are lost during preparation, and PRP does not contain red and white blood cells, which are known to affect significantly platelet function [40]. Therefore, whole blood is preferred as a matrix to measure platelet aggregation and determine the antiplatelet effect of pharmacological intervention [41]. We suggest that evaluation of platelet function in PRP should be abandoned and replaced with methods using whole blood such as impedance aggregometry that is frequently used and has been shown to be reliable method to measure baseline platelet aggregation and the effect of pharmacological interventions such as serotonin antagonism [42].
Desired tests and approaches for a pharmacodynamic perspective
Desired tests
It is becoming increasingly clear that at present a comprehensive set of pharmacodynamic methods for anticoagulant treatment is lacking. This is evident from the observations that traditional tests such as the PT and APTT are insensitive to therapeutic concentrations of fondaparinux, and reversible factor Xa inhibitors express poorly in those tests. It can be argued that the majority of the tests provide information on the pharmacokinetics of drugs rather than information on their effects. For instance, measurement of anti-Xa activity is in essence no more than measuring the plasma concentration of the drug and does not provide any information that can be related to individual clinical responses.
Also, at present it seems to have been insufficiently realized that the APTT and PT should not be used to evaluate the anticoagulant effects of drugs other than unfractionated heparin and vitamin K antagonists, respectively. The general approach in drug development and clinical trials of targeting drugs at doubling the APTT or PT negates the notion that different drugs at similar APTT or PT prolongations most likely influence the coagulation system differently and provide no objective information on the similarity of efficacy or safety. We suggest that trials with non-like compounds (pentasaccharide vs. low molecular weight heparin) using a similar read-out (APTT) deliver at best insufficient and possibly even erroneous information.
Phenotype
The traditional clotting methods use time to clotting as the phenotype, while intuitively other characteristics of the clot would be more appropriate. Recent studies have focused more on the clot by measuring fibre qualities, clot strength, porosity and lyzability [43].
We suggest that in this area future pharmacodynamic measures will be found, either in a complex test or as a set of measurements that relate better to the risk of thrombosis and bleeding. The observation that information on clot properties provides better guidance for preventing bleeding and the effectiveness of treatment than clotting time in critically ill patients with bleeding problems [44, 45] may be considered a first step in this direction. This can be taken further when evaluations will also be done that study clot growth/propagation in conditions with flow/circulation because this clinically relevant condition is not mimicked in current in vitro tests [46, 47].
Coupled assays
The recognition that coagulation programmes subsequent fibrinolysis has resulted in interest in using ‘coupled assays’. In these tests coagulation is started high in the cascade followed by the study of lysis, usually evoked by the addition of a plasminogen activator. The start high in the cascade with tissue factor or contact activation allows the expression of coagulation interventions. A typical pattern of turbidity of the clot is shown in Figure 2, where the effect of stimulating TAFI activation by thrombomodulin is indicated and appears to have a large impact on lysis.
Figure 2.
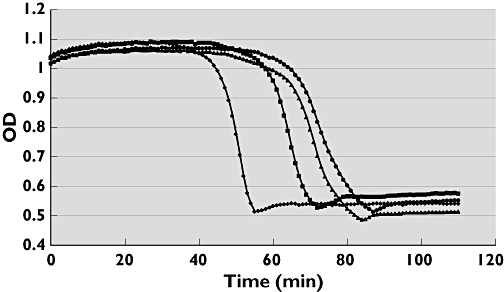
Turbidity read out of clot formation (starting with tissue factor, lipid and calcium addition) and clot dissolution in platelet-poor citrated plasma supplemented with t-PA. Addition of thrombomodulin (reinforcing TAFIa formation) strongly inhibits lysis. From left to right: 0, 1, 3 and 5 nmol l−1 Thrombomodulin
This coupled assay has the advantage of studying the clot phenotype by turbidity. This is a more complex end-point than studying thrombin activity and plasmin activity simultaneously, which also has been employed in a coupled assay format.
The most complex system ex vivo to date is thromboelastography with whole blood, which also incorporates the platelet contribution. A mimic has been developed where plasma is supplemented with standard platelets [48].
New information in thrombin generation tests
The thrombin generation tests add as new information the amount of thrombin formed after clotting. This is a determinant of clot quality and thrombus growth and can be considered as one of the indirect measurements for these parameters. It does, however, not directly address clot architecture, lyzability and propagation, and further developments to capture these aspects are needed. Interestingly, the thrombin generation test captures effects of fondaparinux and rivaroxaban in therapeutically relevant concentrations. In this respect the thrombin generation test carries important pharmacodynamic information.
Pleiotropic effects
Another issue that is generally insufficiently recognized regards the pleiotropic effects of haemostatic factors and of drugs intended to alter haemostasis. Several factors of coagulation have multiple effects in the cascade and effects on specific tissues [49–52]. Most of these effects go unnoticed in present tests and call for a set of tests to be properly delineated. An example is the contribution that the endothelium may have on the anticoagulation pathway. The formation of activated protein C on the cell surface is not expressed explicitly or recognizable in current tests and may be defective without being noticed. This in vivo effect can practically only be addressed by methods using molecular markers reporting on the endothelial contribution. One such marker is the APC-PCI complex which has a continuous level and can report about the potential of this route [53].
Molecular markers
It is also likely that molecular markers such as prothrombin fragment 1 + 2, thrombin-antithrombin complexes, plasmin-antiplasmin complexes, d-dimer, etc. are valuable pharmacodynamic markers. These markers report on the action in vivo in parts of the cascade.
Frequently, this documentation is done in a stable situation where the report is on the continuous action in part of the cascade. The evaluation of molecular markers in dynamic situations is difficult since frequent sampling is necessary.
The continuous action in a stable situation is already present in very young and very healthy individuals and can be viewed as the running of the engine in a free, uncoupled gear condition. It is thought to be necessary for the system to react very rapidly, which would not be possible with the engine shut off, but it needs a degree of activation below a threshold of firing of the system [54]. Consequently, from nearly all parts of the cascade molecular markers can be detected which indicate it is active at all places in the process.
An additional contribution to this continuous action is attributed to normal wear and tear in the body. This increases with age, showing increases in the levels together with increases in inflammatory markers such as interleukin-6 and C-reactive protein [55, 56]. Also in specific chronic diseases, the levels can be higher due to participation of the haemostatic pathways in the chronic processes.
Additionally, the markers from different parts of the cascade can also report on balances and imbalances in the action of the system. Thus the ratio of F 1 + 2 over PAP can report on the balances between coagulation and fibrinolysis activation. The ratio between F 1 + 2 and APC-PCI can report on the balance between coagulation activation and anticoagulation activation [57].
Individual variability = individual treatment?
An under-reported phenomenon, recently highlighted by Ginsburg et al. [58], is the wide distribution seen in several levels of haemostasis variables in the population of which von Willebrand factor is exemplary [59]. The same holds true for a wide spread of molecular markers.
Here, we illustrate this phenomenon for the molecular markers F 1 + 2, TAT, PAP and d-dimer. In a narrowly selected group of young women planning to enrol in a study on oral contraceptives these markers were measured and the concentrations were divided into deciles. The ratio of the medians of the 9th and 2nd decile were 3.5 (F1 + 2), 2.4 (TAT), 2.8 (PAP) and 3.0. This indicates a consistent three-fold difference in the basal activity or set point among the markers. The data for d-dimer are illustrated in Figure 3, showing the distribution in the selected deciles and how far apart they are.
Figure 3.
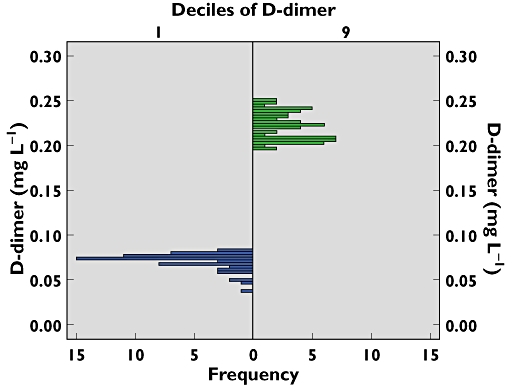
Histogram of d-dimer in the second and ninth decile of F 1 + 2 recorded in young. Women [60]. Each group contained between 50 and 60 individuals. d-dimer is chosen as the most robust analyte. The first and tenth deciles are avoided due to increased variations in the values
Studies have shown that these levels of variables are habitual or stable in a particular individual and have a significant heritability [61]. Also, the stability of the thrombin generation test (longitudinal ETP CV% = 16) and the spread in the population (groups ETP CV% = 23.8) have been documented, illustrating a similar phenomenon for this global test [62]. We have evaluated also the ratios of molecular markers and similarly found that these are stable for defining individuals and also an individual balance between coagulation and anticoagulation and between coagulation and fibrinolysis [57]. This implies that individuals are really individual and, more importantly, differ greatly. This not only applies for baseline concentrations or activity, but also for treatments. In this case it is important to note that oral vitamin K antagonists reduce F 1 + 2 in all subjects, while at the same time the individual variability (CV 30%) in F1 + 2 before treatment and after treatment remained similar [63, 64]. This has been also been reported for factor levels [63]. Apparently, inter-individual variation remains constant during treatment and suggests that inherent differences between individuals are great. This suggests that pre-treatment haemostatic profiling might be an important improvement for each individual's benefit risk assessment of subsequent treatment.
Conclusions
The documentation of the physiology of the haemostatic system and its response to treatment requires further development of pharmacodynamic methods, which most likely concerns a set of in vitro methods combined with molecular markers. We suggest that the haemostatic system should be targeted with individualized treatments. An individual with potent and active clotting may need more intense anticoagulant treatment while persons with weak activity might be more at risk of bleeding and should receive anticoagulant treatment of lower intensity. Presently, treatments are still not individualized but based upon population-based observations. Although these treatment algorithms have proven value, they are far from optimal [65]. This is illustrated schematically in Figure 4 which also represents the situation of the well-known treatment window for VKA anticoagulation. This figure, which is based upon published data [66], shows the risk of thrombosis associated with low intensity treatment and the risk of bleeding with high intensity treatment.
Figure 4.
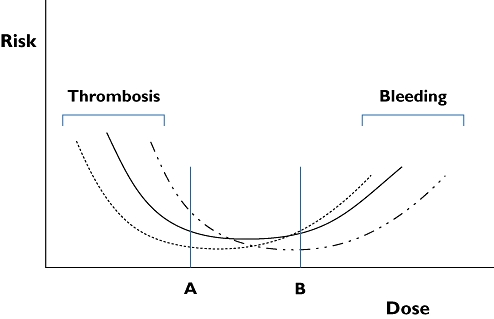
Dose–risk curves for anticoagulant treatment with vitamin K antagonists as reported [66]. The curve for the entire treated population is represented by the solid curve. The dotted curve and the dot-stripe curve represent subpopulations with less or more tendency to clot, respectively. A and B represent two treatment doses. In persons belonging to the subgroup represented by the dotted curve, treatment between doses A and B will be effective, but bleeding risk increases at dose B. For persons making up the subgroup of the dot-stripe curve, dose A is not fully effective, but dose B is safe
The middle curve represents the population mean [66]. We propose that subdividing patients in categories with high (right curve) and low (left curve) coagulation potency/activity can produce different curves. This will result in real individual adjustment of treatment intensity and is likely to increase efficacy and safety.
This introduction emphasizes that it is a challenge to identify and use the most appropriate tools to identify the individual differences before the treatment. Also, the identification and utilization of proper pharmacodynamic tests to estimate the magnitude of intervention in each individual will be challenging. However, it is a desirable future compared with the rather simplistic approach of comparing new (oral thrombin and factor Xa) coagulation inhibitors with existing therapies using reductionistic tests and suggesting that these treatments can be applied in a fixed dose without ever testing for individual effects.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Astrup T. The haemostatic balance. Thromb Diath Haemorrh. 1958;2:347–57. [PubMed] [Google Scholar]
- 2.Shantsila E, Lip GY. The role of monocytes in thrombotic disorders. Insights from tissue factor, monocyte-platelet aggregates and novel mechanisms. Thromb Haemost. 2009;102:916–24. doi: 10.1160/TH09-01-0023. [DOI] [PubMed] [Google Scholar]
- 3.Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–66. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker RC, Smyth S. The evolution of platelet-directed pharmacotherapy. J Thromb Haemost. 2009;7(Suppl 1):266–71. doi: 10.1111/j.1538-7836.2009.03428.x. [DOI] [PubMed] [Google Scholar]
- 5.Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13:1211–20. doi: 10.1111/j.1582-4934.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor SD, Taylor AJ, Williams EC, Winter TC. Coagulation concepts update. AJR Am J Roentgenol. 2009;193:1656–64. doi: 10.2214/AJR.08.2191. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433–46. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 8.Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J Thromb Haemost. 2009;7:4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 9.Yin J, Luo XG, Yu WJ, Liao JY, Shen YJ, Zhang ZW. Antisense oligodeoxynucleotide against tissue factor inhibits human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Cell Physiol Biochem. 2010;25:477–90. doi: 10.1159/000303053. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Lowenberg EC, Crosby JR, MacLeod AR, Zhao C, Gao D, Black C, Revenko AS, Meijers JC, Stroes ES, Levi M, Monia BP. Inhibition of the intrinsic coagulation pathway factor XI by antisense oligonucleotides: a novel antithrombotic strategy with lowered bleeding risk. Blood. 2010;116:4684–92. doi: 10.1182/blood-2010-04-277798. [DOI] [PubMed] [Google Scholar]
- 11.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–33. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 12.McGlasson DL, Fritsma GA. Whole blood platelet aggregometry and platelet function testing. Semin Thromb Hemost. 2009;35:168–80. doi: 10.1055/s-0029-1220325. [DOI] [PubMed] [Google Scholar]
- 13.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 14.de Maat MP, Kluft C, Jespersen J, Gram J. World distribution of factor V Leiden mutation. Lancet. 1996;347:58. [PubMed] [Google Scholar]
- 15.Irdem A, Devecioglu C, Batun S, Soker M, Sucakli IA. Prevalence of factor V Leiden and prothrombin G20210A gene mutation. Saudi Med J. 2005;26:580–3. [PubMed] [Google Scholar]
- 16.Tripodi A, van den Besselaar A. Laboratory monitoring of anticoagulation: where do we stand? Semin Thromb Hemost. 2009;35:34–41. doi: 10.1055/s-0029-1214146. [DOI] [PubMed] [Google Scholar]
- 17.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374–83. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 18.Renne T, Nieswandt B, Gailani D. The intrinsic pathway of coagulation is essential for thrombus stability in mice. Blood Cells Mol Dis. 2006;36:148–51. doi: 10.1016/j.bcmd.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bach J, Endler G, Winkelmann BR, Boehm BO, Maerz W, Mannhalter C, Hellstern P. Coagulation factor XII (FXII) activity, activated FXII, distribution of FXII C46T gene polymorphism and coronary risk. J Thromb Haemost. 2008;6:291–6. doi: 10.1111/j.1538-7836.2008.02839.x. [DOI] [PubMed] [Google Scholar]
- 21.Endler G, Marsik C, Jilma B, Schickbauer T, Quehenberger P, Mannhalter C. Evidence of a U-shaped association between factor XII activity and overall survival. J Thromb Haemost. 2007;5:1143–8. doi: 10.1111/j.1538-7836.2007.02530.x. [DOI] [PubMed] [Google Scholar]
- 22.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84–7. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 23.Bertina RM. Elevated clotting factor levels and venous thrombosis. Pathophysiol Haemost Thromb. 2003;33:395–400. doi: 10.1159/000083835. /2004. [DOI] [PubMed] [Google Scholar]
- 24.Diamant M, Tushuizen ME, Sturk A, Nieuwland R. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 25.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 26.Kluft C, Meijer P. External quality assessment for thrombin generation tests: an exploration. Semin Thromb Hemost. 2010;36:791–6. doi: 10.1055/s-0030-1265296. [DOI] [PubMed] [Google Scholar]
- 27.van Beers EJ, Schaap MC, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, Meijers JC, Biemond BJ. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angles-Cano E, Vivien D. [Cellular microparticles, potential useful biomarkers in the identification of cerebrovascular accidents] Med Sci (Paris) 2009;25:843–6. doi: 10.1051/medsci/20092510843. [DOI] [PubMed] [Google Scholar]
- 29.Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–25. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 30.Gerotziafas GT, Chakroun T, Samama MM, Elalamy I. In vitro comparison of the effect of fondaparinux and enoxaparin on whole blood tissue factor-triggered thromboelastography profile. Thromb Haemost. 2004;92:1296–302. doi: 10.1160/TH03-11-0694. [DOI] [PubMed] [Google Scholar]
- 31.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–61. [PubMed] [Google Scholar]
- 32.Devreese K, Wijns W, Combes I, Van kerckhoven S, Hoylaerts MF. Thrombin generation in plasma of healthy adults and children: chromogenic versus fluorogenic thrombogram analysis. Thromb Haemost. 2007;98:600–13. [PubMed] [Google Scholar]
- 33.Young G, Yonekawa KE, Nakagawa PA, Blain RC, Lovejoy AE, Nugent DJ. Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxaparin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography. Blood Coagul Fibrinolysis. 2007;18:547–53. doi: 10.1097/MBC.0b013e328201c9a9. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen B, Ingerslev J. A direct thrombin inhibitor studied by dynamic whole blood clot formation. Haemostatic response to ex-vivo addition of recombinant factor VIIa or activated prothrombin complex concentrate. Thromb Haemost. 2006;96:446–53. [PubMed] [Google Scholar]
- 35.Young G, Yonekawa KE, Nakagawa PA, Blain RC, Lovejoy AE, Nugent DJ. Differential effects of direct thrombin inhibitors and antithrombin-dependent anticoagulants on the dynamics of clot formation. Blood Coagul Fibrinolysis. 2007;18:97–103. doi: 10.1097/MBC.0b013e3280116c4c. [DOI] [PubMed] [Google Scholar]
- 36.Sorensen B, Ingerslev J. Tailoring haemostatic treatment to patient requirements – an update on monitoring haemostatic response using thrombelastography. Haemophilia. 2005;11(Suppl 1):1–6. doi: 10.1111/j.1365-2516.2005.01156.x. [DOI] [PubMed] [Google Scholar]
- 37.Rand JH, Wu XX, Lapinski R, van Heerde WL, Reutelingsperger CP, Chen PP, Ortel TL. Detection of antibody-mediated reduction of annexin A5 anticoagulant activity in plasmas of patients with the antiphospholipid syndrome. Blood. 2004;104:2783–90. doi: 10.1182/blood-2004-01-0203. [DOI] [PubMed] [Google Scholar]
- 38.Sarich TC, Eriksson UG, Mattsson C, Wolzt M, Frison L, Fager G, Gustafsson D. Inhibition of thrombin generation by the oral direct thrombin inhibitor ximelagatran in shed blood from healthy male subjects. Thromb Haemost. 2002;87:300–5. [PubMed] [Google Scholar]
- 39.Matsuno H, Kozawa O, Okada K, Ueshima S, Matsuo O, Uematsu T. Plasmin generation plays different roles in the formation and removal of arterial and venous thrombus in mice. Thromb Haemost. 2002;87:98–104. [PubMed] [Google Scholar]
- 40.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin Appl Thromb Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 41.Tabuchi A, Taniguchi R, Takahashi K, Kondo H, Kawato M, Morimoto T, Kimura T, Kita T, Horiuchi H. Action of aspirin on whole blood-aggregation evaluated by the screen filtration pressure method. Circ J. 2008;72:420–6. doi: 10.1253/circj.72.420. [DOI] [PubMed] [Google Scholar]
- 42.Moerland M, Kemme MJ, van der Linden M, Burggraaf J. Measurement of collagen- and serotonin-induced platelet aggregation in whole blood. Expert Rev Clin Pharmacol. 2010;3:177–82. doi: 10.1586/ecp.10.2. [DOI] [PubMed] [Google Scholar]
- 43.Undas A, Zalewski J, Krochin M, Siudak Z, Sadowski M, Pregowski J, Dudek D, Janion M, Witkowski A, Zmudka K. Altered plasma fibrin clot properties are associated with in-stent thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:276–82. doi: 10.1161/ATVBAHA.109.194936. [DOI] [PubMed] [Google Scholar]
- 44.Rahe-Meyer N, Solomon C, Winterhalter M, Piepenbrock S, Tanaka K, Haverich A, Pichlmaier M. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 45.Kozek-Langenecker S. Management of massive operative blood loss. Minerva Anestesiol. 2007;73:401–15. [PubMed] [Google Scholar]
- 46.Ataullakhanov FI, Panteleev MA. Mathematical modeling and computer simulation in blood coagulation. Pathophysiol Haemost Thromb. 2005;34:60–70. doi: 10.1159/000089927. [DOI] [PubMed] [Google Scholar]
- 47.Ovanesov MV, Ananyeva NM, Panteleev MA, Ataullakhanov FI, Saenko EL. Initiation and propagation of coagulation from tissue factor-bearing cell monolayers to plasma: initiator cells do not regulate spatial growth rate. J Thromb Haemost. 2005;3:321–31. doi: 10.1111/j.1538-7836.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 48.He S, Zhu K, Skeppholm M, Vedin J, Svensson J, Egberg N, Blomback M, Wallen H. A global assay of haemostasis which uses recombinant tissue factor and tissue-type plasminogen activator to measure the rate of fibrin formation and fibrin degradation in plasma. Thromb Haemost. 2007;98:871–82. [PubMed] [Google Scholar]
- 49.Borensztajn K, Peppelenbosch MP, Spek CA. Factor Xa: at the crossroads between coagulation and signaling in physiology and disease. Trends Mol Med. 2008;14:429–40. doi: 10.1016/j.molmed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–52. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 51.Schoenmakers SH, Reitsma PH, Spek CA. Blood coagulation factors as inflammatory mediators. Blood Cells Mol Dis. 2005;34:30–7. doi: 10.1016/j.bcmd.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Wysoczynski M, Liu R, Kucia M, Drukala J, Ratajczak MZ. Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling. Mol Cancer Res. 2010;8:677–90. doi: 10.1158/1541-7786.MCR-10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strandberg K, Kjellberg M, Knebel R, Lilja H, Stenflo J. A sensitive immunochemical assay for measuring the concentration of the activated protein C-protein C inhibitor complex in plasma: use of a catcher antibody specific for the complexed/cleaved form of the inhibitor. Thromb Haemost. 2001;86:604–10. [PubMed] [Google Scholar]
- 54.Jesty J, Rodriguez J, Beltrami E. Demonstration of a threshold response in a proteolytic feedback system: control of the autoactivation of factor XII. Pathophysiol Haemost Thromb. 2005;34:71–9. doi: 10.1159/000089928. [DOI] [PubMed] [Google Scholar]
- 55.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–6. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 56.Wu TL, Tsao KC, Chang CP, Li CN, Sun CF, Wu JT. Development of ELISA on microplate for serum C-reactive protein and establishment of age-dependent normal reference range. Clin Chim Acta. 2002;322:163–8. doi: 10.1016/s0009-8981(02)00172-9. [DOI] [PubMed] [Google Scholar]
- 57.Kluft C, Stenflo J, Meijer P, Burggraaf J. APC-PCI complex as a new tool for the evaluation of anticoagulant response to endogenous thrombin formation and its sequel in fibrinolysis. JThromb Haemostas. 2010;8(Suppl 1):58. [Google Scholar]
- 58.Westrick RJ, Ginsburg D. Modifier genes for disorders of thrombosis and hemostasis. J Thromb Haemost. 2009;7(Suppl 1):132–5. doi: 10.1111/j.1538-7836.2009.03362.x. [DOI] [PubMed] [Google Scholar]
- 59.Eikenboom JC, Castaman G, Kamphuisen PW, Rosendaal FR, Bertina RM. The factor VIII/von Willebrand factor ratio discriminates between reduced synthesis and increased clearance of von Willebrand factor. Thromb Haemost. 2002;87:252–7. [PubMed] [Google Scholar]
- 60.The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicenter study. Contraception. 2003;67:173–85. doi: 10.1016/s0010-7824(02)00476-6. [DOI] [PubMed] [Google Scholar]
- 61.Vossen CY, Hasstedt SJ, Rosendaal FR, Callas PW, Bauer KA, Broze GJ, Hoogendoorn H, Long GL, Scott BT, Bovill EG. Heritability of plasma concentrations of clotting factors and measures of a prethrombotic state in a protein C-deficient family. J Thromb Haemost. 2004;2:242–7. doi: 10.1111/j.1538-7933.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- 62.Rudez G, Meijer P, Spronk HM, Leebeek FW, ten Cate H, Kluft C, de Maat MP. Biological variation in inflammatory and hemostatic markers. J Thromb Haemost. 2009;7:1247–55. doi: 10.1111/j.1538-7836.2009.03488.x. [DOI] [PubMed] [Google Scholar]
- 63.Watala C, Golanski J, Kardas P. Multivariate relationships between international normalized ratio and vitamin K-dependent coagulation-derived parameters in normal healthy donors and oral anticoagulant therapy patients. Thromb J. 2003;1:1–7. doi: 10.1186/1477-9560-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahashi H, Wada K, Satoh N, Takakuwa E, Furuta R, Yoshino N, Shibata A. Evaluation of oral anticoagulant therapy by measuring plasma prothrombin fragment 1 + 2. Blood Coagul Fibrinolysis. 1993;4:435–9. doi: 10.1097/00001721-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Brummel KE, Paradis SG, Branda RF, Mann KG. Oral anticoagulation thresholds. Circulation. 2001;104:2311–7. doi: 10.1161/hc4401.098492. [DOI] [PubMed] [Google Scholar]
- 66.Torn M, Rosendaal FR. Oral anticoagulation in surgical procedures: risks and recommendations. Br J Haematol. 2003;123:676–82. doi: 10.1046/j.1365-2141.2003.04652.x. [DOI] [PubMed] [Google Scholar]


