Abstract
Conventional anticoagulants have proven efficacy in the management of thromboembolism. Their adverse effects and a narrow therapeutic window, necessitating regular need for monitoring, however, have long been an incentive for the development of safer anticoagulants without compromising efficacy. Over the last decade or so several new parenteral and oral anticoagulants have been launched with efficacy comparable with conventional agents. From fondaparinux to its long acting derivative idraparinux, and the factor Xa inhibitor rivaroxaban to the direct thrombin inhibitor dabigatran, the advent of new anticoagulants is radically changing anticoagulation. For conventional anticoagulants, despite their shortcomings, effective methods of reversing their anticoagulant effects exist. Moreover, strategies to deal with the occurrence of fresh thrombotic events in the face of therapeutic anticoagulation with the conventional agents have also been addressed. Nevertheless, for the new anticoagulants, the optimal management of these complications remains unknown. This review explores these issues in the light of current evidence.
Keywords: bleeding complications, dabigatran, new anticoagulants, rivaroxaban, treatment failure
Introduction
The ever increasing armamentarium of therapeutic modalities has resulted in new challenges, and at some junctures, given us more questions than answers. It is inevitable that the stupendous advancements in the field of haemostasis and thrombosis will re-shape the world of coagulation medicine. This premise is a challenge as well as a reproach, and thus constitutes a rationale for addressing the current management of thrombotic problems, while at the same time inviting approaches for forthcoming demands. In recent years, several novel anticoagulants have been developed. While these agents appear advantageous over conventional anticoagulants, the quest for ‘the ideal anticoagulant’ remains far from over. In addition to being fully effective in preventing and treating thromboembolism an ‘ideal anticoagulant’ should also not cause any bleeding. Its anticoagulant effect should be predictable and thus obviate monitoring of coagulation parameters (Table 1). While offering a number of advantages over conventional anticoagulants the new anticoagulants continue to be associated with a significant incidence of treatment failure and bleeding. An effective approach to deal with these has not yet been developed. With the exception of idrabiotoparinux no specific antidote exists for reversing these agents. Likewise, the optimal management of new or recurrent thrombosis while on therapeutic anticoagulation is unclear. The aim of this review is to discuss possible management strategies for these problems in the light of existing evidence.
Table 1.
Characteristics of an ideal anticoagulant
| 100% efficacy in prevention and treatment of thromboembolism |
| Free of any bleeding complications |
| Oral and parenteral route of administration |
| No requirement for dose adjustment in any situation |
| No requirement for monitoring of anticoagulant effect |
| Cost effectiveness |
| No unexpected toxicity |
New anticoagulants – overview
From initiation of the coagulation cascade involving tissue factor through to culmination in the formation of fibrin, new strategies for anticoagulation have focused on targeting different activated clotting factors (Figure 1). Whatever the approach, the aim of anticoagulation remains limiting thrombin generation. A drug capable of restricting thrombin generation in a controlled fashion would appear to offer a ‘balanced’ way of anticoagulation.
Figure 1.
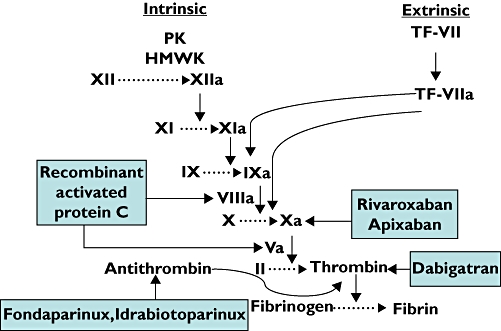
The traditional in vitro coagulation activation showing targets of new anticoagulants. PK = prekallikrein, HMWK = high molecular weight kininogens, TF-VII = tissue factor – factor VI complex, TF-VIIa = tissue factor – activated factor VII complex. Rivaroxaban and apixaban are direct activated factor X (Xa) inhibitors. Dabigatran is a direct thrombin inhibitor. Activated protein C inactivates activated factors VIII and V (VIIIa, Va). Fondaparinux and idrabiotoparinux do not inhibit any coagulation factor but potentiate the activity of antithrombin which, in turn antagonizes thrombin and Xa. Whether inactivation of an activated coagulation factor or potentiation of a naturally occurring anticoagulant (such as antithrombin), the net effect is to limit thrombin generation
The two main groups of new anticoagulants include direct inhibitors of activated factor X (FXa), and thrombin. Other novel strategies for anticoagulation include targeting the activated protein C (aPC) pathway. The physiological mechanism of protein C (PC) activation occurs by an intriguing pathway mediated by thrombin itself. In the microcirculation, thrombin complexes with a transmembrane endothelial glycoprotein, thrombomodulin. The resultant thrombin-thrombomodulin complex causes activation of PC which in association with its cofactor protein S causes proteolytic inactivation of activated factors V (FVa) and VIII (FVIIIa). Essentially this provides an anticoagulation mechanism through inhibition of thrombin generation [1]. As aPC does not completely abolish thrombin generation, the equilibrium of haemostasis achieved appears to be more favourable with a wider therapeutic window. Recombinant aPC has proven value for the treatment of coagulopathy in sepsis and is likely to find more applications. Yet another novel therapeutic method of activation of PC in vivo is by recombinant soluble thrombomodulin. In phase II trials a recombinant form of the extracellular domain of thrombomodulin has shown efficacy for the prevention of venous thromboembolism in total hip replacement surgery patients [2].
Tissue factor, activated factors IX and VII have all been targeted for inhibition to provide anticoagulation. The fact that the thrombin-thrombomodulin complex exerts an anticoagulant effect through activation of the PC pathway has led to engineering of thrombin with selective inhibition of its procoagulant activity [3]. The development of a mutant thrombin molecule with substrate affinity favouring PC effectively creates an intriguing mechanism for anticoagulation and has the potential to find applications where other anticoagulants may be less suitable.
The new parenteral anticoagulants
With all their limitations, heparins have remained the mainstay of offering immediate anticoagulation for more than five decades. Although the development of the synthetic pentasaccharide fondaparinux was a step forward, its parenteral route of administration, dosing frequency and haemostatic complications similar to unfractionated heparin (UFH) and low molecular heparins (LMWHs) [4, 5] limited its main advantage to scarcity of association with heparin induced thrombocytopenia [6]. Its long-acting derivative, idraparinux requiring only once weekly injections addressed the issue of dosing frequency but rather disappointingly failed to show non-inferiority to standard therapy in the treatment of pulmonary embolism [7]. Moreover, the very advantage of long half-life raised concerns about bleeding risk especially in the absence of a specific antidote. Recently, its biotynylated form, idrabiotaparinux, has been shown to have a similar time course of FXa inhibition, efficacy and safety to idraparinux for the treatment of deep venous thrombosis [8]. What is more reassuring is the ability to reverse its anticoagulant effect immediately and specifically by intravenous avidin [9]. Nevertheless, results of two trials show that idraparinux (or idrabiotaparinux) is far from reaching the elusive goal of an ideal anticoagulant [7, 10].
New oral anticoagulants
The direct thrombin inhibitor, ximelagatran, was hailed as a breakthrough in oral anticoagulation but had to be withdrawn due to the high incidence of hepatotoxicity [11]. Several oral anticoagulants with a much safer risk benefit profile have since been developed and have found place in clinical practice. Their mechanism of action is either through direct inhibition of FXa (rivaroxaban, apixaban and endoxaban) or thrombin (dabigatran etexilate and AZD0837). Over the past several years a number of randomized clinical trials have been performed comparing direct FXa and thrombin inhibitors with conventional anticoagulants in different clinical settings. Dabigatran, rivaroxaban and apixaban have all been evaluated in the prevention and treatment of thromboembolic disease including atrial fibrillation, deep vein thrombosis and pulmonary embolism. The main advantages of these agents over conventional anticoagulants include a wide therapeutic window, no requirement for monitoring and stable anticoagulation with little drug and dietary interactions. Whereas the arena of anticoagulation is set to change, important issues have emerged with the use of new agents and demand careful address for ensuring the safety and efficacy of anticoagulation. To understand the problems that may ensue with the use of new anticoagulants it is important to compare their biologic activities and parameters used for monitoring with the conventional anticoagulants.
Biologic activity, monitoring and interactions of conventional anticoagulants
Heparins
Unfractionated and low molecular weight heparins remain the mainstay of immediate management of thromboembolism. Even with intravenous dosing competitive binding of UFH by proteins in the plasma and those secreted by platelets and endothelial cells, causes a highly variable dose–response effect. The resultant narrow therapeutic window requires close laboratory monitoring of the anticoagulant effect of UFH. While monitoring of the anticoagulant effect of UFH is usually with activated partial thromboplastin time (aPTT), in certain situations of apparent heparin resistance aPTT may not reflect the actual degree of anticoagulation and monitoring should be with measurement of anti-Xa activity [12]. On the other hand, using thromboelastography and thrombin generation, Uprichard et al. have recently shown that aPTT may be a more accurate measure of anticoagulant effect of UFH in vivo than anti-Xa assay [13]. Whereas monitoring of LMWHs is not required routinely, anti-Xa assay, rather than aPTT, may be useful for optimizing dose in certain groups of patients such as the elderly, pregnant and in renal failure [14]. In addition to non-genetic factors affecting heparin treatment there is some evidence to suggest that interleukin-1 receptor antagonist polymorphisms may play a role in identifying patients that benefit from LMWH in non-ST-segment elevation acute coronary syndrome [15]. The usefulness of this finding in routine clinical practice awaits further evidence.
Vitamin K antagonists
Warfarin is the most commonly used vitamin K antagonist (VKA). Following oral ingestion it has a 100% bioavailability. It is mainly bound to albumin and its protein binding approaches 99.5%. As only the non-protein-bound fraction is biologically active, another drug binding to albumin can increase the biological activity of warfarin by competitively displacing it from albumin binding sites [16]. Moreover, with the metabolism of warfarin being mainly by the CYP2C9 hepatic microsomal system its in vivo activity is significantly influenced by induction of this enzyme system. While these two factors account for intra-individual variation in the anticoagulant activity, attention has recently focused on pharamacogenomics accounting for inter-individual variation in anticoagulant activity of vitamin K antagonists. Single nucleotide polymorphisms (SNPs) in cytochrome CYP2C9 affect warfarin half-life [17, 18] and SNPs in the gene that codes for vitamin K epoxide reductase (VKOR) determine warfarin sensitivity [19, 20], thus providing a genetic basis to account for the inter-individual variability of warfarin dose requirement.
For patients treated with VKAs laboratory monitoring is most commonly done by measuring the international normalized ratio (INR). Whereas the INR provides an instantaneous measure of the adequacy of anticoagulation in patients established on VKAs, the quality of anticoagulation over a prolonged period of time is assessed by estimating the total proportion of time that INR remains within a pre-determined range. Time-in-therapeutic range has been shown to correlate negatively with major bleeding and a previous meta-analysis by van Walraven et al. has shown that patients maintained on vitamin K antagonists were adequately anticoagulated for only 64% of the time [21]. More recently this has been confirmed for atrial fibrillation patients who seem to spend just one-half of the time within the therapeutic INR [22].
Complications, treatment failures and bleeding with heparins and vitamin K antagonists
Despite proven efficacy for the treatment of venous thromboembolic events (VTEs), the rate of recurrent thrombosis while on therapeutic doses of adjusted dose UFH or LMWH can reach 4% [23, 24]. Contrary to common perception of heparin preventing early recurrence of VTEs, achieving rapid therapeutic anticoagulation with heparin may reduce the recurrence of late VTEs [25]. Carrier et al. have recently shown that further recurrence of VTEs can occur in cancer patients receiving therapeutic dose LMWHs despite dose escalation by 20–25% [26]. At the same time significant bleeding can occur in 5% of patients treated with UFH or LMWHs [27, 28].
Recurrence of VTEs in the face of therapeutic anticoagulation with VKAs has been estimated to be between 2–4% at 3 months [27, 29].
Although influenced by patient characteristics and intensity of anticoagulation, the annual risk of major bleeding in patients with atrial fibrillation on vitamin K antagonists is significant. A meta-analysis of six published clinical trials revealed a rate of 1.8 per 100 patients-years of major bleeding in atrial fibrillation patients under 75 years of age and 3.2 per 100 patient-years in the older group [30].The Stroke Prevention in Atrial Fibrillation II study showed the rate of major bleeding in patients on warfarin to be 2.3% per year [31]. Although the limitations of conventional anticoagulants have long emphasised the need for a safer anticoagulant it is only recently that the quest has met some success, albeit partial. Overcoming some of the existing problems with heparins and VKAs, the new agents have raised new issues that require to be addressed.
Managing complications, treatment failure and bleeding due to conventional anticoagulants
Despite laboratory methods for effective monitoring of conventional anticoagulants, haemorrhagic complications and treatment failure with fresh thrombotic events are significant. Fortunately there are effective strategies for rapid and full reversal of UFH and VKAs. Administration of vitamin K, fresh frozen plasma and prothrombin complex concentrates effectively reverse the anticoagulant effect of VKAs. Protamine sulphate specifically neutralizes UFH although its effect on LMWH reversal may be partial depending on a number of characteristics [32]. On the other hand management of recurrent thrombosis in the face of therapeutic anticoagulation with warfarin remains a challenge. Current strategies include increasing the intensity of anticoagulation with a higher target INR, switching over to UFH/LMWH, or adding aspirin. Recurrence while on therapeutic LMWH is more challenging and is addressed by increasing the dose, changing the LMWH preparation or switching over to VKA.
Biologic activity, monitoring and interactions of new anticoagulants
Fondaparinux, idraparinux, and its biotynylated form, idrabiotaparinux, constitute the main new parenteral anticoagulants. Fondaparinux is a synthetic pentasaccharide that selectively inhibits factor Xa by binding to antithrombin. It induces a critical conformational change in antithrombin that potentiates the natural inhibitory effect of antithrombin against factor Xa by a factor of 300. Its half-life is about 17 h [33, 34].
Idraparinux is a hypermethylated analogue of fondaparinux. Due to a very high affinity for antithrombin its half-life is prolonged to ∼80 h. While this addressed the frequency of administration, concerns about excessive bleeding due to its long half-life led to the development of its biotynylated form, idrabiotaparinux. The rationale behind this modification is that avidin, which specifically binds biotin, can be administered intravenously to target and neutralize idrabiotaparinux [9].
New oral anticoagulants are poised to change radically the field of anticoagulation. With a mechanism of action distinct from VKAs these agents target a specific activated coagulation factor.
Dabigatran
Dabigatran is a potent direct thrombin inhibitor that selectively and reversibly inhibits both free and clot-bound thrombin. Unlike irreversible thrombin-binding drugs its anticoagulant effect is more predictable. In addition to preventing venous thrombosis, in vitro evidence suggests that dabigatran, by virtue of its inhibition of tissue factor-induced platelet aggregation, may be effective against arterial thrombosis [35]. As it is a highly polar zwitterion molecule, oral absorption does not occur [36]. Its prodrug dabigatran etexilate was developed specifically for this purpose and is absorbed rapidly with a median tmax of 2 h [37]. The conversion of dabigatran etexilate to dabigatran is by microsomal carboxylesterases [36]. The bioavailability of dabigatran after administration of its prodrug is about 7%, accounting for the high doses that are needed to maintain therapeutic plasma concentrations [36, 38]. Due to moderate tissue distribution it has a high volume of distribution of 60–70 l [39]. Dabigatran exhibits ∼35% binding to plasma proteins [38]. Its half-life is between 7–17 h with renal clearance accounting for 80% elimination [40]. These have practical therapeutic implications for managing haemostatic complications, as haemodialysis can be utilized for accelerating plasma clearance especially in renal impairment.
Dabigatran does not affect the CYP2C9 hepatic microsomal system, nor is it metabolized to any significant extent by these enzymes [36]. This renders interactions between dabigatran etexilate and drugs metabolized by these enzymes unlikely. Dabigatran is a substrate for the P-glycoprotein transporter system. However, co-administration of other P-glycoprotein substrates such as digoxin, while affecting area under the drug plasma concentration–time curve (AUC), has not been shown to result in a significant alteration of the coagulation parameters of aPTT, PT or ecarin clotting time (ECT) [41].
Due to the effect of dabigatran on thrombin-mediated conversion of fibrinogen to fibrin, it causes prolongation of all routine coagulation assays. Although the effect of increasing plasma concentrations of the drug is dissimilar on various coagulation tests the maximal effect on clotting parameters closely reflects the peak plasma concentrations, indicating that inhibition of thrombin is a direct effect linked to the central plasma compartment [42]. Peak anticoagulant effect is achieved within 2 h of oral administration of dabigatran etexilate and remains at ∼50% after 12 h. Unlike VKAs, interpretation of coagulation assays in patients taking dabigatran should be done in relation to the time of blood sampling to the administration of the dose.
While a single oral dose leads to rapid, dose-dependent prolongation in mean aPTT, PT, thrombin time (TT) and ECT, routine monitoring of the anticoagulant effect is not necessary. Within the range of plasma concentrations of dabigatran generally found in patients, aPTT is not a sensitive measure of anticoagulation, providing only a qualitative, and not a quantitative measure, of anticoagulant activity [43, 44]. PT, on the other hand, is little affected by dabigatran, with only modest elevations at therapeutic concentrations [40].
As with other direct thrombin inhibitors, the ECT gives a more precise description of the anticoagulant effect, being more sensitive and accurate than aPTT. It has been shown that the commercially available Hemoclot Thrombin Inhibitor Assay (Hyphen BioMed, France), may provide emergency laboratory coagulation assessment in the presence of dabigatran. This assay covers a wide concentration range (0–4000 nm) and calibration is possible with hirudin or lyophilized dabigatran [42]
Rivaroxaban
Factor Xa is positioned at the beginning of the common pathway of coagulation, and its inhibition, by limiting the amplification of coagulation that ensues in a progressive fashion down the clotting cascade, may achieve more effective anticoagulation [45]. While heparins and fondaparinux exhibit an indirect anti-FXa activity, rivaroxaban, apixaban and endoxaban are direct FXa inhibitors.
Rivaroxaban is a highly selective, reversible, oral direct FXa inhibitor. It inhibits prothrombinase-bound and clot-associated FXa [46]. Following oral administration its bioavailability is 80% with peak plasma concentrations occurring between 2.5 to 4 h [47]. Plasma protein binding, mainly with albumin, is high at approximately 95%. The mean terminal half-life is 3.2–11 h. In humans, the elimination of rivaroxaban exhibits a dual mode. Renal and faecal eliminations of the unchanged drug account for almost half of the excretion of a dose. The other half undergoes metabolic transformation by CYP3A4 in the CYP450 system and also via CYP-independent mechanisms. Rivaroxaban is also a substrate for the transporter protein P-glycoprotein and breast cancer resistance protein [48]. Co-administration of rivaroxaban with strong CYP3A4 inducers such as rifampicin may lead to decreased rivaroxaban concentration and should be done with caution. With the exception of strong CYP3A4 and P-glycoprotein inhibitors, such as azole antimycotics or human immunodeficiency protease inhibitors, no clinically significant drug interactions are known to occur with rivaroxaban [49].
Rivaroxaban causes prolongation of PT and aPTT in a concentration dependent fashion and exhibits a greater sensitivity for PT [50]. These clotting times are, however, not useful for measuring the pharmacodynamic effects of rivaroaxaban, as the composition of the reagents utilized influences the reactivity of rivaroxaban. Moreover, the conversionof PT values in seconds to INR, cannot be applied to rivaroxaban as this parameter is only calibrated and validated for vitamin K antagonists [51, 52]. Although monitoring of coagulation parameters is not indicated routinely during treatment with rivaroxaban a quantitative determination of plasma concentration of rivaroxaban may be needed in certain situations such as in severe overdose or evaluation of compliance. While a modified PT could be used for the measurement of rivaroxaban it is not specific [51]. More recently an anti-Factor Xa assay that uses rivaroxaban-containing plasma calibrators has been developed and may provide the optimal method for determining plasma rivaroxaban concentrations [53].
Complications and treatment failures with new anticoagulants
Based on results of large phase III trials for prevention of VTE in relation to knee or hip arthroplasty, dabigatran and rivaroxaban have been approved for thromoboprophylaxis in these settings [54–58]. In randomized trials these agents have been compared with warfarin for the prevention of stroke and systemic thromboembolism in patients with AF [59, 60], and dabigatran has been approved for this indication in the USA, Canada and Japan. Additionally, dabigatran and rivaroxaban have been shown to be effective for the treatment of acute symptomatic VTE in randomized trials [61, 62]. Whereas these studies have established the efficacy of these agents in prevention and treatment of thromboembolism, the primary efficacy outcome of stroke, systemic embolism and recurrent VTE and the principal safety outcome of major bleeding have not been insignificant.
Randomized trials comparing the efficacy of dose adjusted warfarin to dabigatran [59] and rivaroxaban [60] for the prevention of stroke and systemic embolism in atrial fibrillation showed the primary outcome of stroke or systemic embolism per year was 1.54% in the lower dose dabigatran group, 1.11% in the higher dose dabigatran group [59] and 2.12% in the rivaroxaban group [60]. At the same time major bleeding events were comparable with warfarin in both studies [59, 60]. In the RE-COVER study comparing the efficacy of dabigatran with warfarin in the treatment of acute symptomatic VTE, 2.4% of patients receiving dabigatran had recurrent VTE as compared with 2.1% receiving warfarin. Major bleeding episodes were noted in 1.6% patients who received dabigatran and 1.9% in the warfarin group [61]. In a recently reported study comparing rivaroxaban with standard treatment (enoxaprin-VKA) for the treatment of acute symptomatic DVT the incidence of recurrent VTE was 2.1% in the rivaroxaban group and 3.0% in the standard treatment group. Major bleeding occurred in 0.8% and 1.2% in rivaroxaban and standard treatment group respectively [62]. Encouraging as these may seem the results raise important issues that need to be addressed.
Managing complications and treatment failure of new anticoagulants
New thrombotic events
If adequately anticoagulated, the occurrence of thromboembolism (whether primary or recurrent) constitutes failure of anticoagulation. For conventional anticoagulants several therapeutic strategies, with variable success have been employed in this situation. The optimal management of such an event occurring with the new anticoagulants is unclear and the options include the following:
Dose escalation. The risk of VTE appears to be low with higher doses of dabigatran. This was first demonstrated in the BISTRO-I trial [63] and later confirmed in the BISTRO-II trial [64] when a significant dose-dependent decrease in VTE was observed with increasing doses of dabigatran. In the RENOVATE trial the primary efficacy outcome of total VTE and all cause mortality was 6.0% in the dabigatran etexilate 220 mg group vs. 8.6% in the dabigatran etexilate 150 mg group [54]. Similar results were echoed in the RE-MODEL trial, with primary efficacy outcome (composite of total VTE, venographic or symptomatic, and mortality during treatment) occurring in 36.4% of the dabigatran etexilate 220 mg group and 40.5% of the 150 mg group [55]. The RE-LY trial also showed fewer thromboembolic events with higher dose dabigatran vs. lower dose [59]. While there is no evidence for efficacy of higher doses in cases of thrombotic events occurring while on treatment with dabigatran, a reciprocal relationship between dose and primary thrombotic events invites consideration of this approach. On the contrary, phase II trials showed no correlation of the primary efficacy endpoint of VTE and all-cause mortality with the dose of rivaroxaban [65, 66] and therefore dose escalation may not be effective in patients treated with rivaroxaban who develop a thrombotic event.
Switching over to an alternative anticoagulant. While this approach has some validity to address treatment failure with conventional anticoagulants its efficacy is not known in patients treated with the new oral anticoagulants. So far there has been no head-to-head comparison between dabigatran and rivaroxaban. What value substitution of rivaroxaban for dabigatran and vice versa will have when one agent fails is currently unknown and no recommendations can be made at present. Intriguingly, a recent meta-analysis and adjusted indirect comparison of dabigatran and rivaroxaban has suggested rivaroxaban to be superior to dabigatran in preventing VTE (RR 0.50, 95% CI 0.37–1.64) albeit at the expense of a higher bleeding tendency [67]. Switching over to conventional anticoagulants (vitamin K antagonists or heparins – UFH or LMW) is another option but is hampered by absence of evidence at the present time. This option will also raise interesting questions about the optimal target aPTT, INR or anti-Xa assay should a switch be made to one of the conventional anticoagulants.
Adding an antiplatelet agent. Addition of aspirin to warfarin has been advocated to reduce subsequent cardiovascular events in patients with antiphospholipid syndrome [68, 69]. The value of this approach is not known in patients treated with the new oral anticoagulants.
Other theoretical options include concomitant use of an oral vitamin K antagonist, LMWH or even combining dabigatran and rivaroxaban.
The use of pharmacogenomics, with the rationale of finding the optimal dose of warfarin, and thus preventing haemorrhagic complications, is well described. Likewise, this approach may also have some utility for identifying a subgroup of patients likely to benefit from treatment with LMWHs in non-ST segment acute coronary syndromes. Similar approaches do not yet complement treatment with new anticoagulants and the main issue is reversing the anticoagulant effect.
Reversing the anticoagulant effect of the new anticoagulants
The incidence of bleeding with anticoagulants remains significant with its attendant morbidity and mortality [70–72]. Reversal of the anticoagulant effect is necessary not only for management of acute bleeding but also to allow for elective and emergency invasive procedures. Unlike heparins and VKAs, reversal of the new anticoagulants is fraught with challenges. With the exception of idrabiotaparinux there is no specific antidote available for reversal of the new parenteral or oral anticoagulants.
Elective surgery
For patients undergoing surgical interventions temporary cessation of anticoagulation should be considered. Dabigatran should be stopped at least 24 h prior to elective surgery. In patients with normal renal function, this allows the plasma dabigatran concentrations to fall to 25% of the steady-state trough concentrations, with a decrease to 5–10% at 48 h. Depending on the nature of surgery, renal function, and individual bleeding risk it may be necessary to discontinue dabigatran 2–4 days prior to surgery [42].
Although no specific information is available about discontinuation of rivaroxaban prior to elective surgery, based on half-life studies a 24 h gap would seem appropriate. As neither directly measured plasma concentrations nor PT prolongation in seconds predict bleeding in an individual patient treated with rivaroxaban, there is little utility of coagulation tests for assessment of haemostasis prior to surgery in patients taking rivaroxaban [50, 73, 74].
Management of overdose
Although there is no in vivo evidence as yet, in vitro studies have shown oral activated charcoal could be used to neutralize recent ingestion of overdose quantities of dabigatran etexilate before it is absorbed in the gut. In addition, dabigatran could theoretically be adsorbed from plasma via haemoperfusion over a charcoal filter [75]. Although the use of activated charcoal has also been suggested for managing overdose of rivaroxaban [49], the approach is not evidence-based yet.
Management of bleeding
As they have a relatively short half-life, discontinuation of dabigatran and rivaroxaban results in a fairly quick drop in plasma concentrations and is sufficient for mild bleeding. Management of moderate to severe bleeding includes general supportive measures with fluid and blood product replacement.
Oral activated charcoal should be given if less than 2 h have elapsed since the last dose of dabigatran. Because of the predominant renal excretion of dabigatran adequate diuresis should be ensured, and if these measures prove ineffective haemodialysis should be considered.
There is no specific antidote for antagonizing the effect of dabigatran or rivaroxaban. In the event of life-threatening bleeding, patients on dabigatran should be considered for charcoal filtration, although this recommendation is based on limited non-clinical data [42].
Recombinant factor VIIa (rFVIIa) has been used for reversal of new parenteral anticoagulants, including LMWHs, fondaparinux, idraparnux and direct thrombin inhibitors [76]. However, the evidence is limited to case reports and small laboratory investigations. Findings have also been inconsistent for reversal of the anticoagulant effect of melagatran, the active compound of ximelagatran [77, 78]. At present there are no published clinical data on the use of rFVIIa for reversal of the new oral anticoagulants. van Ryn et al. have shown rFVIIa to be effective in preserving haemostasis in a rat tail model of template bleeding due to high dose of dabigatran [79]. While a wide dose range of 20–120 µg kg–1 has been used for reversing the anticoagulant effect of parenteral anticoagulants, no specificications for the dose of rFVIIa for the reversal of the new oral anticoagulants can be made at the present time.
Prothrombin complex concentrates (PCCs) have a well established role for antagonizing the effect of VKAs [80]. Likewise, activated prothrombin complex concentrates (APCCs) have been successfully used in haemophilia patients with inhibitory antibodies [81]. PCCs and APCCs have both been shown ex vivo to reduce significantly prolongation of bleeding time associated with high dose dabigatran [43, 79].
In the absence of alternative therapies the use of rFVIIa, PCC or APCC should be considered for the management of severe and life-threatening bleeding in patients receiving the new oral anticoagulants.
Antagonizing the anticoagulant activity of direct factor Xa inhibitors may be possible through administration of activated factor X (FXa). A truncated form of catalytically inactive FXa that maintains high affinity for FXa inhibitors has shown, in vivo and in vitro, to reverse dose-dependently FXa inhibitory activity in plasma, as measured by anti-FXa units, tissue factor-initiated thrombin generation and clotting assays [82, 83].
New anticoagulants and neuraxial anaesthesia
Like other anticoagulants, regional anaesthesia in patients receiving full therapeutic doses of new oral anticoagulants is contraindicated [84]. Catheter manipulation and removal carry similar risks to insertion and should be timed to take place at the nadir of the concentration of the anticoagulant. Based on the fact that only 25% of the active drug remains after two half-lives have elapsed, Rosencher et al. recommend delaying removal of a neuraxial catheter until at least after this period of time. As the formation of a stable clot takes 8 h the authors propose restarting anticoagulation to be left until 8 h minus the time to reach maximum activity (tmax). The approach appears to be a reasonable compromise between the risk of bleeding and VTE [85].
Conclusion
The new anticoagulants have obvious advantages over conventional agents, including rapid onset of action, predictable therapeutic effect, lack of requirement for monitoring and limited interactions with other drugs. While emerging clinical evidence continues to foster the confidence of physicians in their use, new questions have surfaced that need careful address.
Potential advantages may turn out to be disadvantageous in some patients. For example, a short half-life may only require cessation of drug for the management of haemorrhagic complication but at the same time it demands strict adherence to the drug for clinical efficacy. Similarly, the lack of requirement of monitoring seems extremely attractive. However, not only does it prevent tailoring of the intensity of anticoagulation but also makes determination of the adequacy of anticoagulation unreliable.
Unlike heparins and VKAs, no specific antidote is as yet available for the reversal of the anticoagulant effect of any of the new anticoagulants.
With the emergence of further novel anticoagulants the arena of haemostasis will continue to witness dynamic changes.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Clouse LH, Comp PC. The regulation of hemostasis: the protein C system. N Engl J Med. 1986;314:1298–304. doi: 10.1056/NEJM198605153142006. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Comp P, Douketis J, Royds R, Yamada K, Gent M. Dose-response study of recombinant human soluble thrombomodulin (ART-123) in the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2005;3:962–8. doi: 10.1111/j.1538-7836.2005.01251.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsiang M, Paborsky LR, Li WX, Jain AK, Mao CT, Dunn KE, Lee DW, Matsumura SY, Matteucci MD, Coutré SE, Leung LL, Gibbs CS. Protein engineering thrombin for optimal specificity and potency of anticoagulant activity in vivo. Biochemistry. 1996;35:16449–57. doi: 10.1021/bi9616108. [DOI] [PubMed] [Google Scholar]
- 4.Büller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg-Segers AE, Cariou R, Leeuwenkamp O, Lensing AW, Matisse Investigators Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 5.Büller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, Segers AE, Cariou R, Leeuwenkamp O, Lensing AW, Matisse Investigators Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–73. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin TE, Cook RJ, Marder VJ, Sheppard JA, Moore JC, Eriksson BI, Greinacher A, Kelton JG. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791–6. doi: 10.1182/blood-2005-05-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gogh Investigators. Buller HR, Cohen AT, Davidson B, Decousus H, Gallus AS, Gent M, Pillion G, Piovella F, Prins MH, Raskob GE. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med. 2007;357:1094–104. doi: 10.1056/NEJMoa064247. [DOI] [PubMed] [Google Scholar]
- 8.Equinox Investigators. Efficacy and safety of once weekly subcutaneous idrabiotaparinux in the treatment of patients with symptomatic deep venous thrombosis. J Thromb Haemost. 2011;9:92–9. doi: 10.1111/j.1538-7836.2010.04100.x. doi: 10.1111/j.1538-7836.2010.04100.x. [DOI] [PubMed] [Google Scholar]
- 9.Paty I, Trellu M, Destors JM, Cortez P, Boëlle E, Sanderink G. Reversibility of the anti-FXa activity of idrabiotaparinux (biotinylated idraparinux) by intravenous avidin infusion. J Thromb Haemost. 2010;8:722–9. doi: 10.1111/j.1538-7836.2010.03746.x. [DOI] [PubMed] [Google Scholar]
- 10.Amadeus Investigators. Bousser MG, Bouthier J, Büller HR, Cohen AT, Crijns H, Davidson BL, Halperin J, Hankey G, Levy S, Pengo V, Prandoni P, Prins MH, Tomkowski W, Torp-Pedersen C, Wyse DG. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet. 2008;371:315–21. doi: 10.1016/S0140-6736(08)60168-3. [DOI] [PubMed] [Google Scholar]
- 11.Testa L, Bhindi R, Agostoni P, Abbate A, Zoccai GG, van Gaal WJ. The direct thrombin inhibitor ximelagatran/melagatran: a systematic review on clinical applications and an evidence based assessment of risk benefit profile. Expert Opin Drug Saf. 2007;6:397–406. doi: 10.1517/14740338.6.4.397. [DOI] [PubMed] [Google Scholar]
- 12.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:141S–59S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 13.Uprichard J, Manning RA, Laffan MA. Monitoring heparin anticoagulation in the acute phase response. Br J Haematol. 2010;149:613–9. doi: 10.1111/j.1365-2141.2010.08129.x. [DOI] [PubMed] [Google Scholar]
- 14.Baglin T, Barrowcliffe TW, Cohen A, Greaves M, British Committee for Standards in Haematology Guidelines on the use and monitoring of heparin. Br J Haematol. 2006;133:19–34. doi: 10.1111/j.1365-2141.2005.05953.x. [DOI] [PubMed] [Google Scholar]
- 15.Ray KK, Francis S, Crossman DC. A potential pharmacogenomic strategy for anticoagulant in non-ST-segment elevation acute coronary syndromes: the role of interleukin-1 receptor antagonist genotype. J Thromb Haemost. 2005;3:287–91. doi: 10.1111/j.1538-7836.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 16.Yacobi A, Udall JA, Levy G. Serum protein binding as a determinant of warfarin body clearance and anticoagulant effect. Clin Pharmacol Ther. 1976;19(5 Pt 1):552–8. doi: 10.1002/cpt1976195part1552. [DOI] [PubMed] [Google Scholar]
- 17.Shikata E, Ieiri I, Ishiguro S, Aono H, Inoue K, Koide T, Ohgi S, Otsubo K. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma-glutamyl carboxylase) gene variants with warfarin sensitivity. Blood. 2004;103:2630–5. doi: 10.1182/blood-2003-09-3043. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, Kim RB, Roden DM, Stein CM. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, Grandone E, Margaglione M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–9. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 20.Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–33. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 21.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and meta-regression. Chest. 2006;129:1156–66. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 22.Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15:244–52. doi: 10.18553/jmcp.2009.15.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells PS, Anderson DR, Rodger MA, Forgie MA, Florack P, Touchie D, Morrow B, Gray L, O'Rourke K, Wells G, Kovacs J, Kovacs MJ. A randomized trial comparing two low-molecular-weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 2005;165:733–8. doi: 10.1001/archinte.165.7.733. [DOI] [PubMed] [Google Scholar]
- 24.Girard P, Mathieu M, Simonneau G, Petitpretz P, Cerrina J, Herve P, Rosso J, Musset D, Mensch J, Duroux P. Recurrence of pulmonary embolism during anticoagulant treatment: a prospective study. Thorax. 1987;42:481–6. doi: 10.1136/thx.42.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein PD, Hull RD, Matta F, Yaekoub AY. Anticoagulant therapy for acute venous thromboembolism: what we think we know and what the data show for the timing of recurrent events. Clin Appl Thromb Hemost. 2009;15:609–12. doi: 10.1177/1076029609349500. [DOI] [PubMed] [Google Scholar]
- 26.Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760–5. doi: 10.1111/j.1538-7836.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 27.Juergens CP, Semsarian C, Keech AC, Beller EM, Harris PJ. Hemorrhagic complications of intravenous heparin use. Am J Cardiol. 1997;80:150–4. doi: 10.1016/s0002-9149(97)00309-3. [DOI] [PubMed] [Google Scholar]
- 28.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, ADVANCE-3 Investigators Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 29.Hull R, Hirsh J, Jay R, Carter C, England C, Gent M, Turpie AG, McLoughlin D, Dodd P, Thomas M, Raskob G, Ockelford P. Different intensities of oral anticoagulant therapy in the treatment of proximal-vein thrombosis. N Engl J Med. 1982;307:1676–81. doi: 10.1056/NEJM198212303072704. [DOI] [PubMed] [Google Scholar]
- 30.van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, Koudstaal PJ, Chang Y, Hellemons B. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 31.The Stroke Prevention in Atrial Fibrillation Investigators. Bleeding during antithrombotic therapy in patients with atrial fibrillation. Arch Intern Med. 1996;156:409–16. [PubMed] [Google Scholar]
- 32.Crowther MA, Berry LR, Monagle PT, Chan AK. Mechanisms responsible for the failure of protamine to inactivate low-molecular-weight heparin. Br J Haematol. 2002;116:178–86. doi: 10.1046/j.1365-2141.2002.03233.x. [DOI] [PubMed] [Google Scholar]
- 33.Turpie AG, Gallus AS, Hoek JA, Pentasaccharide Investigators A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med. 2001;344:619–25. doi: 10.1056/NEJM200103013440901. [DOI] [PubMed] [Google Scholar]
- 34.Olson ST, Björk I, Sheffer R, Craig PA, Shore JD, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992;267:12528–38. [PubMed] [Google Scholar]
- 35.van Ryn J, Kink-Eiband M, Hauel N. Effects of dabigatran, a direct thrombin inhibitor, as compared to the direct factor Xa inhibitors, rivaroxaban and apixaban, on tissue factor-induced human platelet aggregation in platelet rich plasma. Blood. 2007;110:558a. [abstract no. 1884] [Google Scholar]
- 36.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–95. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 37.Stangier J, Eriksson BI, Dahl OE. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–63. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- 38.Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–99. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- 39.Sanford M, Plosker GL. Dabigatran etexilate. Drugs. 2008;68:1699–709. doi: 10.2165/00003495-200868120-00007. [DOI] [PubMed] [Google Scholar]
- 40.Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stangier J, Stähle H, Rathgen K, Reseski K, Körnicke T. No interactions of the oral direct thrombin inhibitor dabigatran etexilate and digoxin. J Thromb Haemost. 2007;5(Suppl. 2) P-W-672. [Google Scholar]
- 42.van Ryn J, Stangier J, Haertter S, Liesenfeld K-H, Wienen W, Feuring M, Clemens A. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson SC, Mattsson C, Eriksson UG, Sarich TC, Wåhlander K, Eliasson A, Karlson BW, Sheth SB, Held P. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res. 2005;115:9–18. doi: 10.1016/j.thromres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Walenga JM, Jeske WP, Hoppensteadt D, Fareed J. Factor Xa inhibitors: today and beyond. Curr Opin Investig Drugs. 2003;4:272–81. [PubMed] [Google Scholar]
- 46.Depasse F, Busson J, Mnich J, Le Flem L, Gerotziafas GT, Samama MM. Effect of BAY 59-7939 – a novel, oral, direct factor Xa inhibitor – on clot-bound factor Xa activity in vitro. J Thromb Haemost. 2005;3(Suppl. 1) Abstract number: P1104. [Google Scholar]
- 47.Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–80. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 48.Weinz CT, Kubitza SD, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37:1056–64. doi: 10.1124/dmd.108.025569. [DOI] [PubMed] [Google Scholar]
- 49.Xarelto. Summary of product characteristics. Available at: http://www.xarelto.com/html/downloads/Xarelto_Summary_of_Product_Characteristics_May2009.pdf (last accessed 8 April 2011)
- 50.Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, Straub A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 – an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21. doi: 10.1111/j.1538-7836.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 51.Samama MM, Martinoli JL, LeFlem L. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–25. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 52.Smith SA, Morrissey JH. Thromboplastin composition affects the sensitivity of prothrombin time (PT) clotting tests to direct Factor Xa inhibitors. Blood. 2007;110 Abstract 928. [Google Scholar]
- 53.Samama MM, Amiral J, Guinet C, Perzborn E, Depasse F. An optimised, rapid chromogenic assay, specific for measuring direct factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104:1078–9. doi: 10.1160/TH10-03-0204. [DOI] [PubMed] [Google Scholar]
- 54.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 55.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Büller HR, RE-MODEL Study Group Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 56.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W. RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 57.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG. RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 58.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–9. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 59.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 60.Off orbit? ROCKET AF: rivaroxaban noninferior to warfarin, but superiority analyses at odds. Available at: http://www.theheart.org/article/1148785.do (last accessed 27 March 2011)
- 61.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ, RE-COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 62.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 63.Eriksson BI, Dahl OE, Ahnfelt L, Kälebo P, Stangier J, Nehmiz G, Hermansson K, Kohlbrenner V. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost. 2004;2:1573–80. doi: 10.1111/j.1538-7836.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- 64.Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kälebo P, Reilly P, BISTRO II Study Group A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–11. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 65.Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kälebo P, Misselwitz F, Gent M, OdiXa-Knee Study Group BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost. 2005;3:2479–86. doi: 10.1111/j.1538-7836.2005.01602.x. [DOI] [PubMed] [Google Scholar]
- 66.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Muehlhofer E, Kälebo P. Dose-escalation study of rivaroxaban (BAY 59-7939) – an oral, direct Factor Xa inhibitor – for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res. 2007;120:685–93. doi: 10.1016/j.thromres.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 67.Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism – systematic review and adjusted indirect comparison. J Clin Pharm Ther. 2011;36:111–24. doi: 10.1111/j.1365-2710.2010.01162.x. [DOI] [PubMed] [Google Scholar]
- 68.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002;360:109–13. doi: 10.1016/S0140-6736(02)09409-6. [DOI] [PubMed] [Google Scholar]
- 69.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–74. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 70.Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–6. doi: 10.1016/j.amjcard.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 71.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 72.Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB, III, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–8. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 73.Perzborn E, Roehrig S, Straub A, Kubitza D, Mueck W, Laux V. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler Thromb Vasc Biol. 2010;30:376–81. doi: 10.1161/ATVBAHA.110.202978. [DOI] [PubMed] [Google Scholar]
- 74.Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F. The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov. 2011;10:61–75. doi: 10.1038/nrd3185. [DOI] [PubMed] [Google Scholar]
- 75.van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. [Abstract no 1065]. In: 51st ASH Annual Meeting and Exposition[website]. New Orleans (LA): American Society of Hematology; 2009. Available at: http://ash.confex.com/ash/2009/webprogram/Paper21383.html (last accessed 8 April 2011)
- 76.Young G, Yonekawa KE, Nakagawa PA, Blain RC, Lovejoy AE, Nugent DJ. Recombinant activated factor VII effectively reverses the anticoagulant effects of heparin, enoxaparin, fondaparinux, argatroban, and bivalirudin ex vivo as measured using thromboelastography. Blood Coagul Fibrinolysis. 2007;18:547–53. doi: 10.1097/MBC.0b013e328201c9a9. [DOI] [PubMed] [Google Scholar]
- 77.Wolzt M, Levi M, Sarich TC, Boström SL, Eriksson UG, Eriksson-Lepkowska M, Svensson M, Weitz JI, Elg M, Wåhlander K. Effect of recombinant factor VIIa on melagatran-induced inhibition of thrombin generation and platelet activation in healthy volunteers. Thromb Haemost. 2004;91:1090–6. doi: 10.1160/TH03-09-0605. [DOI] [PubMed] [Google Scholar]
- 78.Sørensen B, Ingerslev J. A direct thrombin inhibitor studied by dynamic whole blood clot formation. Haemostatic response to ex-vivo addition of recombinant factor VIIa or activated prothrombin complex concentrate. Thromb Haemost. 2006;96:446–53. [PubMed] [Google Scholar]
- 79.van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant Factor VIIa or activated prothrombin complex concentrate. Haematologica. 2008;93(Suppl. 1):148. abstract 0370. [Google Scholar]
- 80.Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137–43. doi: 10.1002/ajh.21046. [DOI] [PubMed] [Google Scholar]
- 81.Astermark J, Rocino A, Von Depka M, Van Den Berg HM, Gringeri A, Mantovani LG, Morado M, Garrido RP, Schiavoni M, Villar A, Windyga J, EHTSB Current use of by-passing agents in Europe in the management of acute bleeds in patients with haemophilia and inhibitors. Haemophilia. 2007;13:38–45. doi: 10.1111/j.1365-2516.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 82.Lu G, DeGuzman F, Lakhotia S, Hollenbach SJ, Phillips DR, Sinha U. Recombinant antidote for reversal of anticoagulation by Factor Xa inhibitors. Blood. 2008;112 Abstract 983 (abstract) [Google Scholar]
- 83.Lu G, Luan P, Hollenbach S, Abe K, DeGuzman F, Siu G, Hutchaleelaha A, Inagaki M, Conley PB, Phillips DR, Sinha U. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors. J Thromb Haemost. 2009;7(Suppl. 2) Abstract OC-TH-107 (abstract) [Google Scholar]
- 84.Gogarten W, Vandermeulen E, Van Aken H, Kozek S, Llau JV, Samama CM, European Society of Anaesthesiology Regional anaesthesia and antithrombotic agents: recommendations of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2010;27:999–1015. doi: 10.1097/EJA.0b013e32833f6f6f. [DOI] [PubMed] [Google Scholar]
- 85.Rosencher N, Bonnet MP, Sessler DI. Selected new antithrombotic agents and neuraxial anaesthesia for major orthopaedic surgery: management strategies. Anaesthesia. 2007;62:1154–60. doi: 10.1111/j.1365-2044.2007.05195.x. [DOI] [PubMed] [Google Scholar]


