Abstract
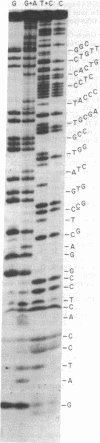
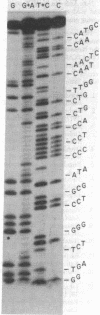
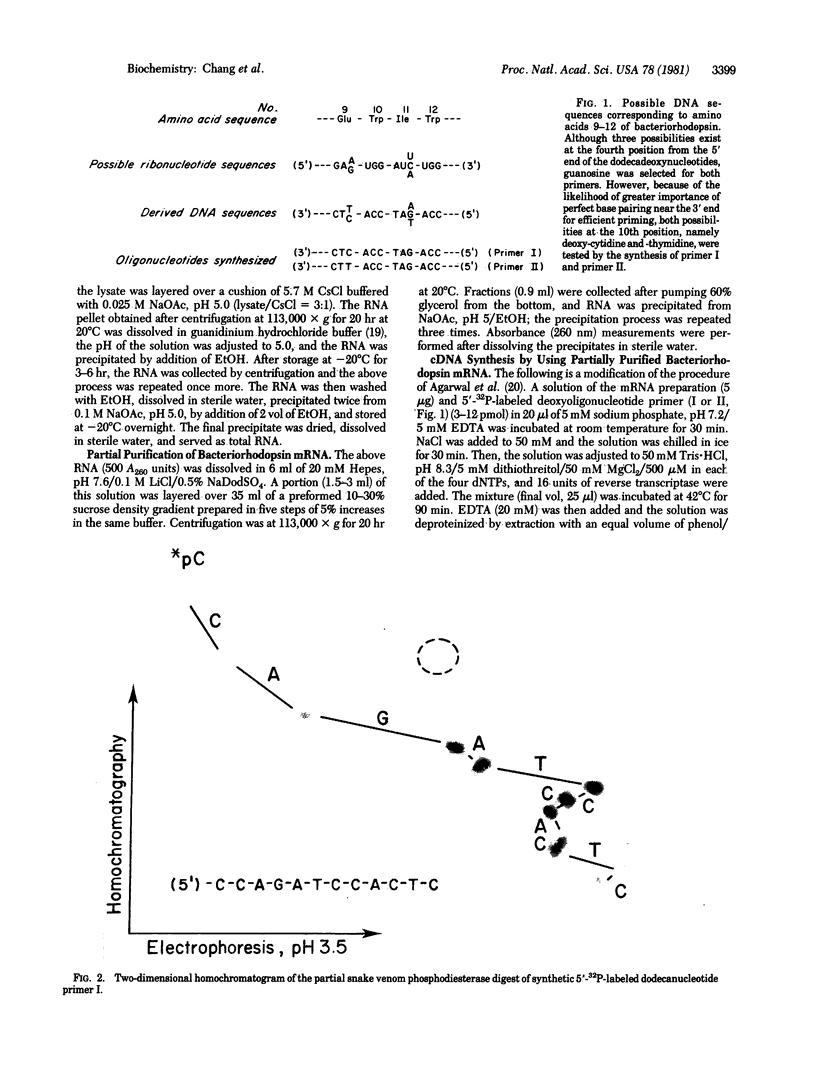
mRNA for bacteriorhodopsin from Halobacterium halobium has been partially purified. By using this mRNA as template in the presence of reverse transcriptase RNA-dependent DNA nucleotidyltransferase and a 5'-[32P] synthetic oligodeoxyribonucleotide corresponding to amino acids 9-12 of bacteriorhodopsin as primer, we have isolated the major 5'-[32P]cDNA product, approximately 80 nucleotides long, and determined its sequence. Based on the cDNA sequence, the 5'-proximal sequence of bacteriorhodopsin mRNA is G-C-A-U-G-U-U-G-G-A-G-U-U-A-U-U-G-C-C-A-A-C-A-G-C-A-G-U-G-G-A-G-G-G-G-G-U-A-U-C -G-C-A-G-G-C-C-C-A-G-A-U-C-A-C-C-G-G-A-C-G-U-C-C-G. This includes the expected sequence for amino acids 1-8 and shows that bacteriorhodopsin is synthesized as a precursor that is at least 13 amino acids longer (Met-Leu-Glu-Leu-Leu-Pro-Thr-Ala-Val-Glu-Gly-Val-Ser) at the NH2 terminus. Agarose/urea gel electrophoresis of the partially purified mRNA showed several bands; of these, a major one hybridized with 5'-[32P]cDNA. These results suggest that the bacteriorhodopsin mRNA in the partially purified preparation is homogeneous in size and that it constitutes a substantial portion of the RNA preparation subjected to electrophoresis.
Full text
PDF
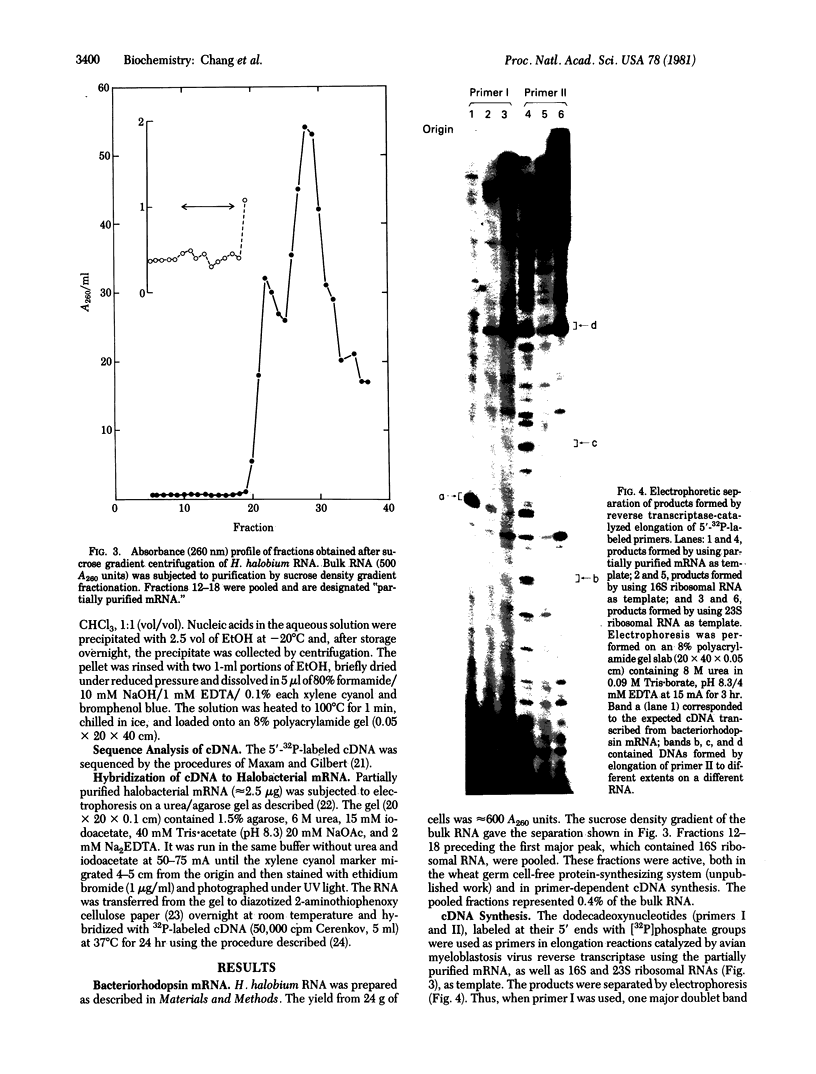


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Bhanot O. S., Khan S. A., Chambers R. W. A new system for studying molecular mechanisms of mutation by carcinogens. J Biol Chem. 1979 Dec 25;254(24):12684–12693. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dellweg H. G., Sumper M. Identification of a bacterio-opsin species with a N-terminally extended amino acid sequence. FEBS Lett. 1980 Jul 28;116(2):303–306. doi: 10.1016/0014-5793(80)80668-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber G. E., Gray C. P., Wildenauer D., Khorana H. G. Orientation of bacteriorhodopsin in Halobacterium halobium as studied by selective proteolysis. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5426–5430. doi: 10.1073/pnas.74.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Khorana H. G. Delipidation of bacteriorhodopsin and reconstitution with exogenous phospholipid. Proc Natl Acad Sci U S A. 1980 Jan;77(1):323–327. doi: 10.1073/pnas.77.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Phillips S., Edgell M. H., Gillam S., Jahnke P., Smith M. Mutagenesis at a specific position in a DNA sequence. J Biol Chem. 1978 Sep 25;253(18):6551–6560. [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Locker J. Analytical and preparative electrophoresis of RNA in agarose-urea. Anal Biochem. 1979 Oct 1;98(2):358–367. doi: 10.1016/0003-2697(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Razin A., Hirose T., Itakura K., Riggs A. D. Efficient correction of a mutation by use of chemically synthesized DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4268–4270. doi: 10.1073/pnas.75.9.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Jay E., Bahl C. P., Wu R. A reliable mapping method for sequence determination of oligodeoxyribonucleotides by mobility shift analysis. Anal Biochem. 1976 Jul;74(1):73–93. doi: 10.1016/0003-2697(76)90311-0. [DOI] [PubMed] [Google Scholar]