Abstract
Prostate cells contain specific receptors for 1α,25-dihydroxyvitamin D [1α,25(OH)2D] or calcitriol, the active form of vitamin D. 1α,25(OH)2D is known to inhibit the proliferation and invasiveness of prostate cancer cells. These findings support the use of 1α,25(OH)2D for prostate cancer therapy. However, 1α,25(OH)2D can cause hypercalcemia, analogs of 1α,25(OH)2D that are less calcemic but exhibit potent antiproliferative activity would be attractive as therapeutic agents. To accomplish these goals, different strategies, based on metabolism, molecular mechanism of actions, and structural modeling, have been taken to modify the structure of vitamin D molecule with the aims to improve the efficacy and decrease the toxicity of vitamin D to treat different diseases. During the past four decades, over 3,000 analogs have been synthesized. In this paper, we discuss the development and the biological analysis of a unique class of vitamin D analogs with a substitution at the carbon 2 of 19-nor-1α,25(OH)2D3 molecule for potential application to the prevention and treatment of prostate cancer as well as other cancers.
1. Introduction
Prostate cancer is the most prevalent nonskin cancer among older men, with 190,000 new cases in 2010, and the second leading cause of cancer deaths among US men after lung cancer with approximately 30,000 deaths projected in 2011 [1]. Androgen deprivation is a common practice for tumors that are ineligible for or fail to respond to surgery or radiation therapy. Although the majority of men respond to androgen deprivation, the median duration of response is only about 2 years [2]. Great efforts have been made to develop new therapies to prolong the survival of patients with prostate cancer that fails to response to androgen deprivation therapy, such as docetaxel- or carbazitaxel-based chemotherapy, immunotherapy with sipuleucel-T, or abiraterone acetate. However, their effectiveness is limited to only a few months gain in survival [3].
It is now well established that the growth of prostate cells is regulated not only by androgens but also by vitamin D. Human prostate cells express vitamin D receptor (VDR) for 1α,25-dihydroxyvitamin D [1α,25(OH)2D] (Figure 1), the active form of vitamin D [4]. Numerous reports have demonstrated that 1α,25(OH)2D stimulates differentiation and inhibits the proliferation, invasiveness, and metastasis of prostate cancer cells [4]. These findings strongly support the use of vitamin D-based therapies for prostate cancer treatment once androgen deprivation has failed. However, the results of early clinical trials using 1α,25(OH)2D3 (Calcitriol) indicated that the hormone caused serious hypercalcemic and hypercalciuric side effects [5, 6]. Therefore, analogs of 1α,25(OH)2D with less calcemic activity but with potent antiproliferative activity would be attractive agents.
Figure 1.

Structures of 1α,25(OH)2D3, 2α-3-hydroxypropoxy-1α,25(OH)2D3 (O2C3) and 19-nor-2α-3-hydroxypropyl-1α,25(OH)2D3 (MART-10).
2. History of the Synthesis of Vitamin D Analogs
Vitamin D was discovered a century ago because of an epidemic of a childhood bone disease, rickets [7]. It was concluded that the primary function of vitamin D in humans was to enhance the intestinal absorption of calcium and phosphate, two major ingredients of bones, to maintain their serum concentrations at a sufficient level to facilitate bone calcification and other cellular functions [8]. It was later realized that vitamin D itself was not active and required a series of hydroxylation steps first in the liver and then in the kidneys to form 25-hydroxyvitamin D [25(OH)D] and 1α,25(OH)2D, respectively, before it became active [8]. Although vitamin D was widely used in the 1940s to treat various forms of skin diseases, such as lupus vulgaris [9], cutaneous tuberculosis [10], and psoriasis [11], it was not until late 1970s that vitamin D receptors were shown to be present in many cells and tissues which were not known to be associated with calcium and phosphorus homeostasis [12]. Subsequently, it was shown that 1α,25(OH)2D was capable of inhibiting the growth of various types of cancer cells [13] and promoting the differentiation of promyelocytic leukemia cells (HL-60) to form mature macrophages [14]. The in vitro cell culture studies were followed by animal studies using xenograft mouse model and chemically induced cancer model to demonstrate the effectiveness of 1α,25(OH)2D in inhibiting the tumor cell growth [4, 15, 16]. However, 1α,25(OH)2D at high doses which were usually required to inhibit tumor cell growth in vivo animal models also raised serum calcium level and decreased the body weight of the animals [16]. In human clinical trials, hypercalcemia and hypercalciuria were also found to be the major side effects of 1α,25(OH)2D when it was administered systemically [5, 6]. To overcome these drawbacks, attempts have been made to synthesize vitamin D analogs that retain most of the nonclassical activities of 1α,25(OH)2D but have much lower calcemic activity in vivo. Several synthetic vitamin D analogs have been demonstrated to exert promising anticancer effects with reduced calcemic consequence and even greater antiproliferative activity than 1α,25(OH)2D [16]. Among those vitamin D analogs, Seocalcitol (EB1089, Leo Pharmaceutical Products) is one of the most studied synthetic analogs [17–19]. A considerable number of in vitro and in vivo studies have been carried out with EB1089 and show that the analog is more potent than 1α,25(OH)2D with respect to the regulation of cancer cell growth and differentiation, and the effect of EB1089 on calcium metabolism in vivo is approximately 50% less than that of 1α,25(OH)2D [16]. The anticancer effects of EB1089 were also demonstrated in vivo in a rat model of mammary gland carcinoma without inducing hypercalcemia [17, 18]. Similar effects were seen in an in vivo prostate cancer study where EB1089 inhibited prostate cancer cell proliferation and reduced tumorigenesis as well as tumor metastases [19]. Clinical trials with 1α-hydroxyvitamin D2 (Hectorol) marketed by Genzyme have been conducted in hormone refractory prostate cancer patients [20, 21]. In a phase I trial, the authors reported that Hectorol was well tolerated with the main toxicities being hypercalcemia and renal insufficiency, and the side effects were reversible with drug discontinuation [20]. In a follow-up phase II study, they observed disease stability >6 months in 30% of the patients and the median survival of 21 months, which is higher than the 17.7 months predicted by the survival nomogram for that patient group [21]. Although the results are less than conclusive, the encouraging findings do warrant further studies with vitamin D analogs. Other vitamin D analogs or structural VDR activators, such as Maxacalcitol (OCT) (Chugai Pharmaceutical Co. Ltd.) [22], 16-ene analogs (Hoffmann LaRoche, Inc.) [23], 19-nor analogs (Hoffmann LaRoche, Inc.) [24], 1α-hydroxyvitamin D5 [25], and LG190119 (Ligand Pharmaceuticals Inc.) [26], have been developed and tested in preclinical studies. These compounds may have promise as therapeutic agents for cancer and other diseases, with fewer side effects than 1α,25(OH)2D3 and 1α-hydroxyvitamin D2. Other vitamin D analogs which have shown some promising in vitro biological activities include C-20 cyclopropylcalcitriol [27], elocalcitol [28], Gemini vitamin D analogs [29].
3. Development of the Less Calcemic 19-Norvitamin D Analogs
19-Norvitamin D compounds in which the ring A methylene group on C-19 is replaced with two hydrogen atoms were known to be in existence in 1983 when 19-nor-10-ketovitamin D derivatives were first isolated and identified from a mixture of vitamin D or 25(OH)D solution incubated with bovine rumen microbes [30]. Later, 19-nor-1α,25(OH)2D3 was synthesized by Perlman et al. to study the structure-activity relationship of 1α,25(OH)2D3 molecule [31]. They reported that this analog induced the differentiation of human leukemia HL-60 cells similar to 1α,25(OH)2D3 with little or no calcemic activity [31]. The findings led to the synthesis of 19-nor-1α,25(OH)2D2 and the evaluation of its activity by Slatopolsky et al. [32]. Both 19-nor-1α,25(OH)2D2 and 19-nor-1α,25(OH)2D3 have potency similar to 1α,25(OH)2D3 in inducing CYP24A1 promoter activity in a transcription assay and in suppressing parathyroid hormone secretion in hemodialysis patients with secondary hyperparathyroidism, without inducing hypercalcemia or hyperphosphatemia [30–33]. Today, 19-nor-1α,25(OH)2D2, also called Zemplar or paricalcitol, is an FDA-approved drug for the treatment of secondary hyperparathyroidism. Subsequently, different modifications of the A-ring, including the synthesis of C-2 modified 19-norvitamin D compounds, were accomplished by a significant number of synthetic organic chemists [34–41].
The first synthesis of 19-norvitamin D reported by DeLuca's group in 1990 is a direct synthesis starting from 25-hydroxyvitamin D3 [31]. Subsequently, a convergent synthetic route was described by the same group, and the method has become one of the standard ones for the synthesis of new 19-norvitamin D analogs [42]. The convergent method consists of a coupling reaction between an A-ring phosphine oxide with a C1–C7 carbon unit and an 8-keto-CD-ring with a C17 side chain such as 25-hydroxy Grundmann's ketone.
During the past decade, systematic synthesis of vitamin D3 analogs with C2-modification has been attempted, and a number of C2-modified analogs with a greater VDR agonistic activity than 1α,25(OH)2D3 have been successfully synthesized [34, 35, 42–44]. For example, a substitution with 2α-methyl, 2α-(3-hydroxypropyl), or 2α-(3-hydroxypropoxy) group increased its binding affinity for the VDR two- to fourfold compared to 1α,25(OH)2D3 [43–45]. Similarly, several highly potent VDR antagonists, which belong to a series of TEI-9647 analogs with C2α functionalization as well as the 24-alkyl modification on the lactone ring, have been synthesized [46]. The mechanism of the enhanced C2α-effects on VDR binding affinity has been revealed by an X-ray co-crystallographic analysis of the VDR-ligand complexes [47]. The study shows that the terminal hydroxy group of 2α-(3-hydroxypropyl) or 2α-(3-hydroxypropoxy) substituent plays an important role in expelling the water molecules in the ligand binding domain of the VDR to form hydrogen bonds with arginine-274 residue of the VDR molecule to stabilize the VDR-ligand complex [47]. Knowing the advantage of modifying 1α,25(OH)2D3 molecule with “2-substitution” to enhance VDR binding affinity and “19-demethylenation” to eliminate calcemic potential, we, therefore, set forth to synthesize C2-substituted 19-nor-1α,25(OH)2D3 analogs using the convergent synthetic approach developed by DeLuca's laboratory [42]. However, during the synthesis of 19-nor-2α-3-hydroxypropyl-1α,25(OH)2D3 (MART-10) and 19-nor-2β-3-hydroxypropyl-1α,25(OH)2D3 (MART-11), we quickly realized that the typical coupling reaction between the 2-substituted 19-nor-A-ring part and the 8-keto-CD-ring part, that is, C7-C8 connection based on the Horner-Wadsworth-Emmons reaction, was problematic because of the large 1,2-steric repulsion between 1α-siloxy and the phenyl groups on phosphorus atom present in the oxaphosphetane transition state [34]. We decided to connect one of the double bonds of the target diene of MART-10/MART-11 between the C5 (A-ring) and C6 (two carbons elongated CD-ring from the 8-keto group) positions using the Julia coupling approach, and the reactions turned out to be successful (Figure 2). Finally, the target products were separated using a reversed phase HPLC to obtain two diastereomers, MART-10 (2α-form) and MART-11 (2β-form) [48].
Figure 2.
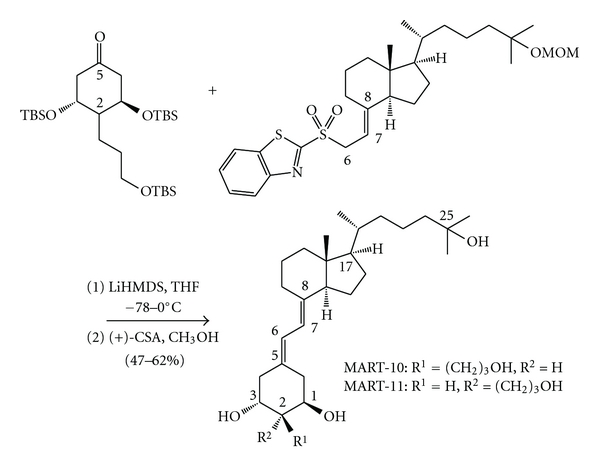
Julia coupling reaction between the A-ring C5 and the CD-ring C6 positions. TBS: tert-butyldimethylsilyl group as a protecting group, MOM: methoxymethyl group as a protecting group, LiHMDS: lithium hexamethyldisilazide, THF: tetrahydrofuran, (+)-CSA: (+)-10-camphorsulfonic acid. The structures are written with steroidal numbering.
The MART-10 and MART-11 obtained were then studied for their VDR binding property using a calf thymus vitamin D receptor preparation and HL-60 differentiation potency. We found that MART-10 and MART-11 had a binding affinity equal to 100% and 3% of the parent hormone 1α,25(OH)2D3, respectively. However, we observed a 36-fold and 7-fold greater activity in the induction of HL-60 cell differentiation by MART-10 and MART-11, respectively, than by 1α,25(OH)2D3 [35, 49]. The discrepancy between VDR binding and differentiation activity can be explained at least in part by a greater ability in recruiting coactivators, such as hTIF-2 and hSRC-1, by MART-10 and MART-11 than by 1α,25(OH)2D3 as determined by a high-throughput screening method developed in our laboratory to study the interaction between human VDR and cofactors [50].
4. The Antiproliferative Activity of 19-Norvitamin D Analogs in Prostate Cells
The antiproliferative activity of 19-norvitamin D analogs was first studied in LNCaP prostate cancer cells and in primary cultures of prostate cancer cells using 19-nor-1α,25(OH)2D2 and 19-nor-1α,25(OH)2D3 [50, 51]. It was reported that 19-nor-1α,25(OH)2D2 had antiproliferative activity comparable to 1α,25(OH)2D3, as determined by 3H-thymidine incorporation [51]. Similarly, 19-nor-1α,25(OH)2D3 was found to be equipotent to 1α,25(OH)2D3 in the primary cultures of prostate cancer cells and LNCaP prostate cancer cells (Figure 3) [52]. After we obtained a series of 19-nor-1α,25(OH)2D3 analogs modified at C-2 position with different hydrocarbon moieties, we began to study their antiproliferative activity in PZ-HPV-7 prostate cells, a cell line derived from the epithelial zone of a normal prostate and obtained from ATCC. Among them, 19-nor-2α-3-hydroxypropyl-1α,25(OH)2D3 (MART-10) and 19-nor-2β-3-hydroxypropyl-1α,25(OH)2D3 (MART-11) were found to be about 500- to 1,000-fold more active than 1α,25(OH)2D3 [53].
Figure 3.
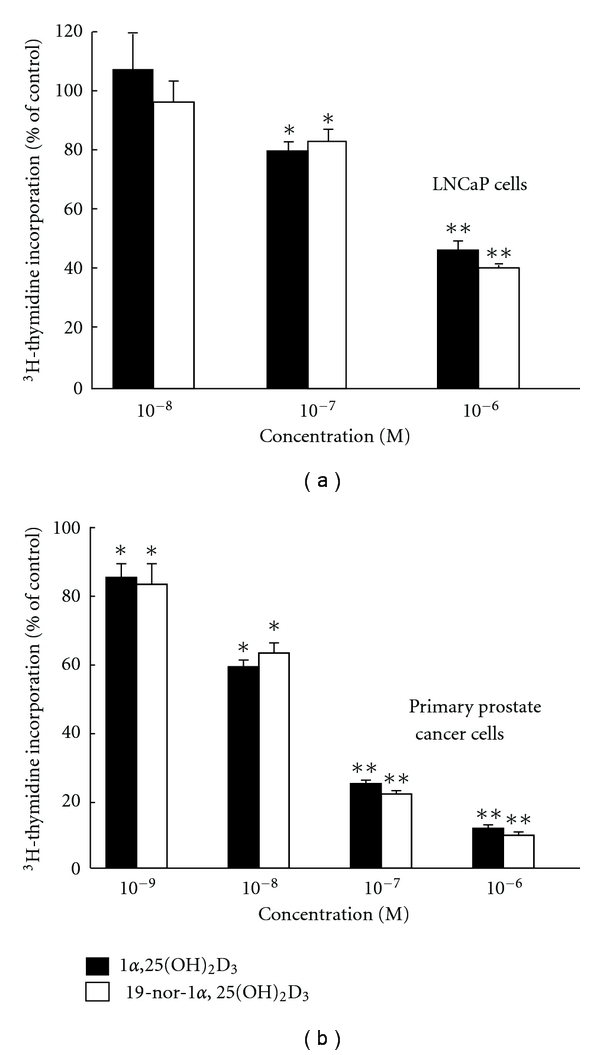
Comparison of the effect of 1α,25(OH)2D3 and 19-nor-1α,25(OH)2D3 on the 3H-thymidine incorporation in (a) LNCaP cells and (b) the primary cultures of prostate cancer cells. Results are presented as the means ± SD of 5–8 determinations. There is no difference between 1α,25(OH)2D3 and 19-nor-1α,25(OH)2D3 at any dose levels examined. *P < 0.05, **P < 0.001 versus controls.
Comparison of the inhibitory effect of MART-10 with 1α,25(OH)2D3 on the cellular proliferation was then carried out in androgen-dependent LNCaP and androgen-independent PC-3 prostate cancer cells by hemocytometer cell counting. Similar to the findings using PZ-HPV-7 cells, MART-10 is about 1,000-fold more active than 1α,25(OH)2D3 in inhibiting LNCaP (Figure 4) [54] and PC-3 prostate cancer cell proliferation (D. Iglesias-Gato et al., unpublished data).
Figure 4.

The dose-dependent effects of 1α,25(OH)2D3 and MART-10 on LNCaP cell proliferation. LNCaP cells were treated with ethanol vehicle or the indicated concentrations of 1α,25(OH)2D3 and MART-10 for one week and then trypsinized and cell counted with hemocytometer. The results are expressed as the percent of control of the means ± SD of 3 determinations from a representative experiment. The experiment was repeated 3 times with similar results. *P < 0.05 compared to the previous doses.
5. Metabolism of MART-10 by 24-Hydroxylase (24-OHase or CYP24A1)
CYP24A1 is one of the three major enzymes involved in the metabolism of vitamin D endocrine system. The gene encoding this enzyme is highly inducible by 1α,25(OH)2D3 or its analogs, and, therefore, the induction of this gene has been used as an index for the biological potency of new analogs [55, 56]. Most importantly, CYP24A1 serves as a principle mechanism to terminate the biological actions of 1α,25(OH)2D3 or its analogs through 24-hydroxylation-dependent catabolic pathway [57]. Because of this important role, specific inhibitors of CYP24A1 have been developed and used to enhance and prolong the actions of the natural hormone [57]. Alternatively, analogs which are more resistant to the catabolic degradation of CYP24A1 will have longer half-life and potentially more bioavailablity than 1α,25(OH)2D3.
In a series of experiments comparing the expression of CYP24A1 in response to 1α,25(OH)2D3 and MART-10 treatment in LNCaP and PC-3 prostate cancer cells, we observed that MART-10 was capable of inducing CYP24A1 expression at a lower concentration and to a greater extent [54] and with a longer duration than 1α,25(OH)2D3 (D. Iglesias-Gato et al., unpublished observation). The longer duration suggests that MART-10 is more resistant to CYP24A1 degradation. To find out whether this was the case, we then used a cell-free CYP24A1 reconstituted system [54] to determine the k cat/K m value, an indicator of enzyme susceptibility, of MART-10 or 2α-(3-hydroxypropoxy)-1α,25(OH)2D3 (O2C3) (Figure 1) used as a substrate. We found that MART-10 had a k cat/K m of 0.33 which is about 1/10 of what was found with O2C3 (k cat/K m = 3.0). Since the k cat/K m for O2C3 is about 1/50 of 1α,25(OH)2D3 [58], the k cat/K m for MART-10 is about 1/500 of 1α,25(OH)2D3. The k cat/K m data suggest that the addition of 3-hydroxypropyl group at carbon 2 in the MART-10 molecule may hinder the contact of the side-chain of MART-10 molecule to the heme group of CYP24A1 and results in a very poor 24-hydroxylation and, in turn, much less degradation of MART-10 by CYP24A1. The conclusion is supported by a docking model of MART-10 sit inside the substrate-binding pocket of the human CYP24A1 (Personal communication with Drs. Yamamoto and Sakaki).
6. MART-10 Is a More Potent Inhibitor of Cancer Cell Invasion
In addition to its higher activity in inhibiting prostate cancer cell proliferation, MART-10 is about 10-fold more active than 1α,25(OH)2D3 in inhibiting PC-3 cell invasion (Figure 5) [53]. Similar results were obtained with 19-nor-2β-3-hydroxypropyl-1α,25(OH)2D3 (unpublished observation). MART-10 also exhibited a greater downregulation of matrix metalloproteinase-9 (MMP-9) expression at both the transcriptional and translational levels (D. Iglesias-Gato et al., unpublished observation). Since MMP-9 is an enzyme involved in the cell invasion pathway [59, 60], the greater downregulation of MMP-9 activity may be responsible for the more potent anti-invasion effect observed in the presence of MART-10. In turn, the greater effect on MMP-9 gene expression and the expression of CYP24A1 and genes involved in cell proliferation may be due to its more profound VDR transactivation activity in prostate cancer cells [54, 61].
Figure 5.

Effect of 1α,25(OH)2D3, and 19-nor-2α-3-hydroxypropyl-1α,25(OH)2D3 (MART-10) on the invasion of PC-3 cells. The results are presented as the means ± SD of 3 determinations. *Comparison between control and 1α,25(OH)2D3 or MART-10; **Comparison between 1α,25(OH)2D3 and MART-10.
7. MART-10 Binds to Vitamin-D-Binding Protein (DBP) with a Lower Affinity Than 1α,25(OH)2D3
The bioavailability of MART-10 in circulation was examined by measuring its binding affinity to serum DBP (Kd, defined as the concentration of 1α,25(OH)2D3 or MART-10 at which a 50% reduction in [3H]-25(OH)D3 binding to DBP was observed). The Kds for MART-10 and 1α,25(OH)2D3 are 17.5 μM and 0.67 μM, respectively, indicating that the binding affinity of MART-10 for DBP is about 25-fold less than that for 1α,25(OH)2D3 (Figure 6) [54]. The lower DBP binding affinity for MART-10 will allow more MART-10 to be translocated into the target cells, including the prostate.
Figure 6.
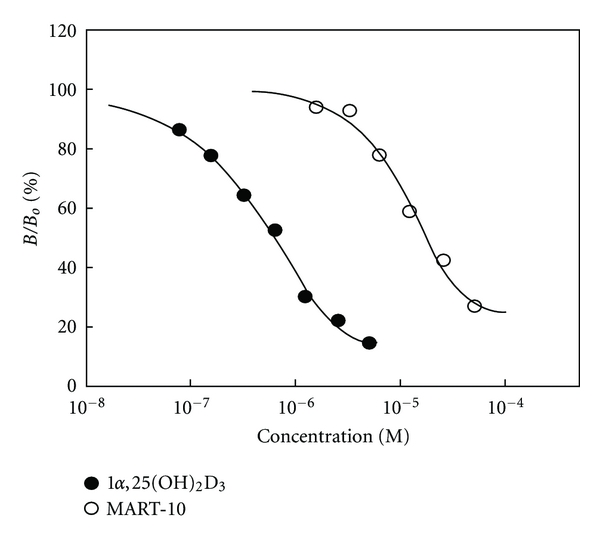
Binding of MART-10 and 1α,25(OH)2D3 to vitamin D binding protein (DBP). The binding affinity of 1α,25(OH)2D3 and MART-10 to vitamin-D-binding protein (DBP) was determined by the displacement of [3H]-25(OH)D3 from rat serum DBP by indicated concentrations of MART-10 and 1α,25(OH)2D3. The results are expressed as the percentage of displaced [3H]-25(OH)D3 (B) over total specific bound of [3H]-25(OH)D3 (Bo).
8. Conclusion
Vitamin D has been discovered as an antirachitic agent for almost a century. For more than half a century since its discovery, vitamin D was believed to be involved only in calcium and phosphate homeostasis. The realization that vitamin D (vitamin D2 and vitamin D3) itself was not active and required two successive hydroxylation steps to produce its active form, 1α,25(OH)2D3, led to the finding in 1979 that VDR was present in many tissues not related to calcium and phosphate metabolism. Subsequently, many non-classical actions of 1α,25(OH)2D3 were revealed, including antiproliferation, anti-invasion, proapoptosis, prodifferentiation, immune regulation, and so forth, (Figure 7). Although 1α,25(OH)2D3 exhibited potent antitumor effects on prostate cancer models, hypercalcemia and hypercalciuria side effects were quickly realized in animal models and human clinical trials. The lethal side effects, thus, limit the application of 1α,25(OH)2D3 clinically. Consequently, several thousand vitamin D analogs were synthesized with an intention to eliminate or lessen the side effects and at the same time to enhance their antitumor activity. So far, none of the analogs have shown clinically satisfactory results. In this paper we describe the synthesis of a novel analog of 1α,25(OH)2D3, called MART-10 and present in vitro data using normal, androgen-dependent LNCaP, and androgen-independent PC-3 cell culture models. Comparing to 1α,25(OH)2D3, MART-10 is 10 times more active in stimulating VDR transactivation in LNCaP cells, about 500- to 1000-fold more active in inhibiting the proliferation of these three types of prostate cells, 10 times more potent in inhibiting PC-3 invasion, at least 500-fold more resistant to CYP24A1-dependent degradation and has about 25-fold lower binding affinity to DBP (Table 1). In addition, MART-10 did not raise serum calcium when it was injected into rats [D. Iglesias-Gato et al., unpublished observation]. The unique properties of MART-10 suggest that this analog has a potential as a new regimen for prostate cancer treatments through all stages of the disease.
Figure 7.
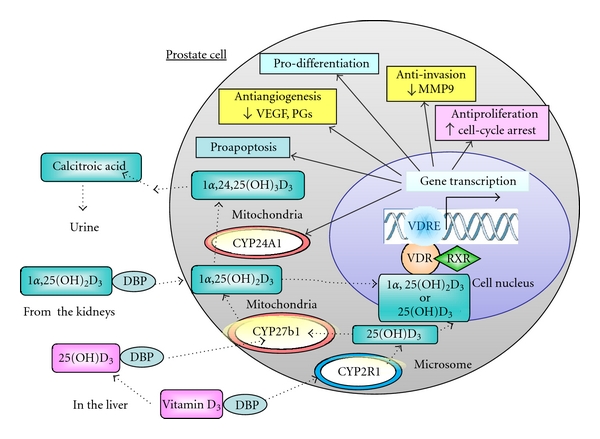
Metabolism and the nonclassical actions of vitamin D in prostate cells. Prostate cells express vitamin D 25-hydroxylase (25-OHase, or CYP2R1, a microsomal enzyme), 1α-OHase (or CYP27B1, a mitochondrial enzyme), and 24-OHase (or CYP24A1, a mitochondrial enzyme) and, therefore, are capable of synthesizing 1α,25(OH)2D3 from vitamin D3. Binding of 1α,25(OH)2D3 or 25(OH)D3 to the vitamin D receptor (VDR) causes the VDR to heterodimerize with the retinoid X receptor (RXR). The VDR-RXR heterodimer binds to specific vitamin D response elements in the promoter region of vitamin-D-responsive genes and induces gene transcription. The gene products include proteins involved in its own metabolism (CYP24A1), cell-cycle arrest, apoptosis, differentiation, anti-invasion, antiangiogenesis, and many other actions.
Table 1.
Comparison of biological activity between 1α,25(OH)2D3 and MART-10 in prostate cancer cells.
| Anti-proliferation | Anti-invasion | CYP24A1, K cat/K m | DBP Binding | |
|---|---|---|---|---|
| 1α,25(OH)2D3 | 1 | 1 | 1 | 1 |
| MART-10 | 1,000 | 100 | 1/500 | 1/25 |
Abbreviations
- 1α,25(OH)2D3:
1α,25-dihydroxyvitamin D3
- VDR:
Vitamin D receptor
- DBP:
Vitamin D binding protein
- MMP-9:
Matrix metalloproteinase-9
- O2C3:
2α-(3-hydroxypropoxy)-1α,25(OH)2D3
- MART-10:
19-nor-2α-(3-hydroxypropyl)-1α,25(OH)2D3
- MART-11:
19-nor-2β-(3-hydroxypropyl)-1α,25(OH)2D3.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer Journal for Clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kirby RS. Recent advances in the medical management of prostate cancer. British Journal of Clinical Practice. 1996;50(2):88–93. [PubMed] [Google Scholar]
- 3.Beltran H, Beer TM, Carducci MA, et al. New therapies for castration-resistant prostate cancer: efficacy and safety. European Urology. 2011;60(2):279–290. doi: 10.1016/j.eururo.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Miller GJ. Vitamin D and prostate cancer: biologic interactions and clinical potentials. Cancer and Metastasis Reviews. 1999;17(4):353–360. doi: 10.1023/a:1006102124548. [DOI] [PubMed] [Google Scholar]
- 5.Osborn JL, Schwartz GG, Smith DC, Bahnson R, Day R, Trump DL. Phase II trial of oral 1,25-dihydroxyvitamin D (calcitriol) in hormone refractory prostate cancer. Urologic Oncology. 1995;1(5):195–198. doi: 10.1016/1078-1439(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 6.Gross C, Stamey T, Hancock S, Feldman D. Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol) Journal of Urology. 1998;159(6):2035–2040. doi: 10.1016/S0022-5347(01)63236-1. [DOI] [PubMed] [Google Scholar]
- 7.McCollum EV, Simmonds N, Becker JE, Shipley PG, Bunting RW. Studies on experimental rickets. XXI. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. Journal of Biological Chemistry. 1922;53:293–312. [PubMed] [Google Scholar]
- 8.DeLuca HF, Schnoes HK. Metabolism and mechanism of action of vitamin D. Annual Review of Biochemistry. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- 9.Airey FS. Vitamin D as a remedy for lupus vulgaris. Medical World. 1946;64:807–810. [PubMed] [Google Scholar]
- 10.Charpy J, Dowling GB, et al. Vitamin D in cutaneous tuberculosis. Lancet. 1947;2(6472):p. 398. [PubMed] [Google Scholar]
- 11.Holcik LJ. Treatment of psoriasis with large doses of vitamin D2. Ceskoslovenska Dermatologie. 1949;24(4):145–149. [PubMed] [Google Scholar]
- 12.Stumpf WE, Sar M, Reid FA, et al. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 13.Colston K, Hirst M, Feldman D. Organ distribution of the cytoplasmic 1,25-dihydroxycholecalciferol receptor in various mouse tissues. Endocrinology. 1980;107(6):1916–1922. doi: 10.1210/endo-107-6-1916. [DOI] [PubMed] [Google Scholar]
- 14.Abe E, Miyaura C, Sakagami H. Differentiation of mouse myeloid leukemia cells induced by 1α,25-dihydroxyvitamin D3 . Proceedings of the National Academy of Sciences of the United States of America. 1981;78(8):4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends in Endocrinology and Metabolism. 2003;14(9):423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Molecular Aspects of Medicine. 2008;29(6):433–452. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Colston KW, Mackay AG, James SY, Binderup L, Chander S, Coombes RC. EB1089: a new vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochemical Pharmacology. 1992;44(12):2273–2280. doi: 10.1016/0006-2952(92)90669-a. [DOI] [PubMed] [Google Scholar]
- 18.Valrance ME, Brunet AH, Welsh J. Vitamin D receptor-dependent inhibition of mammary tumor growth by EB1089 and ultraviolet radiation in vivo. Endocrinology. 2007;148(10):4887–4894. doi: 10.1210/en.2007-0267. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia V, Saini MK, Shen X, et al. EB1089 inhibits the parathyroid hormone-related protein-enhanced bone metastasis and xenograft growth of human prostate cancer cells. Molecular Cancer Therapeutics. 2009;8(7):1787–1798. doi: 10.1158/1535-7163.MCT-09-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, Oettel K, Ripple G, et al. Phase I trial of 1α-hydroxyvitamin D2 in patients with hormone refractory prostate cancer. Clinical Cancer Research. 2002;8(9):2820–2827. [PubMed] [Google Scholar]
- 21.Liu G, Wilding G, Staab MJ, et al. Phase II study of 1α-hydroxyvitamin D2 in the treatment of advanced androgen-independent prostate cancer. Clinical Cancer Research. 2003;9(11):4077–4083. [PubMed] [Google Scholar]
- 22.Abe J, Morikawa M, Miyamoto K, et al. Synthetic analogues of vitamin D3 with an oxygen atom in the side chain skeleton. A trial of the development of vitamin D compounds which exhibit potent differentiation-inducing activity without inducing hypercalcemia. FEBS Letters. 1987;226(1):58–62. doi: 10.1016/0014-5793(87)80550-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhou JY, Norman AW, Lübbert M, Collins ED, Uskokovic MR, Koeffler HP. Novel vitamin D analogs that modulate leukemic cell growth and differentiation with little effect on either intestinal calcium absorption or bone calcium mobilization. Blood. 1989;74(1):82–93. [PubMed] [Google Scholar]
- 24.Asou H, Koike M, Elstner E, et al. 19-nor vitamin-D analogs: a new class of potent inhibitors of proliferation and inducers of differentiation of human myeloid leukemia cell lines. Blood. 1998;92(7):2441–2449. [PubMed] [Google Scholar]
- 25.Mehta RG, Moriarty RM, Mehta RR, Penmasta R, Lazzaro G, Constantinou A. Prevention of preneoplastic mammary lesion development by a novel vitamin D analogue, 1α-hydroxyvitamin D5. Journal of the National Cancer Institute. 1997;89(3):212–218. doi: 10.1093/jnci/89.3.212. [DOI] [PubMed] [Google Scholar]
- 26.Boehm MF, Fitzgerald P, Zou A, et al. Novel nonsecosteroidal vitamin D mimics exert VDR-modulating activities with less calcium mobilization than 1,25-dihydroxyvitamin D3 . Chemistry and Biology. 1999;6(5):265–275. doi: 10.1016/S1074-5521(99)80072-6. [DOI] [PubMed] [Google Scholar]
- 27.Uskokovic MR, Manchand P, Marczak S, et al. C-20 cyclopropyl vitamin D3 analogs. Current Topics in Medicinal Chemistry. 2006;6(12):1289–1296. doi: 10.2174/156802606777864962. [DOI] [PubMed] [Google Scholar]
- 28.Adorini L, Penna G, Amuchastegui S, et al. Inhibition of prostate growth and inflammation by the vitamin D3 receptor agonist BXL-628 (elocalcitol) Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):689–693. doi: 10.1016/j.jsbmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Okamoto R, Haritunians T, et al. Novel Gemini vitamin D3 analogs have potent antitumor activity. Journal of Steroid Biochemistry and Molecular Biology. 2008;112(1–3):151–156. doi: 10.1016/j.jsbmb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napoli JL, Sommerfeld JL, Pramanik BC, et al. 19-Nor-10-ketovitamin D derivatives: unique metabolites of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 . Biochemistry. 1983;22(15):3636–3640. doi: 10.1021/bi00284a015. [DOI] [PubMed] [Google Scholar]
- 31.Perlman KL, Sicinski RR, Schnoes HK, DeLuca HF. 1α,25-Dihydroxy-19-nor-vitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Letters. 1990;31(13):1823–1824. [Google Scholar]
- 32.Slatopolsky E, Finch J, Ritter C, et al. A new analog of calcitriol, 19-Nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hypercalcemia. American Journal of Kidney Diseases. 1995;26(5):852–860. doi: 10.1016/0272-6386(95)90455-7. [DOI] [PubMed] [Google Scholar]
- 33.Llach F, Keshav G, Goldblat MV, et al. Suppression of parathyroid hormone secretion in hemodialysis patients by a novel vitamin D analogue: 19-nor-1,25-dihydroxyvitamin D2 . American Journal of Kidney Diseases. 1998;32(2, supplement 2):S48–S54. doi: 10.1053/ajkd.1998.v32.pm9808143. [DOI] [PubMed] [Google Scholar]
- 34.Sicinski RR, Rotkiewicz P, Kolinski A, et al. 2-Ethyl and 2-ethylidene analogues of 1α,25-dihydroxy-19-norvitamin D3: synthesis, conformational analysis, biological activities, and docking to the modeled rVDR ligand binding domain. Journal of Medicinal Chemistry. 2002;45(16):3366–3380. doi: 10.1021/jm020007m. [DOI] [PubMed] [Google Scholar]
- 35.Ono K, Yoshida A, Saito N, et al. Efficient synthesis of 2-modified 1α,25-dihydroxy-19-norvitamin D3 with Julia olefination: high potency in induction of differentiation on HL-60 cells. Journal of Organic Chemistry. 2003;68(19):7407–7415. doi: 10.1021/jo034787y. [DOI] [PubMed] [Google Scholar]
- 36.Kittaka A, Saito N, Honzawa S, et al. Creative synthesis of novel vitamin D analogs for health and disease. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):269–276. doi: 10.1016/j.jsbmb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Toyoda A, Nagai H, Yamada T, et al. Novel synthesis of 1α,25-dihydroxy-19-norvitamin D from 25-hydroxyvitamin D. Tetrahedron. 2009;65(48):10002–10008. [Google Scholar]
- 38.Hanazawa T, Wada T, Masuda T, Okamoto S, Sato F. Novel synthetic approach to 19-nor-1α,25-dihydroxyvitamin D3 and its derivatives by Suzuki-Miyaura coupling in solution and on solid support. Organic Letters. 2001;3(24):3975–3977. doi: 10.1021/ol016908r. [DOI] [PubMed] [Google Scholar]
- 39.Huang P, Sabbe K, Pottie M, Vandewalle M. A novel synthesis of 19-nor 1α,25-dihydroxyvitamin D3 and related analogues. Tetrahedron Letters. 1995;36(45):8299–8302. [Google Scholar]
- 40.Shimizu M, Miyamoto Y, Takaku H, et al. 2-Substituted-16-ene-22-thia-1α,25-dihydroxy-26,27-dimethyl-19-norvitamin D3 analogs: synthesis, biological evaluation, and crystal structure. Bioorganic and Medicinal Chemistry. 2008;16(14):6949–6964. doi: 10.1016/j.bmc.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 41.Glebocka A, Sicinski RR, Plum LA, Clagett-Dame M, DeLuca HF. New 2-alkylidene 1α,25-dihydroxy-19-norvitamin D3 analogues of high intestinal activity: synthesis and biological evaluation of 2-(3′-alkoxypropylidene) and 2-(3′-hydroxypropylidene) derivatives. Journal of Medicinal Chemistry. 2006;49(10):2909–2920. doi: 10.1021/jm051082a. [DOI] [PubMed] [Google Scholar]
- 42.Perlman KL, Swenson RE, Paaren HE, Schnoes HK, DeLuca HF. Novel synthesis of 19-nor-vitamin D compounds. Tetrahedron Letters. 1991;32(52):7663–7666. [Google Scholar]
- 43.Saito N, Honzawa S, Kittaka A. Recent results on A-ring modification of 1α,25-dihydroxyvitamin D3: design and synthesis of VDR-agonists and antagonists with high biological activity. Current Topics in Medicinal Chemistry. 2006;6(12):1273–1288. doi: 10.2174/156802606777864953. [DOI] [PubMed] [Google Scholar]
- 44.Saito N, Suhara Y, Kurihara M, et al. Design and efficient synthesis of 2α-(ω-hydroxyalkoxy)- 1α,25-dihydroxyvitamin D3 analogues, including 2-epi-ED-71 and their 20-epimers with HL-60 cell differentiation activity. Journal of Organic Chemistry. 2004;69(22):7463–7471. doi: 10.1021/jo0491051. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi E, Nakagawa K, Suhara Y, et al. Biological activities of 2α-substituted analogues of 1α,25-dihydroxyvitamin D3 in transcriptional regulation and human promyelocytic leukemia (HL-60) cell proliferation and differentiation. Biological and Pharmaceutical Bulletin. 2006;29(11):2246–2250. doi: 10.1248/bpb.29.2246. [DOI] [PubMed] [Google Scholar]
- 46.Saito N, Matsunaga T, Saito H, et al. Further synthetic and biological studies on vitamin D hormone antagonists based on C24-alkylation and C2α-functionalization of 25-dehydro-1α- hydroxyvitamin D3-26,23-lactones. Journal of Medicinal Chemistry. 2006;49(24):7063–7075. doi: 10.1021/jm060797q. [DOI] [PubMed] [Google Scholar]
- 47.Hourai S, Fujishima T, Kittaka A, et al. Probing a water channel near the A-ring of receptor-bound 1α,25-dihydroxyvitamin D3 with selected 2α-substituted analogues. Journal of Medicinal Chemistry. 2006;49(17):5199–5205. doi: 10.1021/jm0604070. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida A, Ono K, Suhara Y, Saito N, Takayama H, Kittaka A. Efficient and convergent coupling route for the short-step synthesis of enantiopure 2α- and 2β-alkylated 1α,25-dihydroxy-19-norvitamin D3 analogues. Synlett. 2003;(8):1175–1179. [Google Scholar]
- 49.Arai MA, Kittaka A. Novel 2-alkyl-1α,25-dihydroxy-19-norvitamin D3: efficient synthesis with Julia olefination, evaluation of biological activity and development of new analyzing system for co-activator recruitment. Anticancer Research. 2006;26(4):2621–2631. [PubMed] [Google Scholar]
- 50.Arai MA, Takeyama KI, Ito S, Kato S, Chen TC, Kittaka A. High-throughput system for analyzing ligand-induced cofactor recruitment by vitamin D receptor. Bioconjugate Chemistry. 2007;18(3):614–620. doi: 10.1021/bc0601121. [DOI] [PubMed] [Google Scholar]
- 51.Chen TC, Schwartz GG, Burnstein KL, Lokeshwar BL, Holick MF. The in vitro evaluation of 25-hydroxyvitamin D3 and 19-nor-1α,25- dihydroxyvitamin D2 as therapeutic agents for prostate cancer. Clinical Cancer Research. 2000;6(3):901–908. [PubMed] [Google Scholar]
- 52.Chen TC, Holick MF, Lokeshwar BL, Burnstein KL, Schwartz GG. Evaluation of vitamin D analogs as therapeutic agents for prostate cancer. Recent results in cancer research. In: Reichrath J, Friedrich M, Tilgen W, editors. Vitamin D Analogs in Cancer Prevention and Therapy. Vol. 164. Berlin, Germany: Springer; 2003. pp. 273–288. [DOI] [PubMed] [Google Scholar]
- 53.Chen TC, Persons KS, Zheng S, et al. Evaluation of C-2-substituted 19-nor-1α,25-dihydroxyvitamin D3 analogs as therapeutic agents for prostate cancer. Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3-5):717–720. doi: 10.1016/j.jsbmb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Flanagan JN, Zheng S, Chiang KC, et al. Evaluation of 19-nor-2α(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 as a therapeutic agent for androgen-dependent prostate cancer. Anticancer Research. 2009;29(9):3547–3553. [PubMed] [Google Scholar]
- 55.Ohyama Y, Ozono K, Uchida M, et al. Identification of a vitamin D-responsive element in the 5′-flanking region of the rat 25-hydroxyvitamin D3 24-hydroxylase gene. Journal of Biological Chemistry. 1994;269(14):10545–10550. [PubMed] [Google Scholar]
- 56.Flanagan JN, Young MV, Persons KS, et al. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Research. 2006;26(4):2567–2572. [PubMed] [Google Scholar]
- 57.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochimica et Biophysica Acta. 2011;1814(1):186–199. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Abe D, Sakaki T, Kusudo T, et al. Metabolism of 2α-propoxy-1α,25-dihydroxyvitamin D3 and 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 by human CYP27A1 and CYP24A1. Drug Metabolism and Disposition. 2005;33(6):778–784. doi: 10.1124/dmd.104.003038. [DOI] [PubMed] [Google Scholar]
- 59.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(10):4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao BY, Yeh SD, Lee YF. 1α,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis. 2006;27(1):32–42. doi: 10.1093/carcin/bgi170. [DOI] [PubMed] [Google Scholar]
- 61.Polly P, Herdick M, Moehren U, Baniahmad A, Heinzel T, Carlberg C. VDR-Alien: a novel, DNA-selective vitamin D3 receptorcorepressor partnership. FASEB Journal. 2000;14(10):1455–1463. doi: 10.1096/fj.14.10.1455. [DOI] [PubMed] [Google Scholar]


