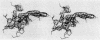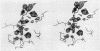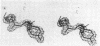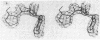Abstract
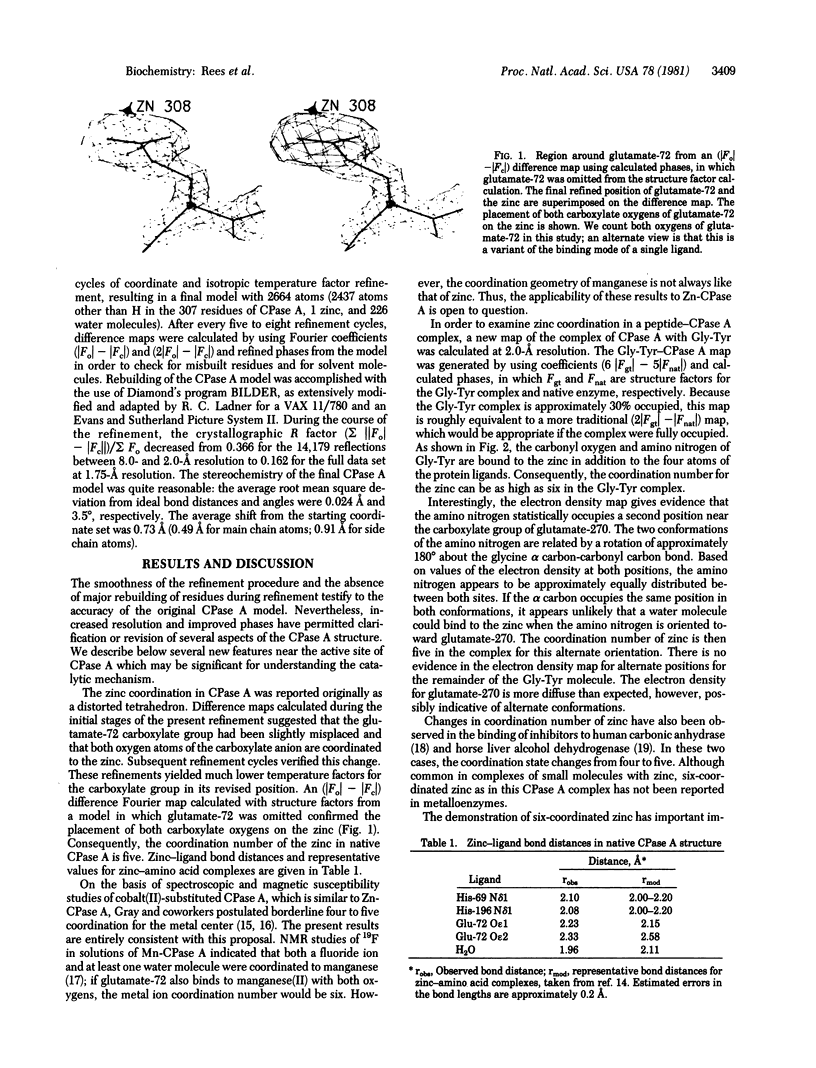
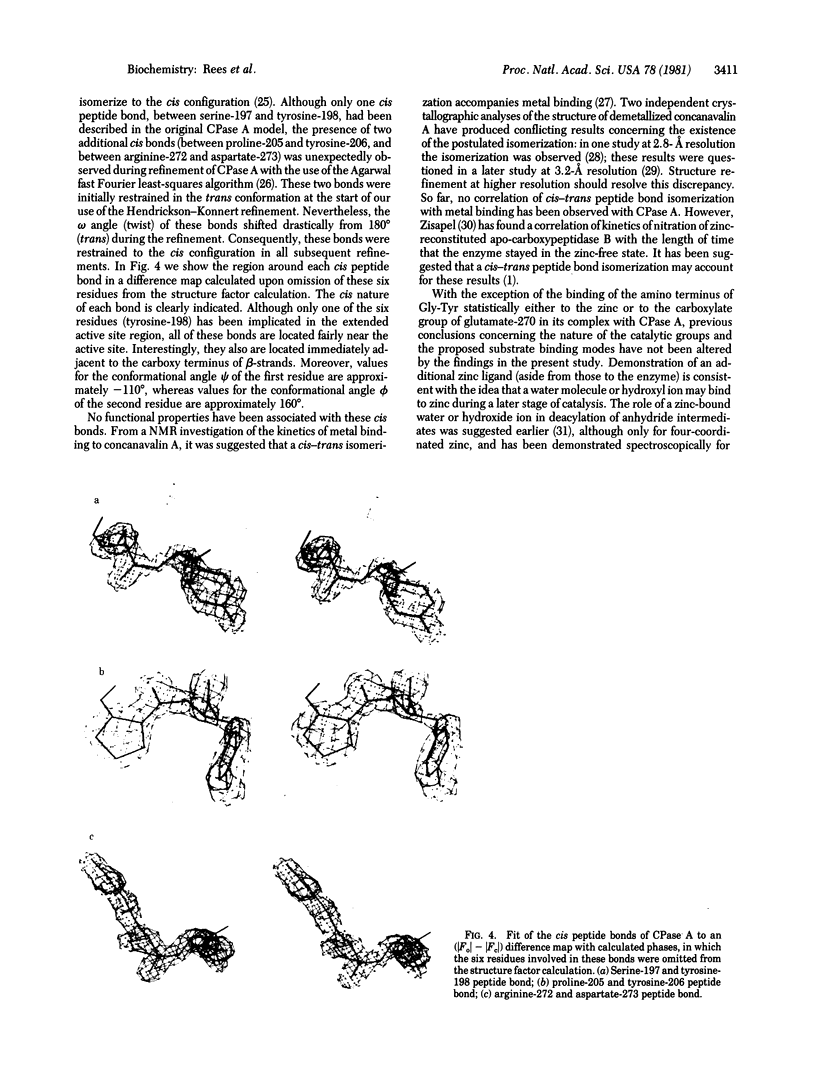
The structure of the metalloenzyme carboxypeptidase A (peptidyl-L-amino-acid hydrolase, EC 3.4.17.1) has been refined at 1.75 A by a restrained least-squares procedure to a conventional crystallographic R factor of 0.162. Significant results of the refined structure relative to the catalytic mechanism are described. In the native enzyme, the zinc coordination number is five (two imidazole N delta 1 nitrogens, the two carboxylate oxygens of glutamate-72, and a water molecule). In the complex (at 2.0-A resolution) of carboxypeptidase A with the dipeptide glycyl-L-tyrosine, however, the water ligand is replaced by both the carbonyl oxygen and the amino nitrogen of the dipeptide. The amino nitrogen also statistically occupies a second position near glutamate-270. Consequently, the coordination number of zinc may vary from five to six in carboxypeptidase A-substrate complexes. Implications of these results for the catalytic mechanism of carboxypeptidase A are discussed. In addition, three cis peptide bonds, none of which involves proline as the amino nitrogen donor, have been located fairly near the active site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiwe T., Bränden C. I. X-ray investigation of the binding of 1,10-phenanthroline and imidazole to horse-liver alcohol dehydrogenase. Eur J Biochem. 1977 Jul 1;77(1):173–179. doi: 10.1111/j.1432-1033.1977.tb11655.x. [DOI] [PubMed] [Google Scholar]
- Breslow R., Wernick D. L. Unified picture of mechanisms of catalysis by carboxypeptidase A. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1303–1307. doi: 10.1073/pnas.74.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H. C. Crystal structures of metal-peptide complexes. Adv Protein Chem. 1967;22:257–424. doi: 10.1016/s0065-3233(08)60043-1. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Petef M., Fridborg K., Cid-Dresdner H., Lövgren S. Structure and function of carbonic anhydrases. Imidazole binding to human carbonic anhydrase B and the mechanism of action of carbonic anhydrases. FEBS Lett. 1977 Jan 15;73(1):115–119. doi: 10.1016/0014-5793(77)80027-6. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. Carboxypeptidase A mechanisms. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3875–3878. doi: 10.1073/pnas.77.7.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Low P. S., Somero G. N. Protein hydration changes during catalysis: a new mechanism of enzymic rate-enhancement and ion activation/inhibition of catalysis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3305–3309. doi: 10.1073/pnas.72.9.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Wyluda B. J., Yamane T. Nuclear magnetic resonance study of the binding of fluoride ions to carboxypeptidase A. J Mol Biol. 1970 Jul 14;51(1):15–30. doi: 10.1016/0022-2836(70)90266-4. [DOI] [PubMed] [Google Scholar]
- QUIOCHO F. A., RICHARDS F. M. INTERMOLECULAR CROSS LINKING OF A PROTEIN IN THE CRYSTALLINE STATE: CARBOXYPEPTIDASE-A. Proc Natl Acad Sci U S A. 1964 Sep;52:833–839. doi: 10.1073/pnas.52.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Mitra A. K. An explanation for the rare occurrence of cis peptide units in proteins and polypeptides. J Mol Biol. 1976 Oct 15;107(1):85–92. doi: 10.1016/s0022-2836(76)80019-8. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. Changes in the three-dimensional structure of concanavalin A upon demetallization. Proc Natl Acad Sci U S A. 1978 May;75(5):2286–2290. doi: 10.1073/pnas.75.5.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Honzatko R. B., Lipscomb W. N. Structure of an actively exchanging complex between carboxypeptidase A and a substrate analogue. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3288–3291. doi: 10.1073/pnas.77.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Lipscomb W. N. Structure of the potato inhibitor complex of carboxypeptidase A at 2.5-A resolution. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4633–4637. doi: 10.1073/pnas.77.8.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. C., Root C. A., Gray H. B. Electronic spectral and magnetic susceptibility studies of nickel[II] and cobalt [II] carboxypeptidase A complexes. J Am Chem Soc. 1975 Jan 8;97(1):21–26. doi: 10.1021/ja00834a006. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. C., Root C. A., Wang R. H., Cerdonio M., Gray H. B. The nature of the ground states of cobalt(II) and nickel(II) carboxypeptidase A. Proc Natl Acad Sci U S A. 1973 Jan;70(1):161–163. doi: 10.1073/pnas.70.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham M., Yonath A., Sussman J. L., Moult J., Traub W., Kalb A. J. Crystal structure of demetallized concanavalin A: the metal-binding region. J Mol Biol. 1979 Jun 25;131(2):137–155. doi: 10.1016/0022-2836(79)90070-6. [DOI] [PubMed] [Google Scholar]
- Zisapel N. Structural changes in metalloenzyme in the course of metal substitution: carboxypeptidase B. Biochem Biophys Res Commun. 1978 Mar 15;81(1):28–34. doi: 10.1016/0006-291x(78)91626-1. [DOI] [PubMed] [Google Scholar]