Abstract
One novel electrochemical immunosensor was constructed by immobilizing capture antibody of alpha-fetoprotein (AFP Ab1) on a nafion/nanogold-particle modified glassy carbon electrode. With a sandwich immunoassay, one DNA-derived magnetic nanoprobe, simplified as DNA/(ZMPs—HRP-AFP Ab2)n, was employed for the detection of AFP. The fabricated procedure of the proposed biosensor was characterized by cyclic voltammetry and electrochemical impedance spectroscopy. The performance and factors influencing the performance of the biosensor were also evaluated. Under optimal conditions, the developed biosensor exhibited a well-defined electrochemical behavior toward the reduction of AFP ranging from 0.01 to 200 ng/mL with a detection limit of 4 pg/mL (S/N = 3). The biosensor was applied to the determination of AFP in serum with satisfactory results. It is important to note that the sandwich nanochainmodified electro-immunosensor provided an alternative substrate for the immobilization of other tumor markers.
1. Introduction
Alpha-fetoprotein (AFP) is a biomarker for cancer diagnosis. High levels of AFP can indicate an increased risk of liver cancer [1, 2]. Immunoassay is one of the most powerful analytical tools due to the specificity and sensitivity of antigen-antibody interactions, therefore, it is used extensively for clinical diagnoses [3]. However, the increasing demand for early and ultrasensitive screening of cancer biomarkers is pushing the enhancement of detection sensitivity by signal amplification or novel detection technologies [4, 5]. Compared with conventional immunoassays such as enzyme-linked immunosorbent assay (ELISA) and chemiluminescence immunoassay, electrochemical immunoassay has attracted considerable interest in wide range for its intrinsic advantages such as high sensitivity, low cost, and fast analysis and maybe become a gold standard for clinical detection of AFP. But they have poor detection limits, suffer from non-specific binding, and are not amenable to high-throughput analyses [6, 7]. Recently, the sandwich type of amperometric immunosensor for AFP has attracted much attention for its greatly enhanced sensitivity based on a signal amplification strategy. In order to construct the immunosensor, in recent years, with the development of nanotechnology, a variety of nanoparticles, such as gold nanoparticles (Au NPs) have been applied as the labels which can dramatically enhance the signal intensity of electrochemical immunosensor. In the meantime, Nafion as a conductive polymer was usually employed as a sensor platform to capture a large amount of Au NPs. The formed nanofunctionalized interface can provide an effective matrix for antibody immobilization with good stability and bioactivity [8–11].
Enzyme amplification is one of the commonly used signal amplification systems in sandwich immunoassay [12–15]. Nanozirconium dioxide (nano-ZrO2), as a kind of Lewis acid, can be directly used to fix the antibody and enzyme through combining the carboxylic acid [12, 13] of protein molecules with the phosphoric acid groups [14] (such as Lewis strong alkali) of DNA. Because the antibodies and enzyme are immobilized on nano-ZrO2 with large superficial area, the total amount remarkably increased and can keep the biological activity for a long time [15]. DNA can specifically combine with ZrO2 [10], but if DNA was directly used to fix ZrO2 to make probes, it will be very difficult to separate the probes from the free antibodies which coexist in the suspension. In recent years, nanoferromagnetic probes made of nanoferromagnetic oxide (such as Fe3O4) material have been developed; they can controllably separate under the outer magnetic reaction. This method is convenient, simple, rapid, and thorough and has attracted more and more attention of researchers [15]. Our group have synthesized Fe3O4 (nuclear)/ZrO2 (shell) magnetic nanomaterial probes (ZMPs) which are very suitable for the construction of such magnetic probes [16, 17]. DNA, as DNA-linked long-chain molecules, contains a lot of phosphate groups, which can fix several magnetic beads of HRP-enzyme-labeled AFP antibody, and at the same condition, the current response increased with the incremental concentration of enzyme.
Herein, a novel sandwich electrochemical by immunosensor nanomagnetic probes was fabricated. A self-assembled nafion/Au NPs membrane was formed through the strong interaction between the GCE and nafion surface. Monoclonal mouse anti-human AFP was covalently immobilized on the electrode to serve as the capture antibody. After specifically interacting with AFP in the sample solution, the immunosensor was incubated with DNA-labeled ZMPs/HRP-AFP Ab2 antibody to allow the formation of a sandwiched complex of capture antibody-AFP-DNA/(ZMPs/HRP-AFP Ab2)n. Then, the HRP enzyme enriched at the electrode surface can catalyze the o-phenylene-diamine (OPD) oxidized by peroxide hydantoin (CP) which produced an amplified reduction peak through an electron-transfer reaction. The response current was directly related to the concentration of the analyte human AFP. The preparation and detection principle of the immunosensor electrode was shown in Figure 1.
Figure 1.
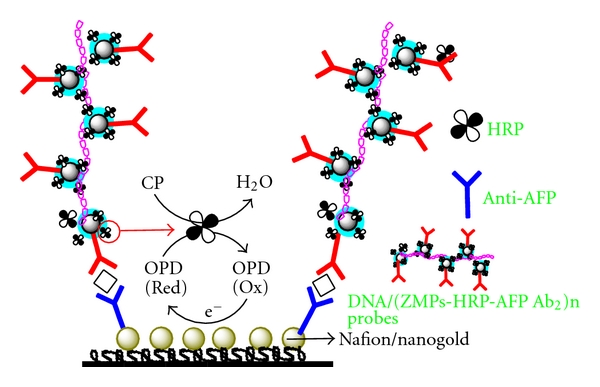
Preparation and detection principle of the GCE/nafion Au NPs/AFP Ab1 immunosensor electrode.
2. Experimental
2.1. Reagents and Chemicals
O-phenylenediamine (OPD) and peroxide hydantoin (CP) were purchased from Shanghai crystal pure reagent Co., LTD. Fe3O4/ZrO2 magnetic particles were made by us. AFP monoclonal antibody solution (AFP Ab1) and AFP ELISA kits were obtained from Sigma company, the ELISA kits contain AFP standard solution and HRP-labeled AFP monoclonal antibody (HRP-AFP Ab2). Calf thymus DNA and bovine serum albumin (BSA) were purchased from Aldrich, USA. All other reagents were of analytical grade. Double-distilled water was used for all experiments.
2.2. Apparatus
Cyclic voltammetry (CV) measurements were performed using a CHI-660B electrochemical workstation (Shanghai CH Instruments Co., China). The CV experiment was performed in a conventional three-compartment electrochemical cell containing a platinum wire as an auxiliary electrode and Ag/AgCl (in saturated KCl) as a reference electrode. A bare or modified glassy carbon electrode (GCE, D = 2 mm) was used as a working electrode. Transmission electron microscopy (TEM) images were obtained using H-7650 type (Hitachi corp., Japan). In addition, the apparatus used in this paper includes S2 RANGER X-ray fluorescence spectrometer (Bruker, Germany), ST-360 microplate reader (Shanghai science biotechnology), NdFeB magnet (Hangzhou Magnet Equipment Ltd., China), and ultrapure water meter (Millipore company, USA).
2.3. The Synthesis and Characterization of ZMPs and DNA/(ZMPs/HRP-AFP Ab)n Probes
Firstly, nano-Fe3O4 particles were synthesized by the method of coprecipitation according to the previous protocol [21]. Then, nano Fe3O4/ZrO2 (ZMPs) magnetic particles were acquired by covering to the Fe3O4 magnetic particles with nano-ZrO2. Transmission electron microscopy (TEM, Figure 2(a)) of Fe3O4/ZrO2 showed that its structure was core-shell type: Fe3O4 is nuclear (black) and ZrO2 (white) is shell. The average size of Fe3O4/ZrO2 (ZMP) magnetic particles was 18.2 nm from the transfer electromicroscope. The X-ray diffraction (XRD) graph of Fe3O4/ZrO2 showed that there are both characteristic peaks of Fe3O4 (2θ = 72.7°) and ZrO2 (2θ = 21.4°) crystals, which indicated that crystal ZrO2 was wrapped in the outer layer of Fe3O4 and was in crystalline state. The X-ray fluorescence spectrum (XRF) of ZMP showed Zr-Kβ (17.4 keV), Zr-Kα (15.7 keV), Zr-Lβ (2.12 ke), Zr-Lα (2.03 keV), Fe-Kβ (7.21 KeV), and Fe-Kα (6.35 keV) peak, which indicated that Fe and Zr both exist in this magnetic particle. AFP Ab2 was added into 5 mL 2 mg/mL solution of ZMPs, and we stirred the mixture constantly for 12 h. The unabsorbed anti-AFP2 was eliminated by the magnetic separation and washed repeatedly for several times. Furthermore, 300 ng/mL HRP was used to block the unreached and nonspecific sites of ZMPs. Its TEM (Figure 2(b)) showed that ZMPs surface was coated with a layer of antibody and HRP. When 1 mg/mL DNA was added to ZMPs-HRP-AFP Ab2 to form the nanochain probes (DNA/(ZMPs-HRP-AFP Ab2)n), its TEM(Figure 2(c)) showed that there are many ZMP particles labeled on DNA chains. When the probes were absorbed by a magnet iron, it showed that it can move in the magnetic field(Figure 2(d)).
Figure 2.
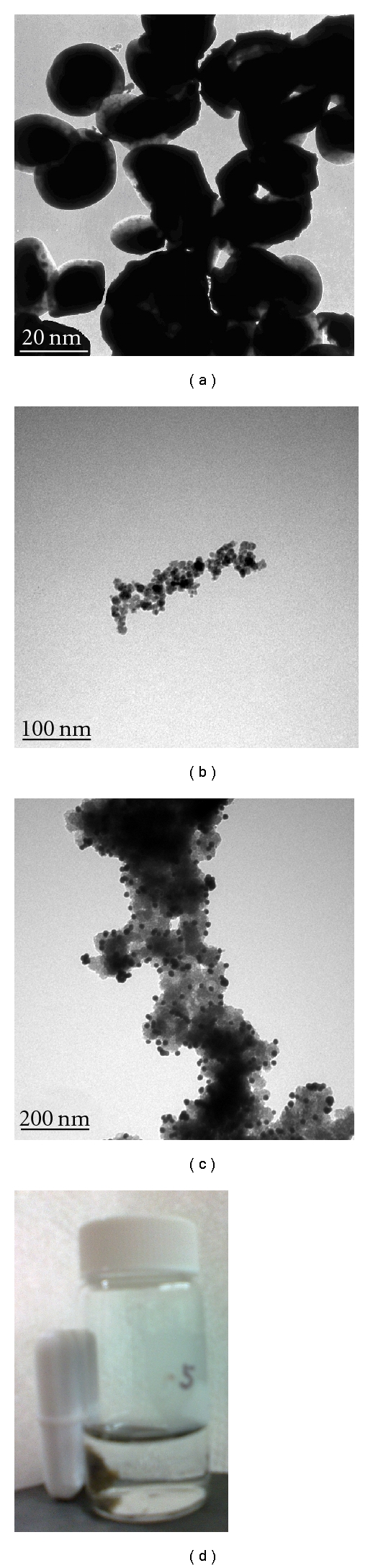
TEM images of (a) ZMP particles, (b) ZMPs-AFP Ab2, (c) DNA/(ZMPs-HRP-AFP Ab2)n, (d) c being absorbed by external magnetic field of DNA/(ZMPs-HRP-AFP Ab2)n probes.
2.4. The Preparation of the Immunosensor
The immunosensor was fabricated and characterized according to the literature [6]. AuNPs/AFP Ab1 (20 μg/mL) was immobilized (by adsorption) onto a GCE/nafion electrode. 50 μL of AuNPs/AFP Ab1 solution was spread onto the cleaned and polished GCE electrode, and the electrode was incubated at 4°C. After the incubation, the electrode was rinsed with distilled water and the electrode was blocked with 2% BSA + 0.05% tween for 30 min at room temperature. The electrode was again rinsed with distilled water to remove any residuals. The AuNPs/Ab1 electrode was then incubated with a 5 μL of AFP antigen for 30 min at room temperature. After the binding of antigen, the electrode was incubated in 50 μL of 5 mg/mL DNA/(HRP-AFP Ab2)n magnetic probe. Finally the electrode was washed to remove unbound conjugate.
3. Results and Discussion
3.1. The Electrochemical Characterization of the Immunosensor
We adopt electrochemical impedance spectrum to investigate the modifying processes of the electrode. As shown in Figure 3, the bare GCE showed a relatively small electron-transfer resistance of Ret (curve a). After nafion/nano Au film was formed on the electrode, a smaller Ret was observed (curve b), which means that nano-Au has high electrical conductivity which could greatly decrease the Ret and facilitate the electron transfer between the electrode and the surface. As the AFP Ab1, antigen, Ab2, HRP-labeled Ab2, and probe-labeled Ab2 could all resist the electron transfer kinetics of redox probe at the electrode interface, an increase tendency of the impedance was observed during their stepwise attachment (curves c, d, and e), which testified the immobilization of these substances on the electrode surface.
Figure 3.
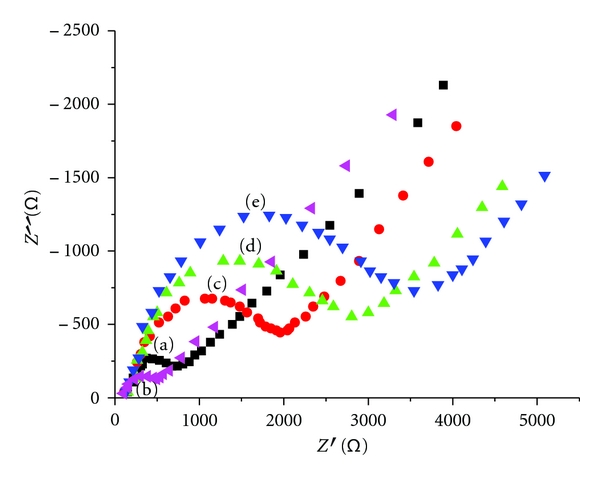
AC impedance spectrum of (a) GCE, (b) GCE/nafion-nano Au, (c) GCE/Nafion-Au NPs/AFP Ab/AFP, and (d) GCE/Nafion-Au NPs/AFP Ab/AFP/(DNA/(ZMPs-HRP-AFP Ab2)n) in 5 mmol/L Fe(CN)63−/4− + 0.5 mol/L KCl solution.
3.2. Electrochemical Behaviors of the Immunosensor
The GCE/Nafion-Au NPs/AFP Ab1 (Figure 4(a)) electrode had no redox peak in the blank PBS between −0.6 V and 0.6 V. When 5 mmol/L CP and 1mmol/L OPD were added into the solution, the modified electrode (Figure 4(b)) has a pair of redox (100 m V/s) at −0.475 V and −0.545 V. Figure 4(d) showed the CV curves of the immunosensor obtained in response to AFP. In the presence of AFP, a distinct catalytic reduction peak was observed (the reduction current of OPD obviously increased, while its oxidation current reduced). In the control experiment, where BSA was used instead of AFP, there was no appreciable current peak in the CV curve (Figure 4(c)), indicating that the immunosensor had a high degree of selectivity for AFP detection.
Figure 4.
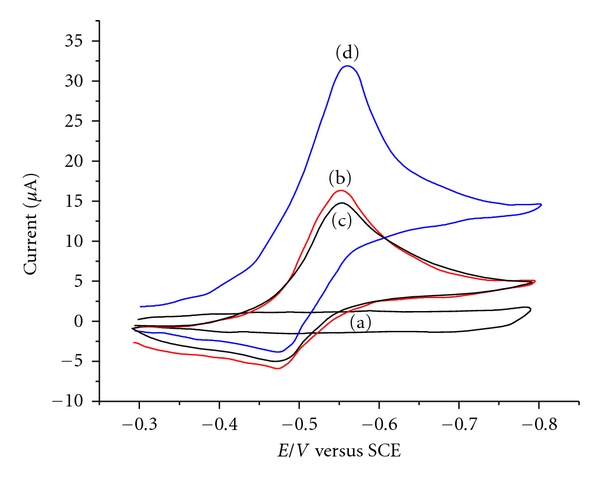
Cyclic voltammograms of (a) GCE/nafion-Au NPs/AFP Ab1 and (b). (a) in 5 mmol/L CP and 1 mmol/L OPD; (c) (a) in response to 1% BSA and (d) 50 ng/mL AFP.
3.3. The Optical Measurements Performed for the Optimization of the Immunosensor
It is important that the amount of capture AFP antibody immobilized onto the ZMPs can play a key role in the analytical characteristics concerning both the range of linearity and sensitivity of the immunoassay. The influence of the amount of AFP Ab1 antibodies was evaluated by immobilizing, in every experiment, 50 μL of different AFP Ab1 solutions prepared in the 0–35 μg/mL concentration range which was employed in this study. The study shows as the DPV peak current for OPD increased sharply with the immobilized AFP Ab1 concentration up to 20 μg/mL. Larger antibody loadings produced a decrease in the voltammetry signal.
The concentration of the HRP immobilized on the ZMPs is critical to the performance of the immunosensor because it can catalyze the reduction current of OPD with CP. The effect of the concentration of HRP was studied. As shown in Figure 5(a), with the increase of the HRP (from 100, 200, 300, 400, and 500 ng/mL), the OD response signals increased. When the concentration reached 300 ng/mL, the OD value became stable which indicated that HRP has reached its saturated adsorption amount on the surface of ZMPs.
Figure 5.
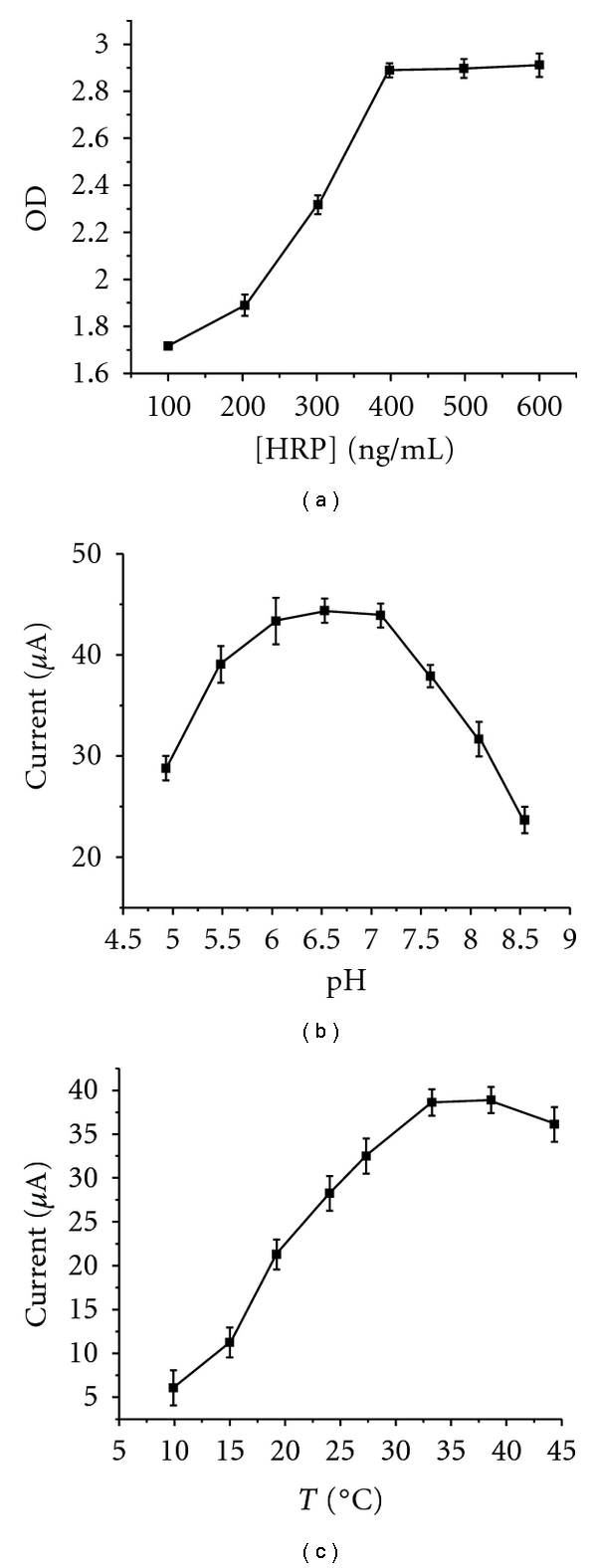
(a) The OD by different amounts of HRP; (b) influence of the pH; (c) incubation time on the response of the immunosensor.
The influence of pH during the binding reaction was studied between 4.8 and 8.5 for the same concentration of AFP (50 ng/mL) in PBS buffer solution with 5 mmol/L CP and 1 mmol/L OPD. The change of capacitance increased with increasing pH from 4.8 to 7.00 and then decreased as the pH increased further. This result shows that the maximum change in capacitive signal occurs at pH 7.00 (Figure 5(b)). Therefore, pH 7.00 of PBS was used as the buffer in the binding reaction. The ratio of the protons participating in the reaction and the electrons transferred in the electrode reaction is 1 : 1. The process of electrode response of OPD is a double-electron process [18–20, 22–24], which is the same with the number of the redox of OPD. The incubation temperature (Figure 5(c)) and time on the catalytic current. We find that the immunosensor possesses good current response signal at room temperature. So all the experiments were completed at room temperature. The incubation time of APF Ab2 with the functionalized DNA/ZMPs was also optimized, and the results showed a maximum ip value for 45 min. Thus, the optimum experiment conditions are pH 7.0, reacted at room temperature for 45 min for detection.
3.4. The Detection of AFP in Real Serum Samples
Under the optimal conditions, the oxidation peak current in DPV of the immunosensor could be utilized as a quantitative measure of AFP concentration. Figure 6(a) depicted the DPV signals of the electrochemical immunosensor in response to human AFP of varying concentrations. It was observed that the sensor gave increased signal with increasing AFP concentration. As shown in the inset of Figure 6(b), the developed immunosensor gave current responses in linear correlation to (AFP) concentrations within the range from 0.01 ng/mL to 200 ng/mL with the detection limit of 4 pg/mL, which were comparable to or even better than those reported previously (Table 1).
Figure 6.
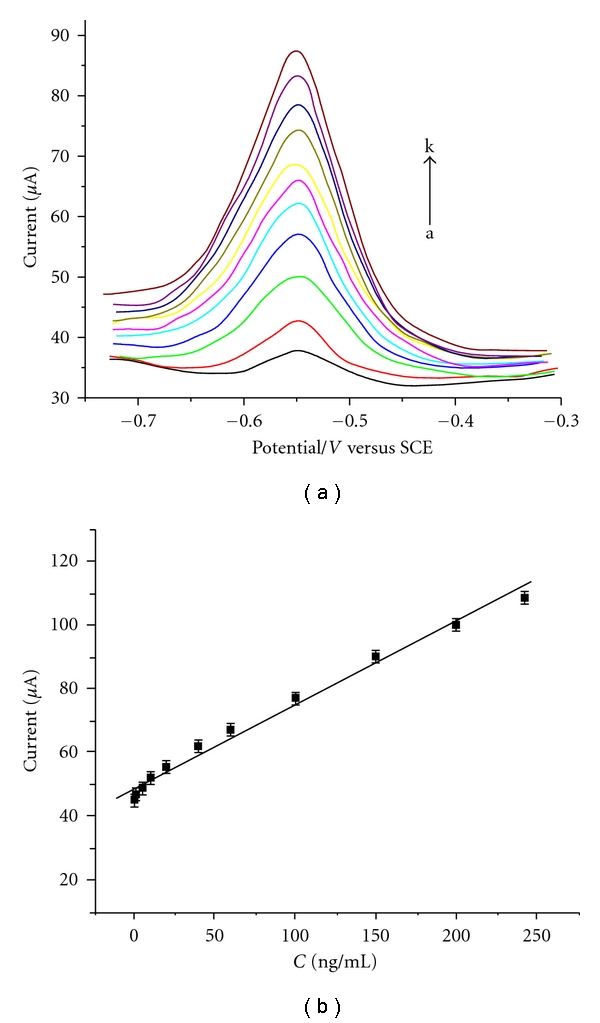
(a) Differential pulse voltammograms of the immunosensor for AFP at various concentrations (from a to k: 0, 1, 5, 10 20, 40, 60, 80, 100, 150, and 200, (b) the calibration curve for the detection of (AFP) under optimal condition.
Table 1.
Comparison of the immunosensor to AFP with others.
| Probes | Detection methods | Linear range | Detection limit | Reference |
|---|---|---|---|---|
| HRP-anti-AFP-GNGs | DPV | 0.02 ~ 4 ng/mL | 10 pg/mL | [18] |
| Ru-silica@Au-anti-AFP | ECL | 0.05 ~ 50 ng/mL | 30 pg/mL | [19] |
| HRP-Ab2-CNSs | DPV | 0.05 ~ 6 ng/mL | 20 pg/mL | [20] |
| HRP-anti-AFP | ELISA | 0.5–16 ng/mL | 0.5 ng/mL | Instruction book |
| ZMPs-AFP2/HRP | CV | 0.01 ~ 200 ng/mL | 4 pg/mL | This method |
3.5. The Precision, Reproducibility, and Stability of the Immunosensor
15 ng/mL and 25 ng/mL AFP were respectively measured for 4 times by the immunosensor which were prepared in different time and different batches. The coefficient of variation obtained in the group was, respectively, 2.3% and 2.2%. It showed that this immunosensor has good preparation repetitiveness. The proposed sensor can be stored in pH 6.5 PBS (4°C) for 45 days, and its signal did not have obvious changes (<5%) which showed the sensor to have good storage stability. Updated electrode can be refreshed to use, the measured results of which on the 100 ng/mL and 15 ng/mL AFP sample were, respectively, 99.3 and 15.7 ng/mL and RSD (n = 3) was, respectively, 3.1% and 3.2%. It suggests that the sensor has good preparation repeatability.
3.6. Reproducibility and Interference
Regeneration allows the sensors to be reused for many times, saving both time and money. Because the interaction between AFP and the immobilized AFP antibody is via electrostatic force [24], AFP can be removed from the electrode's surface by using regeneration solution. We found, at pH 2.50 50 mmol/L glycine-HCl buffer, that most of the AFP can be effectively removed from the AFP Ab1 on the surface of electrodes. But we still found that some of AFP Ab1 on the surface was still occupied by AFP after the regeneration step because there is a reduction of current signals between 10% and 15%. However, if an additional magnetic field (0.3 mT) was vertically added on the surface of the electrode outside the electrolytic cell, then, rinsing the electrode in the same regeneration buffer, the electrode can be regenerated fully. Its detection signal can be completely recovered. This may be due to the superparamagnetism of DNA/ZMPs probes, which can be helpful to remove AFP antigen from AFP Ab1 on the electrode surface through magnetic force. Updated electrode can be employed to do several detections, the measured results of 100 ng/mL and 15 ng/mL AFP samples were, respectively, 99.3 and 15.7 ng/mL, and the RSD is 2.4% and 3.2% respectively. The results indicated that the electrode can be reused with good reproducibility for up to 45 times with an RSD lower than 3.4%.
The effect of substances that might interfere with the response of the biosensor system was also studied. It shows that, when the concentration of the AFP is 5 ng/mL, the changes of signal of the sensor to the major interferences, such as 10 times the cancer embryo antigen (CEA) and hepatitis B virus (HBV) in serum, 200 times the bovine serum albumin, (BSA), glucose, and uric acid, 800 times Na+, Fe2+, Fe3+, Zn2+, and Ca2+, are all lesser than 5% which suggests that the sensor can effectively resist the main interference in human serum.
3.7. The Application of Immunosensor on Detecting AFP in Human Serum
As shown in Table 2, the level of AFP in human serum was determined by this method. In the analysis the serum was diluted about 50 ~ 100 times to reduce the matrix effect. The recoveries were between 95% and 105%. This technique can be applied for the quantitative analysis of the amount of CEA in human serum.
Table 2.
Results for determination of AFP in human serum comparing with ELISA (n = 3).
| Sample | AFP concentration (ng/mL) | ||||
|---|---|---|---|---|---|
| This method | ELISA | Added | Found | Recovery %a | |
| 1 | 5.32 | 5.18 | 5.0 | 10.2 | 97.6 |
| 2 | 9.74 | 10.3 | 9.0 | 18.3 | 95.1 |
| 3 | 20.52 | 21.3 | 20.0 | 40.7 | 100.9 |
aRecovery = 100%∗(found − this method)/added.
4. Conclusions
The immunoassay, based on the highly specific antibody-antigen recognition, can be used in the sensitive quantitative detection of AFP. The greatly enhanced sensitivity relies upon a dual signal-amplification scheme: (1) ZMPs as the enzyme-loading carrier can load many HRP enzyme molecules on each ZMP. Because DNA can combine many ZMP, the labeling protocol allows multiple signals per binding nano chain, (2) nafion/nano Au membrane can provide a high density of primary antibodies because of their high surface area, and (3) The probe can be used in separation, enrichment, and detection. After detection, it can be moved from the surfaces of the electrodes by the external magnetic field to make the surface of the immunosensor update. The described method was shown as acceptable detection with fabrication reproducibility. It is useful for clinical detection of ultra tumor marker levels in human serums.
Acknowledgments
The authors appreciate the support of the National Natural Science Foundation of China (20805024) and the Science and Technology Program of Guangdong, Zhejiang Province and Ningbo (2010A030300006; 2011C23126, 2011A610018, 2009R50025 and 2011C50038).
References
- 1.Zhang YZ, Zhang KY, Ma HY. Electrochemical DNA Biosensors Based on Gold Nanoparticles / Cysteamine / Poly(glutamic acid) Modified Electrode. Am. J. Biomed. Sci. 2009;1(2):115–125. [Google Scholar]
- 2.Wiwanitkit V. Alpha fetoprotein for screening for hepatocellular cancer in populations with viral hepatitis B: an appraisal of Thai reports. Asian Pacific Journal of Cancer Prevention. 2005;6(4):535–536. [PubMed] [Google Scholar]
- 3.Chang HX, Yuan Y, Shi NL, Guan YF. Electrochemical DNA biosensor based on conducting polyaniline nanotube array. Anal Chem. 2007;79(13):5111–5115. doi: 10.1021/ac070639m. [DOI] [PubMed] [Google Scholar]
- 4.Ci YX, Qin Y, Chang WB, Li YZ. Application of a mimetic enzyme for the enzyme immunoassay for α-1-fetoprotein. Analytica Chimica Acta. 1995;300(2):273–276. [Google Scholar]
- 5.Lianidou ES, Ioannou PC, Sacharidou E. Second derivative synchronous scanning fluorescence spectrometry as a sensitive detection technique in immunoassays. Application to the determination of α-fetoprotein. Analytica Chimica Acta. 1994;290(1-2):159–165. [Google Scholar]
- 6.Du D, Wang L, Shao Y, Wang J, Engelhard MH, Lin Y. Functionalized graphene oxide as a nanocarrier in a multienzyme labeling amplification strategy for ultrasensitive electrochemical immunoassay of phosphorylated p53 (s392) Analytical Chemistry. 2011;83(3):746–752. doi: 10.1021/ac101715s. [DOI] [PubMed] [Google Scholar]
- 7.Wei Q, Xiang Z, He J, et al. Dumbbell-like Au-Fe3O4 nanoparticles as label for the preparation of electrochemical immunosensors. Biosensors and Bioelectronics. 2010;26(2):627–631. doi: 10.1016/j.bios.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Ran XQ, Yuan R, Chai YQ, Hong CL, Qian XQ. A sensitive amperometric immunosensor for alpha-fetoprotein based on carbon nanotube/DNA/Thi/nano-Au modified glassy carbon electrode. Colloids and Surfaces B. 2010;79(2):421–426. doi: 10.1016/j.colsurfb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Du D, Zou Z, Shin Y, et al. Sensitive immunosensor for cancer biomarker based on dual signal amplification strategy of graphene sheets and multienzyme functionalized carbon nanospheres. Analytical Chemistry. 2010;82(7):2989–2995. doi: 10.1021/ac100036p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra R, Patel V, Vaqué JP, Gutkind JS, Rusling JF. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Analytical Chemistry. 2010;82(8):3118–3123. doi: 10.1021/ac902802b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui R, Zhu JJ. Fabrication of a novel electrochemical immunosensor based on the gold nanoparticles/colloidal carbon nanosphere hybrid material. Electrochimica Acta. 2010;55(27):7814–7817. [Google Scholar]
- 12.Hu Y, Carr PW. Synthesis and characterization of new zirconia-based polymeric cation-exchange stationary phases for high-performance liquid chromatography of proteins. Analytical Chemistry. 1998;70(9):1934–1942. doi: 10.1021/ac9710240. [DOI] [PubMed] [Google Scholar]
- 13.Sun L, McCormick AV, Carr PW. Study of the irreversible adsorption of proteins on polybutadiene-coated zirconia. Journal of Chromatography A. 1994;658(2):465–473. doi: 10.1016/0021-9673(94)80037-5. [DOI] [PubMed] [Google Scholar]
- 14.Xie Y, Yuan R, Chai YQ. Analysis and test center China; HaikouChina. Immobilization of DNA-Hb on zirconia thin film modified gold electrode for preparation of a hydrogen peroxide biosensor. Chemical Sensors. 2008;18:32–38. [Google Scholar]
- 15.Shang L, Liu X, Zhong J, Fan C, Suzuki I, Li G. Fabrication of ultrathin, protein-containing films by layer-by-layer assembly and electrochemical characterization of hemoglobin entrapped in the film. Chemistry Letters. 2003;32(3):296–297. [Google Scholar]
- 16.Wang J, Chen Q, Zeng C, Hou B. Magnetic-field-induced growth of single-crystalline Fe3O4 nanowires. Advanced Materials. 2004;16(2):137–140. [Google Scholar]
- 17.Yu J, Ju H. Amperometric biosensor for hydrogen peroxide based on hemoglobin entrapped in titania sol-gel film. Analytica Chimica Acta. 2003;486(2):209–216. [Google Scholar]
- 18.Wu YZ, Xu WL, Hou JG, Gan N, Li TH. Determination of trace organophosphous pesticides in vegetables by inductively coupled plasma-atomic emission spectrometry based on magnetic enrichment by nano Fe3O4@ZrO2. Chinese Journal of Pesticide Science. 2010;2:178–184. [Google Scholar]
- 19.Wu J, Tang J, Dai Z. A disposable electrochemical immunosensor for flow injection immunoassay of carcinoembryonic antigen. Biosensors and Bioelectronics. 2006;22(1):102–108. doi: 10.1016/j.bios.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Chen C, Liu S. Enzyme-functionalized silica nanoparticles as sensitive labels in biosensing. Analytical Chemistry. 2009;81(4):1600–1607. doi: 10.1021/ac802345z. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Ju H. Preparation of porous titania sol-gel matrix for immobilization of horseradish peroxidase by a vapor deposition method. Analytical Chemistry. 2002;74(14):3579–3583. doi: 10.1021/ac011290k. [DOI] [PubMed] [Google Scholar]
- 22.Yuan S, Yuan R, Chai Y, et al. Sandwich-type electrochemiluminescence immunosensor based on Ru-silica@Au composite nanoparticles labeled anti-AFP. Talanta. 2010;82(4):1468–1471. doi: 10.1016/j.talanta.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 23.Ou C, Yuan R, Chai Y, Tang M, Chai R, He X. A novel amperometric immunosensor based on layer-by-layer assembly of gold nanoparticles-multi-walled carbon nanotubes-thionine multilayer films on polyelectrolyte surface. Analytica Chimica Acta. 2007;603(2):205–213. doi: 10.1016/j.aca.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 24.Aguilar ZP, Vandaveer WR, Fritsch I. Self-contained microelectrochemical immunoassay for small volumes using mouse IgG as a model system. Analytical Chemistry. 2002;74(14):3321–3329. doi: 10.1021/ac0110348. [DOI] [PubMed] [Google Scholar]


