Abstract
Effects of gustatory nerve transection on salt taste have been studied extensively in rats and hamsters but have not been well explored in the mouse. We examined the effects of chorda tympani (CT) nerve transection on NaCl taste preferences and thresholds in outbred CD-1 mice using a high-throughput phenotyping method developed in our laboratory. To measure taste thresholds, mice were conditioned by oral self-administration of LiCl or NaCl and then presented with NaCl concentration series in 2-bottle preference tests. LiCl-conditioned and control NaCl-exposed mice were given bilateral transections of the CT nerve (LiCl-CTX, NaCl-CTX) or were left intact as controls (LiCl-CNT, NaCl-CNT). After recovery from surgery, mice received a concentration series of NaCl (0–300 mM) in 48-h 2-bottle tests. CT transection increased NaCl taste thresholds in LiCl-conditioned mice and eliminated avoidance of concentrated NaCl in control NaCl-exposed mice. This demonstrates that in mice, the CT nerve is important for detection and recognition of NaCl taste and is necessary for the normal avoidance of high concentrations of NaCl. The results of this experiment also show that the method of high-throughput phenotyping of salt taste thresholds is suitable for detecting changes in the taste periphery in mouse genetic studies.
Keywords: conditioned taste aversion, LiCl, taste preferences, taste threshold
Introduction
Ingested food and drink activate taste receptor cells, most of which are innervated by the chorda tympani (CT), glossopharyngeal (GL), and greater superficial petrosal (GSP) nerves. These gustatory nerves have different receptive fields: the CT innervates fungiform papillae, the GL innervates vallate and foliate papillae, and the GSP innervates the taste buds on the soft palate (Witt et al. 2003). These taste papillae differ in expression of genes involved in taste reception, for example, Tas1r1, Tas1r2, and Pkd113 (reviewed in: Bachmanov and Beauchamp 2007). This probably results in different responsiveness of the gustatory nerves to different taste qualities (e.g., Ninomiya 1998; Inoue et al. 2001; Danilova and Hellekant 2003). Furthermore, the roles of gustatory nerves are not necessarily similar between species. For example, rats and calves (Bos taurus) have stronger neural responses to sweet taste stimuli from the GL in comparison to the CT (Hard af Segerstad and Hellekant 1989; Segerstad and Hellekant 1989), whereas in mice and primates, CT responses to sweeteners are much stronger than GL responses (Shingai and Beidler 1985; Ninomiya et al. 1991; Danilova et al. 2002; Danilova and Hellekant 2003).
Studies with genetically engineered mice are important for understanding molecular mechanisms of salt taste reception (Bosak et al. 2010; Chandrashekar et al. 2010). However, the contributions of different gustatory nerves to salt taste in mice are less clear compared with some other species. The CT is important in the detection and recognition of sodium chloride (NaCl) taste in most mammals examined: rats (Spector and Grill 1992; Sollars and Bernstein 1994; Breslin et al. 1995; Sollars et al. 1996; Blonde et al. 2006; Spector et al. 2010; ), hamsters (Barry et al. 1996), and humans (Just et al. 2006; Karatayli-Ozgursoy et al. 2009). In rats and/or hamsters, transection of the CT diminishes NaCl avoidance in preference tests (Barry et al. 1996), impairs discrimination between NaCl and KCl (Spector and Grill 1992), and increases NaCl taste thresholds (Spector and Grill 1992; St John, Markison, Guagliardo, et al. 1997; Blonde et al. 2006). Much less is known about effects of gustatory nerve transection on salt taste in mice. Although gustatory nerve transection was used in several previous studies with mice (e.g., Uchida et al. 2003; Yasumatsu et al. 2003, 2007; St John and Boughter 2004; Shigemura et al. 2005; Yee et al. 2005), effects on salt taste were examined in only one study: Watanabe et al. (2003) have shown that CT transection has little effect on salt solution intake in the Nax-deficient mouse strain. No studies have examined the effects of nerve section on taste thresholds in any mouse strain.
As mentioned above, rats and mice differ in the relative contribution of the CT and GL nerves to sweet taste responsiveness (Frank et al. 1983; Frank 1991; Danilova and Hellekant 2003), and they may differ in salt taste responsiveness as well. As mice are the predominant model organism used in molecular genetics, it is important that we understand the contributory role of various gustatory nerves in mouse salt taste responses. We therefore conducted the present study to characterize the role of the CT nerve in salt taste perception in mice, with a focus on sensitivity and hedonics.
We measured NaCl taste thresholds using a conditioned taste aversion (CTA)–based method optimized in our laboratory for high-throughput testing of mice. This method is based on similar taste quality perception of NaCl and LiCl (Nachman 1963) and produces thresholds that correspond to taste recognition thresholds in humans (Ishiwatari and Bachmanov 2009). Mice were conditioned by self-administration of LiCl, given a bilateral transection of the CT, and then were tested with a series of ascending NaCl concentrations using 46-h 2-bottle preference tests to determine taste thresholds and to compare them with thresholds of mice that did not have CT surgery. Another group of unconditioned mice underwent a similar procedure but were offered NaCl instead of being exposed to LiCl. Comparison of NaCl preferences in these mice with and without CT transection has characterized the role of CT in salty taste hedonics.
Materials and methods
Subjects
Forty naive outbred CD-1 male mice (Charles River Laboratories) were used. At the beginning of the experiment, they were 8 weeks old and had a mean body weight of 30.2 ± 0.2 g. During experiments, they were housed in individual cages in a temperature-controlled room at 23 °C on a 12:12 h light: dark cycle (7:00 AM on, 7:00 PM off) and had free access to Teklad Rodent Diet 8604 (Harlan), which includes 0.29% sodium. All experimental protocols were approved by the Monell Chemical Senses Center Institutional Animal Care and Use Committee.
Apparatus
Construction of the drinking tubes and cage lids has been described previously (Bachmanov, Tordoff, et al. 1998) and is given in detail on the Monell Mouse Taste Phenotyping Project Web site (Tordoff and Bachmanov 2001; http://www.monell.org/MMTPP/).
Development of CTA
Mice were randomly assigned to 2 treatment groups, NaCl (control group; n = 20) and LiCl (conditioned group; n = 20). All were given deionized water in 2 drinking tubes for 3 days to adapt them to the experimental setting (Table 1). Following adaptation, mice were allowed to drink 150 mM LiCl for the conditioned group or 150 mM NaCl for the control group during two 23-h sessions (days 4 and 6), with the same solution continuously available from both drinking tubes. The 2 conditioning days were separated by a 24-h period of access to water given in both tubes (day 5, recovery). To ensure that an aversion was conditioned, the response to salt taste was assessed in the animal’s home cages in a 46-h 2-bottle test with one tube containing a 150 mM NaCl solution in deionized water and the other tube containing only deionized water (days 7 and 8). The side on which the NaCl solution was presented was counterbalanced among groups and was switched after 23 h. Intake measurements were made by reading fluid volume to the nearest 0.1 mL. NaCl-conditioned mice were indifferent to 150 mM NaCl (mean ± standard error [SE] preference scores were 48 ± 4%) but all LiCl-conditioned mice strongly avoided 150 mM NaCl (mean ± SE preference scores were 2 ± 0.3%; the individual preference scores ranged from 0.9% to 5.7%; see also Figure 1). A one-way analysis of variance (ANOVA) of NaCl preference scores revealed significant effects of treatment group, F1,36 = 128.72, P < 0.001. Thus, mice were successfully conditioned to avoid the taste of NaCl after self-administration of 150 mM LiCl in 2-bottle conditioning sessions.
Table 1.
Experimental schedule of treatments and solution presentation in different groups
| Day | Stage | NaCl-CNT | NaCl-CTX | LiCl-CNT | LiCl-CTX |
| 1–3 | Acclimation | H2O | H2O | H2O | H2O |
| 4 | Conditioning | 150 mM NaCl | 150 mM NaCl | 150 mM LiCl | 150 mM LiCl |
| 5 | Recovery | H2O | H2O | H2O | H2O |
| 6 | Conditioning | 150 mM NaCl | 150 mM NaCl | 150 mM LiCl | 150 mM LiCl |
| 7–8 | Preference testing | 150 mM NaCl versus H2O | 150 mM NaCl versus H2O | 150 mM NaCl versus H2O | 150 mM NaCl versus H2O |
| 9 | CT surgery | Surgery; H2O | No surgery; H2O | Surgery; H2O | No surgery; H2O |
| 10–11 | Recovery | H2O | H2O | H2O | H2O |
| 12–29 | Preference testing | 0–300 mM NaCl versus H2O | 0–300 mM NaCl versus H2O | 0–300 mM NaCl versus H2O | 0–300 mM NaCl versus H2O |
Figure 1.
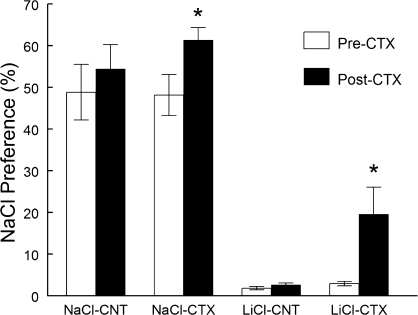
Mean ± standard error of the mean preference scores for 150 mM NaCl tested in 46-h 2-bottle tests before CT transection (on experimental days 7–8) and after CT transection (on days 26–27, during the concentration series testing). N = 10 for each group. Three-way ANOVA detected significant effects of treatment (mice conditioned with 150 mM LiCl had lower preference scores than mice exposed to 150 mM NaCl; F1,36 = 152.5, P < 0.0001), test (preference scores were lower before surgery than after surgery; F1,36 = 15.8, P = 0.0003), and interaction between test and surgery (preference scores before and after surgery were significantly different in CTX mice but not in CNT mice; F1,36 = 6.6; P = 0.01); all other effects and their interactions were not significant (F1,36 < 2.6, P > 0.1). *Significant difference between preference scores before and after CT transection, P < 0.05, Newman–Keuls tests for a 3-way (treatment × surgery × test) interaction.
CT transection surgery
On day 9, mice from both treatment groups (NaCl-exposed controls and LiCl-conditioned mice) were assigned to either CT surgery or no surgery conditions, so as to form a total of 4 groups, NaCl-CNT (NaCl control, no surgery; n = 10), NaCl-CTX (NaCl control, surgery; n = 10), LiCl-CNT (LiCl conditioned, no surgery; n = 10) and LiCl-CTX (LiCl conditioned, surgery; n = 10). Mice given CT surgery were deeply anesthetized with an intraperitoneal injection of a ketamine hydrochloride (42.8 mg/kg), xylazine hydrochloride (8.6 mg/kg), and acepromazine (1.5 mg/kg) mixture (10 mL/kg) and maintained with 1% isoflurane. The animal’s head was tilted 80° away from the surgeon. The auditory meatus was temporarily widened with a No. 7 microforceps and a second microforceps was used to remove the tympanic membrane. Visualization of the CT was achieved by deflection of the malleus rostrally. To prevent regeneration of the transected nerve, its visible part was removed with microforceps along with the malleus and remaining ossicles. Removing the bones eliminated the path the nerve normally follows during development resulting in a physical separation of the peripheral and central ends of the transected CT, which makes nerve regeneration unlikely in the time frame of this experiment (see Discussion for explanation). This procedure was conducted bilaterally. Mice were prophylactically treated with an antibiotic (2.0 mg/kg gentamicin intramuscularly) and given postoperative analgesic treatment (1.0 mg/kg buprenorphine subcutaneously).
In order to limit unnecessary invasive procedures, the surgical controls (NaCl-CNT and LiCl-CNT) in this study did not receive sham surgery. Previous work in rat (St John, Markison, Guagliardo, et al. 1997; St John, Markison, and Spector 1997; St John et al. 2003; Blonde et al. 2006), hamster (Barry et al. 1993, 1996), and mouse (St John and Boughter 2004) has shown that puncture of the tympanic membrane or exposure of the GL does not affect taste responses, and therefore, these procedures are not necessary for surgical control groups.
All mice were given deionized water in 2 drinking tubes on day 9 (surgery) and for the following 2 days (days 10 and 11) to allow the surgical groups to recover from the CTX procedure.
Postoperative preference testing
Starting on day 12, mice were tested with a series of NaCl solutions (0.0, 0.1, 0.3, 1.0, 3.0, 10.0, 30.0, 150.0, and 300.0 mM) presented in ascending order of concentration in 46-h 2-bottle preference tests with NaCl solution in one tube and water in the other tube (days 12–29). The positions of the tubes were switched every 24 h in a left, right, right, left pattern to control for side preferences. Intake measurements were made by reading fluid volume to the nearest 0.1 mL.
Histology
The CT innervates the fungiform papilla of the anterior tongue, and bilateral transection of the CT leads to degeneration of the no longer innervated papillae and the taste buds within (St John et al. 1995; Guagliardo and Hill 2007). Therefore, a decrease in percentage of fungiform papillae with taste pores is indicative of successful CT transection. To establish the effectiveness of CT transection in our study, we counted fungiform taste papilla and taste pores. After the final day of postsurgical testing, mice were euthanized by CO2 asphyxiation and the tongue of each mouse was removed and stored in 4% paraformaldehyde for at least 24 h. For counting taste papilla and taste pores, the anterior tongue was removed and soaked in distilled water for 0.5–1.0 h, immersed in 0.5% w/v methylene blue for 5 min and then briefly rinsed with distilled water. The ventral surface of the tongue was removed taking care to leave intact the pore-rich portion of the epithelium that curves under the front of the tongue. The remaining muscle layer connected to the lingual epithelium was removed by scraping with a no. 15 scalpel blade. The epithelium was pressed between 2 slides in order to observe the fungiform papillae under light microscopy and a 4 × image was recorded. An experiment-blind observer counted numbers of fungiform papillae containing taste pores and papillae devoid of taste pores for all groups; these data were used to calculate the percentage of fungiform papillae containing taste pores.
CTX mice had a significantly lower proportion of fungiform papillae with a taste pore than did CNT mice (41 ± 3% and 67 ± 3%, respectively; effect of nerve section: F1,34 = 29.57, P < 0.0001; 2-way ANOVA). There were no significant effects of treatment (LiCl or NaCl exposure) or treatment × group interaction (P > 0.96). In our study, CTX resulted in smaller reduction of the proportion of fungiform taste buds without taste pores in comparison to previous work in mice (St John and Boughter 2004) and rats (St John and Spector 1998). This discrepancy can be attributed to different species and strains of animals and different staining techniques used. For example, mouse strains differ in effects of taste nerve section on taste papillae numbers (St John and Boughter 2004), and the methylene blue staining method employed in our study was shown to have a small false-positive rate (St John et al. 1995; Parks and Whitehead 1998). The smaller reduction in percentage of taste buds without taste pores could also be due to incomplete sectioning of the CT, but this is unlikely given that we have observed a clear effect of CTX on taste responses. The smaller reduction could, in theory, also be due to CT regeneration by the end of our experiment, but this is again unlikely because our surgical procedure resulted in physical separation of the ends of the transected nerve to prevent regeneration.
Data analysis
There was no significant difference in initial body weights for all groups; thus, there was no need for intake correction according to body weight. Preference scores were calculated as the ratio of the 2-day average solution intake to the 2-day average total fluid (solution + water) intake. Significance of aversion or preference was examined by comparing intakes of a taste solution and water simultaneously presented in a 2-bottle test using paired t-tests.
In the presurgical 2-bottle tests, the 2-day average preference scores of the groups given LiCl and NaCl were analyzed by t-test. In the postsurgical 2-bottle tests, the 2-day average preference scores were analyzed by 3-way mixed-design ANOVA with treatment (LiCl and NaCl) and surgical group (CTX and CNT) as the between-group factors and concentration as a within-group factor (with 9 levels). Preference scores for 150 mM NaCl before and after CT transection were analyzed by 3-way mixed-design ANOVA with treatment (LiCl and NaCl) and surgical group (CTX and CNT) as the between-group factors and test (before and after surgery) as a within-group factor (with 2 levels). Newman–Keuls post hoc tests were used to evaluate differences between individual means.
The taste thresholds for groups and individuals were predicted from the NaCl preference scores using a 3-parameter logistic function modified from Ritz and Streibig (2005). Threshold was defined as the concentration at half performance (preference score of 25%), which approximates the 50% level of correct responses that is often used in psychophysics as a threshold value (Spector 2003; Bufe et al. 2005). The preference scores for all tested concentrations except 0 mM within each treatment group were fit to a regression curve using the function: fx = 50/(1 + exp(b(log(x) − log(c)))). Within the function, (x) is the stimulus concentration, (b) is the slope, and (c) is the threshold concentration. The maximum performance was set to 50% preference as complete indifference and minimum performance was set to 0% preference as complete avoidance in the function. We initially calculated individual taste thresholds for all subjects. However, individual taste threshold values that were higher than the 300 mM NaCl were excluded from data analyses because we considered these values not reliable (i.e., these mice did not avoid any of the NaCl concentrations tested, and so estimated thresholds with values >300 mM were not strongly supported by experimental data). Thresholds were >300 mM in 6 NaCl-CNT mice, all 10 NaCl-CTX mice, none of LiCl-CNT mice, and 2 LiCl-CTX mice. Because most of the mice in the NaCl-CNT and NaCl-CTX groups had thresholds >300 mM, we did not conduct statistical analyses of the individual thresholds in these 2 groups. Preference scores were analyzed in all mice regardless of whether their thresholds were >300 mM or not.
Statistical analyses were conducted using the Statistica software package (StatSoft, Inc., Tulsa, OK; http://www.statsoft.com) for ANOVA and using the statistical language and environment R (R Development Core Team 2010) for evaluating taste thresholds. P < 0.05 was used as the level of statistical significance. All data are presented as mean ± standard error of the mean.
Results
To determine if transection of the CT nerve altered the perception of NaCl taste, both control (NaCl-CNT and NaCl-CTX; Figure 2A) and conditioned (LiCl-CNT and LiCl-CTX; Figure 2B) mice were presented with a concentration series of NaCl (0–300 mM) in nine 46-h 2-bottle preference tests. Three-way ANOVA of NaCl preference scores revealed significant effects of treatment (LiCl-conditioned < NaCl-exposed), surgery (CTX > CNT), and concentration (decrease at higher NaCl concentrations); significant 2-way interactions between treatment and group, concentration and treatment, and concentration and group; and a significant 3-way interaction between treatment, group, and concentration (all P < 0.001).
Figure 2.
Mean ± standard error of the mean NaCl preference scores in mice with bilateral CT transection (CTX; open circles) and control mice (CNT; filled circles) conditioned by exposure to 150 mM NaCl (A) or 150 mM LiCl (B). N = 10 for each group. The curves were fit to the preference scores of each treatment group using the function described in the text (see Materials and Methods). The dotted horizontal line shows a 25% preference score used to calculate the taste threshold (i.e., the stimulus concentration at the intersection of the regression curve with the 25% preference level). *Significant difference between CTX and CNT mice within the same treatment group (i.e., NaCl or LiCl), P < 0.05, Newman–Keuls post hoc tests. +Significant avoidance of a taste solution, P < 0.05, paired t-tests comparing intakes of a solution and water given as 2 choices. (A) CTX mice conditioned with 150 mM NaCl have higher NaCl preference scores at 300 mM relative to CNT mice. The NaCl taste thresholds calculated using group data were 335 mM in the NaCl-CTX mice and 304 mM in the NaCl-CNT mice. (B) CTX mice conditioned with 150 mM LiCl have higher NaCl preference scores at 10, 30, and 150 mM in comparison to CNT mice. The NaCl taste thresholds calculated using group data were 129 mM in the LiCl-CTX mice and 9 mM in the LiCl-CNT mice.
The NaCl-exposed intact mice from the NaCl-CNT group were indifferent to 0–150 mM NaCl and avoided 300 mM NaCl (i.e., drank significantly less 300 mM NaCl than water available as the second choice, P = 0.0003, paired t-test; Figure 2A). Although most rat strains (Tordoff et al. 2008) and some mouse strains (Lush 1991; Bachmanov, Inoue, et al. 1998; Bachmanov et al. 2002) prefer osmotically hypotonic and isotonic NaCl solutions to water, many mouse strains (Lush 1991; Bachmanov, Inoue, et al. 1998; Bachmanov et al. 2002), including CD-1 mice used in this study, do not display preferences at any NaCl concentration. Avoidance of 300 mM NaCl without prior conditioning was commonly found in mice in previous studies (Lush 1991; Bachmanov, Inoue, et al. 1998; Bachmanov et al. 2002), probably because of its aversive sensory (Glendinning et al. 2002) and/or postingestive (Ishiwatari and Bachmanov 2009) properties. NaCl-CTX mice were indifferent to all NaCl concentrations tested, including 300 mM NaCl (P = 0.13, paired t-test). The NaCl-CTX mice had higher preference scores than did NaCl-CNT mice for 300 mM but not for other NaCl concentrations (P < 0.05, post hoc tests). This demonstrates that CT transection eliminated avoidance of 300 mM NaCl in NaCl-exposed mice.
The LiCl-exposed intact mice from the LiCl-CNT group were indifferent to 0–3 mM NaCl and avoided 10–300 mM NaCl (Figure 2B). The LiCl-CTX mice were indifferent to 0–30 mM NaCl and avoided 150 and 300 mM NaCl. The LiCl-CTX mice had higher preference scores than did LiCl-CNT mice for 10, 30, and 150 mM but not for other NaCl concentrations (P < 0.01, post hoc tests).
Although the testing procedure took 18 days, the CTA in the LiCl-CNT mice did not extinguish by the end of testing (Figure 1). All mice in the LiCl-CNT group avoided 150 mM NaCl as strongly as they did in the initial preference test with 150 mM NaCl on days 7–8 (3 ± 0.4% and 2 ± 0.4%, respectively; P = 0.9, post hoc tests; in both tests, the individual preference scores ranged from 1% to 5%). Although in the postsurgical tests LiCl-CTX mice had significantly higher preference scores for the 150 mM solution as compared with presurgical levels (Figure 1) and LiCl-CNT mice (Figure 2B), they significantly avoided this solution (P < 0.05, paired t-test). Furthermore, the LiCl-CTX group had significantly lower 150 mM NaCl preference scores than did the NaCl-CTX group (P = 0.0001, post hoc test; Figures 1 and 2). This indicates that CTA was retained in both LiCl-CNT and LiCl-CTX groups.
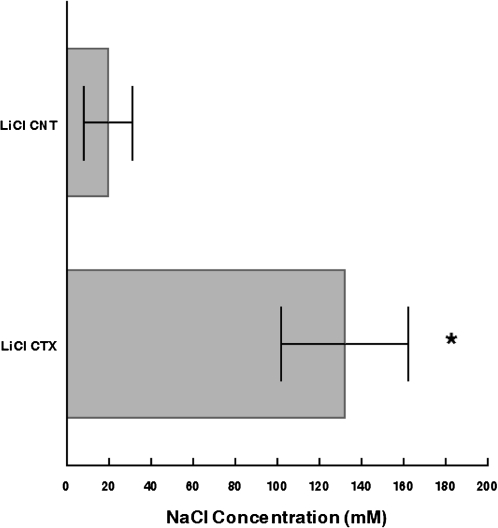
Averaged individual taste thresholds for LiCl-CTX and LiCl-CNT mice were 132 ± 30 mM and 20 ± 12 mM, respectively (Figure 3; P = 0.002, paired t-test). This is consistent with higher preference scores for 10–150 mM NaCl in the LiCl-CTX mice than in LiCl-CNT mice and shows that transection of the CT nerve alters salt taste sensitivity in mice.
Figure 3.
Mice with bilateral transection of the CT nerve exhibit elevated NaCl taste thresholds in comparison to controls. Mean ± standard error of the mean individual taste thresholds for LiCl-CTX (N = 8) and LiCl-CNT (N = 10) mice were 132 ± 30 and 20 ± 12, respectively (*P = 0.002, paired t-test).
Discussion
This is the first study to characterize the effect of the CT transection on salt taste thresholds in mice. We found that CT nerve transection eliminated avoidance of concentrated NaCl in control (NaCl-exposed) mice and increased salt taste thresholds in LiCl-conditioned mice.
Effect of CT transection in control (NaCl-exposed) mice
In NaCl-exposed (i.e., not conditioned) mice, CT transection eliminated avoidance of 300 mM NaCl. This was the highest concentration tested and the only concentration avoided by surgically intact mice. All other NaCl concentrations were neutral to surgically intact mice, and their preferences were not affected by CT transection. Our data correspond to results of experiments with hamsters (Barry et al. 1996) and Fisher 344 rats (Sollars et al. 1991; Sollars and Bernstein 1994), in which CT transection increased preference scores for aversive concentrations of NaCl. In mice, CT transection did not affect preferences for 150 mM NaCl, but this solution was not aversive (Watanabe et al. 2003). Therefore, in all 3 species (mice, rats, and hamsters), CT transection attenuates a natural taste aversion to concentrated NaCl, suggesting that the CT is necessary for the normal avoidance of high concentrations of NaCl.
Electrophysiological studies in mice have shown that the GL has only amiloride-insensitive NaCl responsive fibers (Ninomiya et al. 1991; Ninomiya 1998), but in some mouse strains, the CT has NaCl responsive fibers that are either amiloride-sensitive or amiloride-insensitive (Ninomiya et al. 1989, 1991, 1998; Gannon and Contreras 1995; Treesukosol et al. 2007). Amiloride sensitivity of the CT response to NaCl correlates with behavioral aversion to NaCl in mice (Ninomiya et al. 1989). Therefore, the attenuation of NaCl aversion after CT transection is likely due to the exclusion of the amiloride-sensitive mechanism.
Effect of CT transection in conditioned (LiCl-exposed) mice
The NaCl taste threshold obtained in a group of LiCl-conditioned mice with intact CT was 9 mM, which is similar to the NaCl taste threshold of 4 mM reported in our previous study using mice from the same strain, with similar treatment and calculation procedures (Ishiwatari and Bachmanov 2009). The CT transection dramatically increased NaCl taste threshold (to ∼130 mM). Although mice in the LiCl-CTX group did not avoid 30 mM and lower NaCl concentrations, they showed significant avoidance of 150 mM NaCl (a stimulus perceptually similar to the conditioned stimulus, 150 mM LiCl), which is evidence of continued aversion. These data are similar to results obtained with rats (Spector and Grill 1992; St John, Markison, Guagliardo, et al. 1997; Blonde et al. 2006): in both species, diminished peripheral input caused by CT transection results in higher salt taste thresholds.
Although CT transection increased NaCl taste thresholds, conditioned mice with CT transections still were able to detect and avoid NaCl at higher concentrations (Figure 2B). This residual responsiveness to NaCl in mice with CT transection could be due to several possible mechanisms:
Most likely, gustatory nerves other than the CT (i.e., GL and/or GSP) are contributing to the NaCl taste response in mice, but these other nerves are less sensitive. Electrophysiological studies have shown that the mouse GL is responsive to NaCl (Inoue et al. 2001, 2004), but NaCl responses in the GL have higher threshold than NaCl responses in the CT (Shingai and Beidler 1985; Ninomiya et al. 1991). Consistent with this, the amiloride-sensitive salt taste reception mechanism present in the receptive field of the mouse CT, but not GL nerve (Ninomiya et al. 1991; Ninomiya 1998), is more sensitive than the amiloride-insensitive mechanism present in the receptive fields of both nerves (Chandrashekar et al. 2010; Nelson TM, Bachmanov AA, unpublished data). Therefore, results of our study together with these published data suggest that decreased NaCl taste sensitivity after CT transection is likely due to the exclusion of the amiloride-sensitive mechanism. It is also possible that the amiloride-insensitive component of NaCl taste responses is more sensitive in the CT than in the GL, but we are not aware of any experimental data supporting this possibility.
Alternatively, different gustatory nerves could have similar sensitivity to NaCl, but the increase in NaCl taste thresholds after CT nerve transection could be due to a loss of the combined input of multiple gustatory nerves. Further studies that include the transection of other gustatory nerves singularly and in combination are needed to examine this possibility.
LiCl-CTX mice could also have avoided 150 mM NaCl if they developed CTA not only to salty taste but also to some other chemosensory cues of the conditioned stimulus (e.g., olfactory or viscerosensory). Should this be the case, this would only emphasize the prominent role of the CT in gustatory detection of NaCl, which would be sufficient to reduce sensitivity to NaCl even in presence of its other detectable cues.
In theory, the residual NaCl taste sensitivity in LiCl-CTX mice could be due to nerve regeneration. However, this is unlikely. First, our CT transection surgery was designed to prevent nerve regeneration (see details in Materials and methods). Second, even if CT regeneration would occur in operated mice, its time course would not be sufficiently fast to affect results of our behavioral tests. In several studies of CT regeneration in mice (Yasumatsu et al. 2003, 2007; Shigemura et al. 2005), surgery was designed to facilitate regeneration: the CT was exposed at ∼5 mm rostrally to its entry to the bulla and crushed so that nerve sheath would remain (to facilitate regrowth of neurons along the path of the peripheral part of the nerve). In these studies, the size of the taste buds in the fungiform papillae decreased after the nerve crush, and it returned to presurgical level at 3 weeks after surgery (Shigemura et al. 2005). Taste-evoked nerve activity was only partially restored 3 weeks after surgery, and it returned to a presurgical level only 5 or more weeks after surgery (Yasumatsu et al. 2003, 2007). If CT regeneration would occur in our experiment, it would take an even greater amount of time because we sectioned the nerve more centrally than it was crushed in the cited studies and because we made an effort to prevent nerve regeneration rather than facilitate it. Therefore, even a partial restoration of CT responsiveness due to regeneration in our mice would likely take longer than 21 days (time that our experiment lasted after CT transection). Should CT regeneration have occurred, it would diminish the influence of the CT transection on NaCl taste responses; in other words, without regeneration, the effect of CT transection would be even greater. Therefore, the possibility of CT regeneration in our experiment does not change our conclusions about the role of the CT in NaCl taste perception.
The method of measuring taste thresholds employed here is amenable to high-throughput investigations and so will be particularly useful for genetic studies (Ishiwatari and Bachmanov 2009). The results demonstrate that the technique can detect changes in taste mechanisms that occur in the oral cavity. Therefore, it should be able to detect changes not only due to nerve transection but also due to natural or genetically engineered allelic variants of genes expressed in taste bud cells. Other high-throughput behavioral tests such as 2-bottle preference tests of naive (not conditioned) mice are often used in taste genetics (e.g., Lush 1991; Bachmanov, Tordoff, et al. 1998; Bachmanov et al. 2002; Tordoff et al. 2007), but they are not well suited to study NaCl taste because of possible postingestive effects of NaCl (Rabe and Corbit 1973). This study demonstrates that our technique to measure behavioral NaCl taste thresholds is highly sensitive to changes in the taste periphery and is therefore suitable for genetic studies of salt taste.
Concluding remarks
In summary, the present study demonstrated that the CT plays an important role in salt taste perception by mice and that effects of CT transection are likely mediated by elimination of amiloride-sensitive mechanism of salt taste reception. It also validates the ability of a high-throughput method of assessing taste thresholds (Ishiwatari and Bachmanov 2009) to detect changes in peripheral taste input.
Funding
This work was supported by the National Institutes of Health (NIH) training grant (NIDCD 5T32DC000014-30 to G.J.G.) and NIH grant (R01 DC00882 to A.A.B.). Funding to pay the Open Access publication charges for this article was provided by NIH grant R01 DC00882.
Acknowledgments
The authors thank Drs Steven St John and John Glendinning for valuable insight into performing the surgery and Dr St John for instruction in histology for the validation of nerve transection. We also thank Drs Michael Tordoff and Bruce Kimball for their comments on the manuscript and Caroline Robiolle for her highly skilled technical assistance.
References
- Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2, and NH4Cl solutions by 28 mouse strains. Behav Genet. 2002;32:445–457. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Inoue M, Tordoff MG, Ninomiya Y, Beauchamp GK. Modification of behavioral and neural taste responses to NaCl in C57BL/6 mice: effects of NaCl exposure and DOCA treatment. Physiol Behav. 1998;65:817–822. doi: 10.1016/s0031-9384(98)00239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MA, Larson DC, Frank ME. Loss and recovery of sodium-salt taste following bilateral chorda tympani nerve crush. Physiol Behav. 1993;53:75–80. doi: 10.1016/0031-9384(93)90013-6. [DOI] [PubMed] [Google Scholar]
- Barry MA, Larson DC, Frank ME. Effects of chorda tympani transection on long-term salt preference in hamsters. Physiol Behav. 1996;60:347–352. [PubMed] [Google Scholar]
- Blonde GD, Garcea M, Spector AC. The relative effects of transection of the gustatory branches of the seventh and ninth cranial nerves on NaCl taste detection in rats. Behav Neurosci. 2006;120:580–589. doi: 10.1037/0735-7044.120.3.580. [DOI] [PubMed] [Google Scholar]
- Bosak NP, Inoue M, Nelson TM, Hummler E, Ishiwatari Y, Bachmanov AA. Epithelial sodium channel (ENaC) is involved in reception of sodium taste: evidence from mice with a tissue-specific conditional targeted mutation of the ENaCα gene (Abstract) AChemS XXXII Annual Meeting: 2010. St. Petersburg (FL): Chem Senses. 35:627--644. Full text available online at <http://chemse.oxfordjournals.org/>. [Google Scholar]
- Breslin PA, Spector AC, Grill HJ. Sodium specificity of salt appetite in Fischer-344 and Wistar rats is impaired by chorda tympani nerve transection. Am J Physiol. 1995;269:R350–R356. doi: 10.1152/ajpregu.1995.269.2.R350. [DOI] [PubMed] [Google Scholar]
- Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova V, Danilov Y, Roberts T, Tinti JM, Nofre C, Hellekant G. Sense of taste in a new world monkey, the common marmoset: recordings from the chorda tympani and glossopharyngeal nerves. J Neurophysiol. 2002;88:579–594. doi: 10.1152/jn.2002.88.2.579. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci. 2003;4:5. doi: 10.1186/1471-2202-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- Gannon KS, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol Behav. 1995;57:231–239. doi: 10.1016/0031-9384(94)00279-e. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Guagliardo NA, Hill DL. Fungiform taste bud degeneration in C57BL/6J mice following chorda-lingual nerve transection. J Comp Neurol. 2007;504:206–216. doi: 10.1002/cne.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard af Segerstad CH, Hellekant G. The sweet taste in the calf. II. Glossopharyngeal nerve responses to taste stimulation of the tongue. Physiol Behav. 1989;45:1043–1047. doi: 10.1016/0031-9384(89)90235-7. [DOI] [PubMed] [Google Scholar]
- Inoue M, Beauchamp GK, Bachmanov AA. Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2004;29:789–795. doi: 10.1093/chemse/bjh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Li X, McCaughey SA, Beauchamp GK, Bachmanov AA. Soa genotype selectively affects mouse gustatory neural responses to sucrose octaacetate. Physiol Genomics. 2001;5:181–186. doi: 10.1152/physiolgenomics.2001.5.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, Bachmanov AA. A high-throughput method to measure NaCl and acid taste thresholds in mice. Chem Senses. 2009;34:277–293. doi: 10.1093/chemse/bjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just T, Pau HW, Witt M, Hummel T. Contact endoscopic comparison of morphology of human fungiform papillae of healthy subjects and patients with transected chorda tympani nerve. Laryngoscope. 2006;116:1216–1222. doi: 10.1097/01.mlg.0000224509.61099.29. [DOI] [PubMed] [Google Scholar]
- Karatayli-Ozgursoy S, Ozgursoy OB, Muz E, Kesici G, Akiner MN. Evaluation of taste after underlay technique myringoplasty using whole-mouth gustatory test: smokers versus non-smokers. Eur Arch Otorhinolaryngol. 2009;266:1025–1030. doi: 10.1007/s00405-008-0856-9. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Genetics of perception and communications. New York: Marcel Dekker, Inc; 1991. pp. 227–241. [Google Scholar]
- Nachman M. Learned aversion to the taste of lithium chloride and generalization to other salts. J Comp Physiol Psychol. 1963;56:343–349. doi: 10.1037/h0046484. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y. Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci U S A. 1998;95:5347–5350. doi: 10.1073/pnas.95.9.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Funakoshi M. Strain differences in amiloride inhibition of NaCl responses in mice, Mus musculus. J Comp Physiol A. 1989;166:1–5. doi: 10.1007/BF00190204. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Tanimukai T, Yoshida S, Funakoshi M. Gustatory neural responses in preweanling mice. Physiol Behav. 1991;49:913–918. doi: 10.1016/0031-9384(91)90203-z. [DOI] [PubMed] [Google Scholar]
- Parks JD, Whitehead MC. Scanning electron microscopy of denervated taste buds in hamster: morphology of fungiform taste pores. Anat Rec. 1998;251:230–239. doi: 10.1002/(SICI)1097-0185(199806)251:2<230::AID-AR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rabe EF, Corbit JD. Postingestional control of sodium chloride solution drinking in the rat. J Comp Physiol Psychol. 1973;84:268–274. doi: 10.1037/h0035277. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2010. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Ritz C, Streibig JC. Bioassay analysis using R. J Stat Software. 2005;12:1–22. [Google Scholar]
- Segerstad CH, Hellekant G. The sweet taste in the calf. I. Chorda tympani proper nerve responses to taste stimulation of the tongue. Physiol Behav. 1989;45:633–638. doi: 10.1016/0031-9384(89)90084-x. [DOI] [PubMed] [Google Scholar]
- Shigemura N, Islam AA, Sadamitsu C, Yoshida R, Yasumatsu K, Ninomiya Y. Expression of amiloride-sensitive epithelial sodium channels in mouse taste cells after chorda tympani nerve crush. Chem Senses. 2005;30:531–538. doi: 10.1093/chemse/bji046. [DOI] [PubMed] [Google Scholar]
- Shingai T, Beidler LM. Response characteristics of three taste nerves in mice. Brain Res. 1985;335:245–249. doi: 10.1016/0006-8993(85)90476-7. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Bernstein IL. Gustatory deafferentation and desalivation: effects on NaCl preference of Fischer 344 rats. Am J Physiol. 1994;266:R510–R517. doi: 10.1152/ajpregu.1994.266.2.R510. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Sollars PJ, Bernstein IL. Reversal of the sodium chloride aversion of Fischer 344 rats by chorda tympani nerve transection. Behav Neurosci. 1991;105:603–605. doi: 10.1037//0735-7044.105.4.603. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Tracy CJ, Bernstein IL. Retention of conditioned taste aversion to NaCl after chorda tympani transection in Fischer 344 and Wistar rats. Physiol Behav. 1996;60:65–69. doi: 10.1016/0031-9384(95)02235-x. [DOI] [PubMed] [Google Scholar]
- Spector AC. Psychophysical evaluation of taste function in nonhuman mammals. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker, Inc; 2003. pp. 861–879. [Google Scholar]
- Spector AC, Blonde G, Garcea M, Jiang E. Rewiring the gustatory system: specificity between nerve and taste bud field is critical for normal salt discrimination. Brain Res. 2010;1310:46–57. doi: 10.1016/j.brainres.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol. 1992;263:R169–R176. doi: 10.1152/ajpregu.1992.263.1.R169. [DOI] [PubMed] [Google Scholar]
- St John SJ, Boughter JD., Jr The contribution of taste bud populations to bitter avoidance in mouse strains differentially sensitive to sucrose octa-acetate and quinine. Chem Senses. 2004;29:775–787. doi: 10.1093/chemse/bjh082. [DOI] [PubMed] [Google Scholar]
- St John SJ, Garcea M, Spector AC. The time course of taste bud regeneration after glossopharyngeal or greater superficial petrosal nerve transection in rats. Chem Senses. 2003;28:33–43. doi: 10.1093/chemse/28.1.33. [DOI] [PubMed] [Google Scholar]
- St John SJ, Markison S, Guagliardo NA, Hackenberg TD, Spector AC. Chorda tympani transection and selective desalivation differentially disrupt two-lever salt discrimination performance in rats. Behav Neurosci. 1997;111:450–459. [PubMed] [Google Scholar]
- St John SJ, Markison S, Spector AC. Salt discriminability is related to number of regenerated taste buds after chorda tympani nerve section in rats. Am J Physiol. 1995;269:R141–R153. doi: 10.1152/ajpregu.1995.269.1.R141. [DOI] [PubMed] [Google Scholar]
- St John SJ, Markison S, Spector AC. Chorda tympani nerve transection disrupts taste aversion learning to potassium chloride, but not sodium chloride. Behav Neurosci. 1997;111:188–194. doi: 10.1037//0735-7044.111.1.188. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18:4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project [Internet] Monell Chemical Senses Center, Philadelphia, PA.; 2001. Available from: http://www.monell.org/MMTPP/. Last accessed June 15,2011. [Google Scholar]
- Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007;91:620–631. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1799–R1809. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- Uchida N, Kanazawa M, Suzuki Y, Takeda M. Expression of BDNF and TrkB in mouse taste buds after denervation and in circumvallate papillae during development. Arch Histol Cytol. 2003;66:17–25. doi: 10.1679/aohc.66.17. [DOI] [PubMed] [Google Scholar]
- Watanabe U, Shimura T, Sako N, Kitagawa J, Shingai T, Watanabe E, Noda M, Yamamoto T. A comparison of voluntary salt-intake behavior in Nax-gene deficient and wild-type mice with reference to peripheral taste inputs. Brain Res. 2003;967:247–256. doi: 10.1016/s0006-8993(03)02247-9. [DOI] [PubMed] [Google Scholar]
- Witt M, Reutter K, Miller IJ. Morphology of peripheral taste system. In: Doty RL, editor. Handbook of olfaction and gustation. New York: Marcel Dekker; 2003. pp. 651–678. [Google Scholar]
- Yasumatsu K, Katsukawa H, Sasamoto K, Ninomiya Y. Recovery of amiloride-sensitive neural coding during regeneration of the gustatory nerve: behavioral-neural correlation of salt taste discrimination. J Neurosci. 2003;23:4362–4368. doi: 10.1523/JNEUROSCI.23-10-04362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu K, Kusuhara Y, Shigemura N, Ninomiya Y. Recovery of two independent sweet taste systems during regeneration of the mouse chorda tympani nerve after nerve crush. Eur J Neurosci. 2007;26:1521–1529. doi: 10.1111/j.1460-9568.2007.05761.x. [DOI] [PubMed] [Google Scholar]
- Yee C, Bartel DL, Finger TE. Effects of glossopharyngeal nerve section on the expression of neurotrophins and their receptors in lingual taste buds of adult mice. J Comp Neurol. 2005;490:371–390. doi: 10.1002/cne.20670. [DOI] [PubMed] [Google Scholar]