Abstract
A preterm female infant born at 27 weeks of gestation with a birth weight of 990 g developed acute hypotonia, apnea, hypotension and bradycardia mimicking septic shock syndrome at 14h after birth. Laboratory tests indicated a severe hypermagnesemia of 45 mg/dL. The renal function, complete blood count and maternal blood concentrations of magnesium were normal, and the blood cultures were negative. The patient recovered with treatment including exchange transfusion. However, the etiology of the severe hypermagnesemia remains unknown.
Keywords: Hypermagnesemia, Magnesium, Infant, Prematurity
Introduction
Magnesium (Mg) is the fourth most common cation and the second most common intracellular cation1,2). Mg is necessary for muscle contraction and neuronal transmission, and also acts as a cofactor for carbohydrate, protein and energy metabolism1,3,4). Newborn infants with hypermagnesemia might present with respiratory depression, hypotonia, hypotension, and bradycardia mimicking septic shock syndrome5) or gastrointestinal hypomotility mimicking intestinal obstruction6). Neonatal hypermagnesemia can be caused by increased Mg load such as with maternal Mg sulfate administration, newborn Mg therapy7,8), or decreased renal Mg excretion due to prematurity and asphyxia9). However, there is one case report of non-oliguric idiopathic hypermagnesemia developing in a premature infant5). Here, the second case of extreme hypermagnesemia of unknown etiology in an extremely low birth weight infant is reported.
Case report
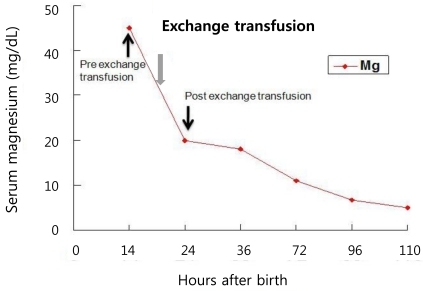
A female infant was born at 27 weeks of gestation with a birth weight of 990 g as a result of premature rupture of membranes and preterm labor. The infant was delivered by spontaneous vaginal delivery, and the Apgar scores at 1 and 5 minutes were 5 and 8, respectively. The baby was intubated and treated with exogenous surfactant for respiratory distress syndrome after admission to the neonatal intensive care unit (NICU). The patient was started on 125 g/L dextrose solution with calcium gluconate, 300 mg/kg per day of body weight per day. The patient was relatively stable and active until 14 hour after birth, when sudden respiratory depression and apnea developed. On physical examination, the patient with plethora was hypotonic with no spontaneous movement. She was afebrile with a body temperature of 36.3℃, and the deep tendon reflexes were diminished. The heart rate decreased from 130 to 140 beats per minute to 80 to 90 beats per minute, and the blood pressure was also decreased from 38/27 mmHg to 32/21 mmHg. Pertinent laboratory data indicated that the patient was severely hypermagnesemic (Table 1). An electrocardiogram was consistent with sinus bradycardia, the head ultrasound were negative for hemorrhage. Complete blood cell counts were: hemoglobin level, 15.4 g/L; hematocrit, 0.479 and; white blood cell count, 8,020/L with 17% neutrophils, 68% lymphocytes; the platelet count, was 239,000/L; the blood urea nitrogen (BUN) and creatinine (Cr) were within normal limits, and the blood cultures were negative. Urine output before the onset of symptoms was normal, transiently decreased for a few hours, and then increased thereafter. There was no history of exposure to magnesium containing compounds administered to either the mother or the baby on the delivery floor or in the NICU. The mother's blood magnesium level was normal (2.3 mg/dL). After the diagnosis of severe hypermagnesemia, treatments with intravenous calcium gluconate, hydration and diuretics were started without symptomatic improvement; the serum Mg concentration remained high. Next, a double volume exchange transfusion with a unit of blood was performed for a rapid decrease of the serum Mg level. Immediately after the exchange transfusion, spontaneous movement was observed, and the bradycardia and hypotension improved in association with the rapid decrease in the serum Mg level (Table 1). Once the patient became hemodynamically stabilized with adequate renal function, there was a continuous decline in the serum Mg level reaching normal values in five days (Fig. 1). The patient was extubated on day 6 and the rest of the hospital stay was uneventful. The baby was discharged on day 65.
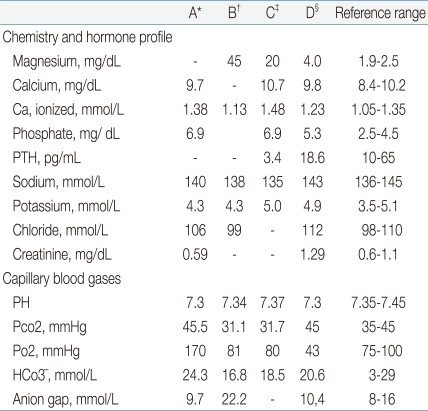
Table 1.
Time Course of Patient's Laboratory Data
PTH, parathyroid hormone.
*On admission. †At the time of the event (14 hours). ‡After the exchange transfusion (24 hours). §On the sixth day after birth.
Fig. 1.
Serum magnesium concentration of our patient at various times after birth. Mg, magnesium.
Discussion
Neonatal hypermagnesemia most commonly occurs after maternal Mg sulfate treatment for preeclampsia10,11). However, in this case, the mother did not have Mg sulfate therapy. Furthermore, no Mg therapy such as parenteral nutrition, antacids, or enemas was performed in the baby. The possibility of exogenous magnesium was considered, but there was no record of magnesium having been administered to the infant and no magnesium - containing fluid in our unit. These findings exclude an increased Mg load as the cause of hypermagnesemia in this patient. Although, the baby was very premature, the initial blood gas was normal and the renal functions including BUN, Cr and urine output during the first 12 hours after birth were normal. These findings exclude decreased renal Mg excretion due to prematurity and asphyxia as the cause of hypermagnesemia in this baby. Taken together, the patient was diagnosed with acute severe hypermagnesemia of unknown etiology.
Potassium is a major intracellular cation, and non-oliguric hyperkalemia observed in extremely low birth weight infants during the first 24 hours after birth might be due to a shift of potassium from the intracellular space to the extracellular space associated with a decrease in Na+, K+-ATPase activity12). Mg is also an essential intracellular cation1). Non-oliguric hypermagnesemia developed in this patient and a patients previously reported5). Overall, these findings suggest the possibility that the idiopathic hypermagnesemia might be attributable to an abnormal massive shift of Mg from the intracellular fluid to the extracellular fluid, and perturbations in the Mg influx/efflux regulation systems13). Further studies are necessary to confirm this possibility.
Although a decreased anion gap might be an indicator of hypermagnesemia, a normal anion gap, as observed in this case, has no documented negative predictive value for the diagnosis of hypermagnesemia14). The finding of transiently reduced parathyroid hormone (PTH) level during severe hypermagnesemia supports the assumption that hypermagnesemia might suppress PTH production and result in lower serum calcium (Ca) concentrations15). Furthermore, the transiently increased ionized Ca (iCa), before the exchange transfusion, suggests that hypermagnesemia might displace bound Ca in the circulation and cause the elevation of the serum iCa concentration.
Clinical signs of neuromuscular depression with floppiness, lethargy, and respiratory depression are frequent manifestations of severe neonatal hypermagnesemia9,10). Acute hypotonia, apnea, hypotension, and refractory bradycardia mimicking a septic shock like syndrome have been reported in premature infants accidentally overdosed with Mg in the parenteral nutrition8).
Treatments such as intravenous calcium gluconate infusion, adequate hydration and loop diuretics were useful in newborn with mild hypermagnesemia with good renal function. Dialysis or exchange transfusion are effective in reducing the serum Mg level and ameliorating the clinical signs of severe hypermagnesemia. Huey et al.5) reported a newborn case with idiopathic extreme hypermagnesemia successfully treated with exchange transfusion. As dialysis is not technically feasible in these micropremies, exchange blood transfusion might be the best therapeutic option for treating severely depressed hypermagnesemic infants5). Citrated donor blood is particularly useful because it will accelerate Mg removal from the baby9). However, hypocalcemia can occur as a common adverse event of exchange transfusion due to the presence of citrate. Thromocyptopenia, metabolic acidosis, bradycardia, apnea and hyponatremia are other complication, and should be monitored16).
Hypermagnesemia has been suggested as a poor prognostic factor; it is associated with a higher mortality rate in critically ill pediatric patients17). However, transient neonatal hypermagnesemia even in severely symptomatic cases has not been associated with long term sequelae8,18). These findings suggest that clinical suspicion, early diagnosis, prompt and appropriate treatment is key factors for the survival and improved outcome in newborns with severe hypermagnesemia.
References
- 1.Musso CG. Magnesium metabolism in health and disease. Int Urol Nephrol. 2009;41:357–362. doi: 10.1007/s11255-009-9548-7. [DOI] [PubMed] [Google Scholar]
- 2.Mofenson HC, Caraccio TR. Magnesium intoxication in a neonate from oral magnesium hydroxide laxative. J Toxicol Clin Toxicol. 1991;29:215–222. doi: 10.3109/15563659109038614. [DOI] [PubMed] [Google Scholar]
- 3.Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35:215–237. v–vi. doi: 10.1016/j.pop.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaze Folefack F, Stoermann Chopard C. Magnesium metabolism disturbances. Rev Med Suisse. 2007;3:605–606. 608, 610–611. [PubMed] [Google Scholar]
- 5.Huey CG, Chan KM, Wong ET, Nelson JM, Durand M. Los Angeles County-University of Southern California Medical Center clinical pathology case conference: extreme hypermagnesemia in a neonate. Clin Chem. 1995;41:615–618. [PubMed] [Google Scholar]
- 6.Sokal MM, Koenigsberger MR, Rose JS, Berdon WE, Santulli TV. Neonatal hypermagnesemia and the meconium-plug syndrome. N Engl J Med. 1972;286:823–825. doi: 10.1056/NEJM197204132861507. [DOI] [PubMed] [Google Scholar]
- 7.Teng RJ, Liu HC, Tsou Yau KI. Neonatal hypermagnesemia: report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1989;30:333–336. [PubMed] [Google Scholar]
- 8.Ali A, Walentik C, Mantych GJ, Sadiq HF, Keenan WJ, Noguchi A. Iatrogenic acute hypermagnesemia after total parenteral nutrition infusion mimicking septic shock syndrome: two case reports. Pediatrics. 2003;112(1 Pt 1):e70–e72. doi: 10.1542/peds.112.1.e70. [DOI] [PubMed] [Google Scholar]
- 9.Avery GB, MacDonald MG, Mullett MD, Seshia MM. Avery's neonatology: pathophysiology and management of the newborn. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 868. [Google Scholar]
- 10.Stone SR, Pritchard JA. Effect of maternally administered magnesium sulfate on the neonate. Obstet Gynecol. 1970;35:574–577. [PubMed] [Google Scholar]
- 11.Lipsitz PJ. The clinical and biochemical effects of excess magnesium in the newborn. Pediatrics. 1971;47:501–509. [PubMed] [Google Scholar]
- 12.Stefano JL, Norman ME. Nitrogen balance in extremely low birth weight infants with nonoliguric hyperkalemia. J Pediatr. 1993;123:632–635. doi: 10.1016/s0022-3476(05)80967-9. [DOI] [PubMed] [Google Scholar]
- 13.Günther T. Mechanisms and regulation of Mg2+ efflux and Mg2+ influx. Miner Electrolyte Metab. 1993;19:259–265. [PubMed] [Google Scholar]
- 14.Ortiz-Interian CJ, Schlessinger FB, Oster JR. Severe hypermagnesemia without reduction in the anion gap. Magnes Trace Elem. 1990;9:110–114. [PubMed] [Google Scholar]
- 15.Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med. 1984;310:1221–1225. doi: 10.1056/NEJM198405103101904. [DOI] [PubMed] [Google Scholar]
- 16.Jackson JC. Adverse events associated with exchange transfusion in healthy and ill newborns. Pediatrics. 1997;99:E7. doi: 10.1542/peds.99.5.e7. [DOI] [PubMed] [Google Scholar]
- 17.Broner CW, Stidham GL, Westenkirchner DF, Tolley EA. Hypermagnesemia and hypocalcemia as predictors of high mortality in critically ill pediatric patients. Crit Care Med. 1990;18:921–928. doi: 10.1097/00003246-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Oren S, Rapoport J, Zlotnik M, Brami JL, Heimer D, Chaimovitz C. Extreme hypermagnesemia due to ingestion of Dead Sea water. Nephron. 1987;47:199–201. doi: 10.1159/000184491. [DOI] [PubMed] [Google Scholar]