Abstract
The objective of this study was to assess whether women who do not take multinutrient supplements during early pregnancy are more susceptible to the effects of low-to-moderate alcohol consumption on preterm birth and small-for-gestational-age birth (SGA) compared to women who do take multinutrients. This analysis included 800 singleton live births to mothers from a cohort of pregnant women recruited for a population-based cohort study conducted in the Kaiser Permanente Medical Care Program in Northern California. Participants were recruited in their first trimester of pregnancy and information about their alcohol use and supplement intake during pregnancy was collected. Preterm birth (n = 53, 7%) was defined as a delivery prior to 37 completed weeks of gestation and SGA birth (n = 124, 16%) was defined as birth weight less than the 10th percentile for the infant’s gestational age and sex compared to US singleton live births. A twofold increase in the odds of SGA birth attributed to low-to-moderate alcohol intake was found among multinutrient supplement non-users (95% CI: 1.1, 5.3). Yet, among multinutrient supplement users, there was no increased risk of an SGA birth for women who drank low-to-moderately compared to women who abstained (aOR: 0.97, 95% CI: 0.6, 1.6). Similar results emerged for preterm birth. Our findings provide marginal evidence that multinutrient supplementation during early pregnancy may modify the risk of SGA births and preterm birth associated with alcohol consumption during pregnancy and may have important implications for pregnant women and women of child-bearing age. However, future research needs to be conducted.
Keywords: Low-to-moderate alcohol use, Multinutrient supplement use, Preterm birth, SGA birth, Multivitamin use
Introduction
It remains controversial as to whether there is a safe level of alcohol consumption during pregnancy. The effects of chronic heavy alcohol consumption have been implicated in contributing to adverse reproductive outcomes and neurobehavioral development [1–3]. However, research on the effects of low-to-moderate drinking during pregnancy has not been as definitive, thus signifying the importance of further investigation.
National surveys have documented an increasing trend in alcohol use among both pregnant women and women of child-bearing age [4–7]. In a recent study, 59% of pregnant women participants reported drinking in the past year [7]. The rates of reported past-year alcohol use among women of child-bearing age have ranged between 60 and 70% [6, 7], with nearly a third of the women reporting monthly alcohol use in one study [6]. Given that a woman’s knowledge of her pregnancy status may not occur until several weeks after conception and that 50% of the pregnancies in the US are not planned [8], there exists a high likelihood that many women may unintentionally expose their fetus to alcohol. These statistics contribute to the importance of research on the effects of low-to-moderate alcohol consumption.
The conclusions from a recent systematic review of the literature suggest low-to-moderate alcohol consumption may not have adverse effects on reproductive outcomes such as preterm birth [9]. Results from a majority of the studies reviewed demonstrated no adverse effects [10–17] and one study documented a protective effect against preterm birth [18]. In fact, historically, up through the 1970s ethanol was given to women to control against preterm delivery [19]. Similar non-adverse findings have been noted between low-to-moderate alcohol consumption and small-for-gestational-age (SGA) births, as well [10, 11, 17, 18, 20, 21]. However, studies have not assessed whether certain populations such as women who do not take multinutrients during early pregnancy are more vulnerable to preterm birth or SGA birth associated with low-to-moderate alcohol intake. Further clarification of these null findings would help identify whether or not pregnant multivitamin non-users are a susceptible group who would benefit from intervention.
Several areas of research support the possibility that women who do not take multinutrients during early pregnancy may be at a differential risk of adverse pregnancy outcomes attributed to low-to-moderate alcohol consumption. First, it has been documented that alcohol consumption, in general, can lead to various multinutrient deficiencies [22–26]. Second, animal models suggest that deficiencies of these nutrients during pregnancy may result in adverse fetal outcomes [27–30]. Finally, epidemiological studies have reported a protective effect of multinutrients against preterm birth [31, 32] and SGA birth [31]. The current study examines the relationship between multinutrient supplement use during early pregnancy, alcohol use and preterm and SGA birth. It was hypothesized that an increased risk of preterm birth and SGA birth attributed to low-to-moderate alcohol consumption would emerge only among the multinutrient supplement non-users.
Materials and Methods
Study Sample
This analysis includes 800 singleton live births to mothers from a cohort of 1,063 pregnant women originally recruited for a population-based cohort study conducted in the Kaiser Permanente Medical Care Program (KPMCP) in Northern California (Fig. 1). All KPMCP women members who lived in San Francisco County and parts of San Mateo County who had a positive pregnancy test at one of two San Francisco KPMCP facilities from October 1996 to October 1998 were identified through the computerized laboratory database as potential eligible subjects. A woman’s second pregnancy, if any, during the study period was not eligible for the study to avoid non-independent observations. All women submitting a urine sample for a pregnancy test were given a flyer describing the purpose and procedures of the study and a postage-paid and self-addressed return refusal card. Women with positive pregnancy tests who did not return a refusal postcard were contacted by a trained female interviewer to determine their eligibility for the study. English-speaking women who intended to carry their pregnancy to term and whose gestational age at the pregnancy test was less than or equal to 10 complete weeks were eligible for the study. The median gestational age at study entry was 40 days. The original study recruited women to evaluate the relationship between electromagnetic field exposure during pregnancy and miscarriage [33]. Figure 1 includes a detailed overview of the recruitment process and study sample.
Fig. 1.
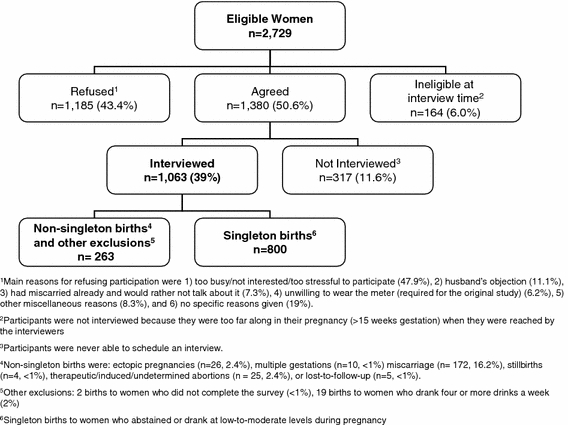
Recruitment process and study sample
In-person interviews were conducted by trained interviewers. Eighty-two percent of the interviews were completed within the first trimester of pregnancy. The remainder were completed prior to 18 weeks of pregnancy. Written informed consent was obtained from each participant. Institutional Review Board approval for this study was obtained from Kaiser Permanente’s Human Subjects Committee.
Measures
Preterm Birth
Preterm birth was defined as a delivery prior to 37 completed weeks of gestation. Gestational age was ascertained for participants through one of the following methods: physician-recorded gestation in the KPMCP automated databases, reviewing medical charts, or telephoning women (<7%). Gestational age at delivery was ascertained by telephone for those women who delivered outside of the Kaiser system for reasons predominately due to their insurance status. Ultrasound dating of pregnancy is routinely done within KPMCP.
Gestational age was ascertained for all 800 singleton live births.
Small for Gestational Age (SGA)
SGA was defined as birth weight less than the 10th percentile for the infant’s gestational age and sex compared to US single live births as documented by Alexander et al. [34]. Birth weight was obtained through the same methods conducted to ascertain gestational age. The final sample for SGA births was 790 as the birth weight of ten live births was not collected.
Low-to-Moderate Alcohol Use
Women were asked the “total number of beers (one beer is equal to 12 oz)”, “total number of glasses of wine or champagne (one glass is equal to 4 oz)”, and “total number of mixed drinks (one drink is equal to 1 oz of hard liquor) consumed since becoming pregnant or since your last menstrual period, LMP”. The average number of drinks consumed per week was calculated by adding the total number of drinks consumed, and dividing by the gestational age in weeks at the interview date. Based on previous research [15, 16, 35] and our sample size, low-to-moderate alcohol consumption was defined as consuming less than four drinks a week. Nineteen women reported drinking four or more drinks a week and were not included in this analysis. None of the nineteen women had a preterm birth, and only two had an SGA birth. The final sample consisted of 308 low-to-moderate drinkers and 492 abstainers.
Multinutrient Supplement Use
Participants were asked if they had taken any vitamins, including multiple, prenatal and single vitamins or any other type of supplements since becoming pregnant or their LMP. Women were asked about the type and brand of supplement for each supplement they reported taking. As multivitamins and prenatal vitamins are similar in their content of vitamins and other multinutrients, all women who reported taking either a multivitamin or a prenatal vitamin during pregnancy were considered multinutrient supplement users (n = 573). Each response to the type and brand of supplement used was reviewed and final categorization was conducted after ensuring (via familiarity with brands, internet searches, and visits to stores) that the type and brand reported were prenatal or multivitamin supplements.
Covariates
Participants were asked whether they used any illicit drugs, engaged in regular exercise (physical activity for 30 min at least three times a week), smoked, or consumed caffeine during pregnancy, and their height and weight. Pre-pregnancy Body Mass Index (BMI) was calculated (kg/m2) and categorized into two categories (1) underweight/normal ≤24.9 and 2) overweight/obese 25+. Other demographic characteristics considered were race (Black, Asian, White/Hispanic/Other), education (≤ some college/technical training vs. graduated from college or more education) and marital status (married/partnered versus single). Women were also asked about income and classified into four categories: <$35 k, $35k–$59 k, $60 k+ and non-responders (forty-two or 5% of the women). Age was dichotomized as less than or equal to 35 and 36 or older, because pregnancies occurring among women aged 36 or older are considered high risk. Finally, women were asked whether the current pregnancy was intended and about previous history of preterm or low birth weight birth. Information about previous SGA birth(s) was not collected.
Data Analysis
Pearson chi-square tests were performed to test differences in categorical variables. All confounders (variables significantly associated (P ≤ 0.05) with both alcohol consumption and either preterm birth or SGA birth), alcohol consumption, multinutrient supplement use, as well as the interaction term for alcohol consumption and multinutrient supplement use were included in the respective multivariable model. Tests for interaction generally have less power to test for statistical significance [36]. Therefore prior to any data analysis, it was determined that a corresponding P value of <0.10 for the Wald statistic on the interaction term would be the cut-off at which we would report the relationship between alcohol consumption and preterm birth among multinutrient supplement users and non-users separately. The same methods were used in models with SGA as the outcome. Post-hoc analyses were conducted with different cut-offs for low-to-moderate alcohol intake and both preterm birth and SGA birth.
Results
Low-to-moderate alcohol consumption was reported by 39% (n = 308) of the women (Table 1). In addition, 573 (72%) women reported taking multinutrient supplements (prenatal or multivitamin supplements) during early pregnancy, of whom 182 (32%) reported that they started taking them prior to pregnancy. Of the women using multinutrient supplements, 528 (93%) reported daily use. Seven percent (n = 53) of the women in our study had a preterm birth and 16% (n = 124) had an SGA birth (Table 2). Of the 53 preterm births, nine (18%) were very preterm (<33 weeks).
Table 1.
Demographic characteristics, pregnancy behaviors, pregnancy history by alcohol intake and multinutrient use
| Total sample n = 800 |
Low/moderate alcohol intake n = 308 n (%) |
P valuea | Multinutrient use n = 573 n (%) |
P valueb | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Maternal age 36+ | 135 | 64 (47) | 0.020 | 107 (79) | 0.031 |
| Maternal age <36 | 665 | 244 (37) | 466 (70) | ||
| Marital status | |||||
| Single | 62 | 31 (50) | 0.054 | 43 (69) | 0.222 |
| Married/partner | 737 | 277 (38) | 529 (72) | ||
| Race | |||||
| Black | 57 | 21 (37) | <0.001 | 36 (63) | <0.001 |
| Asian | 232 | 43 (19) | 144 (62) | ||
| White/Hispanic/other | 511 | 244 (48) | 393 (77) | ||
| Income | |||||
| <$35 k | 214 | 62 (29) | <0.001 | 132 (62) | <0.001 |
| $35 K–$59 K | 251 | 85 (34) | 182 (73) | ||
| $60 k+ | 293 | 148 (51) | 237 (81) | ||
| Non-responders | 42 | 13 (31) | 22 (52) | ||
| Education | |||||
| ≤ Some college/technical school | 449 | 142 (32) | <0.001 | 298 (67) | <0.001 |
| College graduate + | 349 | 165 (47) | 273 (78) | ||
| Pregnancy behaviors | |||||
| Illicit drug use | |||||
| Used drugs | 39 | 27 (69) | 0.029 | 22 (56) | 0.031 |
| Did not use drugs | 761 | 281 (37) | 551 (72) | ||
| Smoking status | |||||
| Smoked | 85 | 42 (49) | 0.029 | 60 (71) | 0.823 |
| Did not smoke | 715 | 266 (37) | 513 (72) | ||
| Exercise status | |||||
| Exercised regularly | 234 | 116 (50) | <0.001 | 186 (79) | <0.01 |
| Did not exercise regularly | 564 | 191 (34) | 386 (68) | ||
| Caffeine consumption | |||||
| Caffeine intake | 586 | 249 (42) | <0.001 | 418 (71) | 0.76 |
| No caffeine intake | 214 | 59 (28) | 155 (72) | ||
| Pregnancy intention | |||||
| Did not plan this pregnancy | 267 | 115 (43) | 0.06 | 174 (65) | 0.004 |
| Planned this pregnancy | 533 | 193 (36) | 399 (75) | ||
| Body mass index | |||||
| Underweight/normal | 536 | 211 (39) | 0.433 | 386 (69) | 0.916 |
| Overweight/obese | 239 | 87 (36) | 173 (72) | ||
| Previous pregnancy history | |||||
| Previous preterm birth | |||||
| Yes | 36 | 12 (33) | 0.514 | 25 (69) | 0.766 |
| No | 764 | 296 (39) | 548 (72) | ||
| Previous low birth weight birth | |||||
| Yes | 30 | 27 (23) | 0.082 | 21 (70) | 0.841 |
| No | 770 | 301 (39) | 552 (72) | ||
| Previous preterm birth/or low birth weight birth | |||||
| Yes | 50 | 15 (30) | 0.202 | 34 (68) | 0.557 |
| No | 750 | 293 (39) | 539 (72) | ||
| Alcohol consumption | |||||
| Low/moderate intake (<4 drinks/week) | 308 | NA | 242 (79) | 0.001 | |
| No alcohol intake | 492 | NA | 331 (67) | ||
| Multinutrient supplements | |||||
| Multinutrient non-user | 227 | 66 (29) | <0.001 | NA | |
| Multinutrient user | 573 | 242 (42) | NA | ||
a P value comparison for low/moderate alcohol intake versus no alcohol intake
b P value comparison for multinutrient use versus non-use
Table 2.
Alcohol consumption by multinutrient use by preterm birth and SGA birth
| Total sample (%) |
Preterm birth n=53 n (%) |
P value | Total sample (%) |
SGA n = 124 n (%) |
P value | |
|---|---|---|---|---|---|---|
| Total sample | 800 | 790 | ||||
| Low-to-moderate alcohol intake | 308 (38) | 17 (6) | 0.32 | 303 (38) | 48 (16) | 0.929 |
| No alcohol intake | 492 (62) | 36 (7) | 487 (62) | 76 (16) | ||
| Multinutrient non-user | 227 (28) | 222 (28) | ||||
| Low-to-moderate alcohol intakea | 66 (29) | 6 (9) | 0.19 | 65 (29) | 15 (23) | 0.04 |
| No alcohol intakea | 161 (71) | 7 (4) | 157 (71) | 19 (12) | ||
| Multinutrient user | 573 (72) | 568 (72) | ||||
| Low-to-moderate alcohol intakeb | 242 (42) | 11 (5) | 0.03 | 238 (42) | 33 (14) | 0.27 |
| No alcohol intakeb | 331 (58) | 29 (9) | 330 (58) | 57 (17) |
aPercent within multinutrient non-uses
bPercent within multinutrient users
Preterm Birth
Low-to-moderate alcohol consumers had a similar proportion of preterm births compared to abstainers (6% vs. 7%, respectively P = 0.320) (Table 2). Marital status and education were the only two potential covariates significantly associated with alcohol use as well as preterm birth. Therefore, they were adjusted for in the multivariable analyses.
The Wald test evaluating the interaction of multinutrient supplement use and low-to-moderate alcohol consumption on preterm birth while adjusting for marital status and education was significant (Wald test: 1.93, df (1), P value = 0.050; Pearson Chi-Square for the goodness-of-fit: 14.35, P value = 0.53). These statistics provide marginal evidence that multinutrient supplements modified the relationship between alcohol consumption during pregnancy and preterm birth. Table 3 displays the adjusted odds ratios for the relationship between alcohol consumption and preterm birth for both users and non-users of multinutrient supplements. Among multinutrient supplement non-users, women who drank alcohol had 2.02 times the odds of preterm birth compared to women who abstained after adjusting for marital status and education, although these results are not significant (95% CI: 0.6, 6.4). Among multinutrient supplement users women who drank had a non significant decreased odds of preterm birth compared to women who abstained (aOR: 0.53, 95% CI: 0.3, 1.1) after adjusting for marital status and education.
Table 3.
Adjusted odds ratios (aORs) for alcohol consumption and preterm birth and SGA birth stratified by multinutrient use status
| Preterm birth1 | SGA2 | |||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
| Total Samplea | 0.73 | 0.4, 1.3 | 1.01 | 0.7, 1.5 |
| Multinutrient Non-userb | 2.02 | 0.6, 6.4 | 2.4 | 1.1, 5.3 |
| Multinutrient Userb | 0.53 | 0.3, 1.1 | 0.97 | 0.6, 1.6 |
1aAdjusted for education, marital status and multinutrient use
1bAdjusted for education and marital status
2aAdjusted for race and multinutrient use
2bAdjusted for race
Additional analyses were conducted to assess the robustness of our findings. Similar trends were found when additionally adjusting for race (aOR for multinutrient supplement non-users: 1.74, 95% CI: 0.54, 5.62 and aOR for multinutrient supplement users: 0.56, 95% CI: 0.27, 1.17). In addition, analyses were conducted with a continuous measure of gestational age. Among multinutrient supplement non-users, a negative coefficient was found for low-to-moderate alcohol consumption (−0.20, 95% CI: −0.85, 0.5) while a positive coefficient was found among multinutrient supplement users (0.28, 95% CI: −0.04, 0.6).
We conducted a final analysis in which we restricted the sample to women who had not had a previous preterm birth (n = 764). The Wald test evaluating the interaction of multinutrient supplement use and low-to-moderate alcohol consumption on preterm birth while adjusting for marital status and education was also significant (Wald test: 2.17, df (1), P value = 0.03). These statistics indicate multinutrient supplements modified the relationship between alcohol consumption during pregnancy and preterm birth, among women who had not had a previous preterm birth. Similar trends emerged in the restriced sample (aOR for multinutrient supplement non-users: 2.2, 95% CI: 0.6, 7.36 and aOR for multinutrient supplement users: 0.44, 95% CI: 0.19, 1.03).
Small-for-Gestational-Age (SGA) Birth
The proportion of SGA births among women who drank low-to-moderate levels of alcohol during pregnancy was similar to women who abstained (16% for both, P = 0.93). Very few covariates were significantly associated with SGA birth (results not shown). Further, race (Black, Asian, White/Hispanic/Other) was the only covariate associated with both SGA and alcohol intake and was the only cofactor included in the multivariable models.
The Wald test evaluating the interaction between low-to-moderate alcohol consumption and multinutrient supplement use in relation to SGA birth was significant (Wald test: 1.96, df (1), P value = 0.050; Pearson Chi-Square for the goodness-of-fit: 8.15, P value = 0.23). These statistics provide marginal evidence that multinutrient supplement use modified the relationship between low-to-moderate alcohol consumption and SGA birth. Among multinutrient supplement non-users, women who drank alcohol had 2.4 times the odds of an SGA birth compared to women who did not drink alcohol (95% CI: 1.1, 5.3) after adjusting for race (Table 3). However, among multinutrient supplement users, the odds of an SGA birth were similar for women who drank low-to-moderately compared to women who abstained (aOR: 0.97, 95% CI: 0.60, 1.58) when controlling for race.
Post-Hoc Analysis
Similar patterns emerged when additional analyses were conducted modifying the cut-point for low-to-moderate consumption from <4 drinks/week to <2 drinks/week and 2–3 drinks/week. A significantly increased risk for preterm birth and SGA birth emerged associated with both categorizations of alcohol consumption (2 drinks/week and 2–3 drinks/week) compared to abstainers among the multinutrient supplement non-users and no adverse effect among multinutrient supplement users.
Discussion
Our findings provide marginal evidence for differential relationships between low-to-moderate alcohol consumption and preterm birth and SGA birth among women who take multinutrient supplements during early pregnancy and those who do not. Our findings suggest that among multinutrient non-users, women who drink low-to-moderate levels of alcohol during early pregnancy have an increased risk of an SGA birth, with a similar trend emerging for preterm birth. However, among women who took multinutrient supplements, an increased risk of preterm birth and SGA birth associated with alcohol use was not observed, thus suggesting a potential mitigating effect by multinutrient supplementation. These findings emerged despite our small sample size in some strata of the analyses. Nevertheless, these findings require further confirmation by future studies.
Previous research has suggested that low-to-moderate alcohol consumption is not associated with an increased risk of preterm birth and SGA births [10–16, 37]. Low-to-moderate consumption has been categorized in a variety of ways, but a protective effect against preterm birth and SGA birth has been consistently reported at levels defined as less than four drinks a week [15, 16]. While our findings are contrary to previous research, they offer a possible explanation; the risk of preterm birth or SGA birth associated with low-to-moderate alcohol consumption is dependent on multinutrient supplement use. Thus, multinutrient supplementation during early pregnancy may play an integral role in the underlying mechanism for the relationship between low-to-moderate alcohol consumption during pregnancy and both preterm and SGA birth. The main contribution of this study is suggesting that future studies evaluate the risk of preterm birth and SGA birth associated with alcohol consumption separately for women taking and not taking multinutrient supplements. The study findings are also supported by animal models and our previous research relating to miscarriage [38–51].
These findings should be interpreted in light of certain limitations. First, we were unable to differentiate between spontaneous and induced preterm births which may also affect our findings. However, the inability to separate subtypes of preterm delivery is likely to have attenuated our observed differences.
The generalizeability of the findings may be limited due to the low response rate for the original study (39%). We can not rule out with certainty that participation was not associated with factors related to both alcohol consumption and multinutrient supplement use; however, due to the prospective nature of the study design, participation was not likely associated with preterm birth or SGA birth. Thus, the impact of the low baseline recruitment rate is reduced. Additionally, the percent of miscarriage among the originally identified eligible non-participants was similar to that of participants (17.2% vs. 16.4%, respectively) further reducing the concern of the generalizeability of the sample. The study was conducted with participants from a managed-care health plan, which may also impact the generalizeability. However, managed care is a major health care model which represents private and public populations, including Medicaid arrangements. Finally, other papers published from these data have reported findings consistent with previous research [33, 52, 53].
The accuracy of self-reported alcohol consumption during pregnancy is always a concern. Comparisons of self-reported pregnancy drinking with the use of vessels (varying sizes of beer, wine and other glasses) to visualize the amount of alcohol consumed have found an underestimation of alcohol consumption resulting from self-report [54]. If the participants in our study under-reported their alcohol consumption during pregnancy, our findings would likely be attenuated due to misclassification of alcohol use.
It was not possible to assess the impact of binge-drinking due to the manner in which alcohol intake was ascertained. However, our post-hoc analyses demonstrated a significantly increased risk for preterm birth and SGA birth associated with <2 drinks a week and 2–3 drinks a week compared to abstainers among the multinutrient supplement non-users and no adverse effect among multinutrient supplement users. Additionally, alcohol exposure was limited to the first trimester of pregnancy. Nevertheless, research has linked oxidative stress in early pregnancy to SGA birth [55]. Oxidative stress is one of the hypothesized biological mechanisms of alcohol’s toxic effects [22–25].
Research assessing the potential role of nutrition as a modifier in the relationship between alcohol consumption and adverse pregnancy outcomes is limited. Our previous research has shown that women who did not take multinutrient supplements were at an increased risk of miscarriage associated with alcohol use during pregnancy [35]. Animal studies have demonstrated that interactions between alcohol and select nutrients can affect fetal development [38–46, 56]. For example, treatment with antioxidants such as Vitamins E and Beta Carotene, has been found to mitigate adverse cellular development attributed to ethanol in rats [41, 48, 49]. More recently, animal research has found choline supplementation and zinc supplementation to attenuate ethanol’s effects on birth weight and brain weight [57] and fetal abnormalilities and spatial memory [51], respectively. Thus it is plausible that multinutrients (which include a variety of vitamins and other multinutrients such as zinc, calcium and choline) may mitigate the risk of adverse pregnancy outcomes associated with alcohol use during pregnancy.
Conclusions
Our findings suggest multinutrient supplementation during early pregnancy may have implications for the risk of alcohol use in relation to preterm delivery and SGA. Thus, our findings imply that women who do not take multinutrient supplements during early pregnancy are especially susceptible to adverse alcohol-related pregnancy outcomes. Although our results need to be further confirmed in future studies, strikingly different trends between users and non-users of multinutrient supplements emerged even after adjustment for possible confounding factors. Previous research in animal models which have demonstrated that supplementation with various multinutrients mitigates the effects of prenatal alcohol exposure on fetal growth and development [41, 48, 49, 57], further support our findings. Maternal nutrition and supplement use should be considered in future research assessing the relationship between drinking during pregnancy and reproductive outcomes. Finally, the high rates of women of child-bearing age who report drinking and the high rate of unintended pregnancies increases both the chances and the numbers of alcohol exposed pregnancies. Therefore, it may be beneficial for public health campaigns to encourage multinutrient supplement use among women of child-bearing age.
Acknowledgments
The authors would like to thank Dr. Sylvia Guendelman for her thoughtful comments and suggestions on earlier versions of this manuscript. In addition, we would like to thank Dr. Mark Hudes for his statistical consult. The original data collection of this study was supported by the California Public Health Foundation. This manuscript was a chapter of Lyndsay Ammon Avalos’s dissertation which was supported by the NIAAA Training Grant: T32-AA007240-29.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jones KL, et al. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/S0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson JL, et al. Teratogenic effects of alcohol on infant development. Alcoholism, Clinical and Experimental Research. 1993;17(1):174–183. doi: 10.1111/j.1530-0277.1993.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Sokol RJ, Miller SI, Reed G. Alcohol abuse during pregnancy: An epidemiologic study. Alcoholism, Clinical and Experimental Research. 1980;4(2):135–145. doi: 10.1111/j.1530-0277.1980.tb05628.x. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Alcohol consumption among women who are pregnant or who might become pregnant—United States, 2002. Morbidity and Mortality Weekly Report. 2004;53(50):1178–1181. [PubMed] [Google Scholar]
- 5.Ebrahim SH, et al. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991–1995. American Journal of Obstetrics and Gynecology. 1999;180(1 Pt 1):1–7. doi: 10.1016/S0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- 6.Nayak MB, Kaskutas LA. Risky drinking and alcohol use patterns in a national sample of women of childbearing age. Addiction. 2004;99(11):1393–1402. doi: 10.1111/j.1360-0443.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 7.Caetano R, et al. The epidemiology of drinking among women of child-bearing age. Alcoholism, Clinical and Experimental Research. 2006;30(6):1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 8.Henshaw SK. Unintended pregnancy in the United States. Family Planning Perspectives. 1998;30(1):24–29. doi: 10.2307/2991522. [DOI] [PubMed] [Google Scholar]
- 9.Henderson J, Gray R, Brocklehurst P. Systematic review of effects of low-moderate prenatal alcohol exposure on pregnancy outcome. BJOG. 2007;114(3):243–252. doi: 10.1111/j.1471-0528.2006.01163.x. [DOI] [PubMed] [Google Scholar]
- 10.Parazzini F, et al. Moderate alcohol drinking and risk of preterm birth. European Journal of Clinical Nutrition. 2003;57(10):1345–1349. doi: 10.1038/sj.ejcn.1601690. [DOI] [PubMed] [Google Scholar]
- 11.Jaddoe VWV, et al. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The generation R study. Annals of Epidemiology. 2007;17(10):834–840. doi: 10.1016/j.annepidem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Lundsberg LS, Bracken MB, Saftlas AF. Low-to-moderate gestational alcohol use and intrauterine growth retardation, low birthweight, and preterm delivery. Annals of Epidemiology. 1997;7(7):498–508. doi: 10.1016/S1047-2797(97)00081-1. [DOI] [PubMed] [Google Scholar]
- 13.Passaro KT, et al. The effect of maternal drinking before conception and in early pregnancy on infant birthweight. The ALSPAC study team. Avon longitudinal study of pregnancy and childhood. Epidemiology. 1996;7(4):377–383. doi: 10.1097/00001648-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Verkerk PH, et al. The effect of moderate maternal alcohol consumption on birthweight and gestational age in a low risk population. Early Human Development. 1993;32(2–3):121–129. doi: 10.1016/0378-3782(93)90006-G. [DOI] [PubMed] [Google Scholar]
- 15.Kesmodel U, Olsen SF, Secher NJ. Does alcohol increase the risk of preterm delivery? Epidemiology. 2000;11(5):512–518. doi: 10.1097/00001648-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Albertsen K, et al. Alcohol consumption during pregnancy and the risk of preterm delivery. American Journal of Epidemiology. 2004;159(2):155–161. doi: 10.1093/aje/kwh034. [DOI] [PubMed] [Google Scholar]
- 17.Day N, et al. Prenatal exposure to alcohol: Effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–541. [PubMed] [Google Scholar]
- 18.McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. American Journal of Public Health. 1992;82(1):87–90. doi: 10.2105/AJPH.82.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs AR, Fuchs F. Ethanol for prevention of preterm birth. Seminars in Perinatology. 1981;5(3):236–251. [PubMed] [Google Scholar]
- 20.Chiaffarino F, et al. Alcohol drinking and risk of small for gestational age birth. European Journal of Clinical Nutrition. 2006;60(9):1062–1066. doi: 10.1038/sj.ejcn.1602419. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson JL, et al. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. Journal of Pediatrics. 1994;124(5 Pt 1):757–764. doi: 10.1016/s0022-3476(05)81371-x. [DOI] [PubMed] [Google Scholar]
- 22.Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: A possible mechanism of toxicity. American Journal of Obstetrics and Gynecology. 2000;182(3):682–688. doi: 10.1067/mob.2000.104201. [DOI] [PubMed] [Google Scholar]
- 23.Henderson GI, et al. In utero ethanol exposure elicits oxidative stress in the rat fetus. Alcoholism, Clinical and Experimental Research. 1995;19(3):714–720. doi: 10.1111/j.1530-0277.1995.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CS. Metabolism of alcohol. Clinics in Liver Disease. 2005;9(1):1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Research Health. 2001;25(3):175–184. [PMC free article] [PubMed] [Google Scholar]
- 26.Church MW, et al. Prenatal cocaine, alcohol, and undernutrition differentially alter mineral and protein content in fetal rats. Pharmacology, Biochemistry and Behavior. 1998;59(3):577–584. doi: 10.1016/S0091-3057(97)00478-4. [DOI] [PubMed] [Google Scholar]
- 27.Chaftez MD. Nutrition and neurotransmitters: The nutrient bases of behavior. Englewood Cliffs, NJ: Prentice Hall; 1990. p. 273. [Google Scholar]
- 28.Meadows NJ, et al. Zinc anc small babies. Lancet. 1981;2(8256):1135–1137. doi: 10.1016/S0140-6736(81)90587-0. [DOI] [PubMed] [Google Scholar]
- 29.Neggers YH, et al. The relationship between maternal serum zinc levels during pregnancy and birthweight. Early Human Development. 1991;25(2):75–85. doi: 10.1016/0378-3782(91)90186-7. [DOI] [PubMed] [Google Scholar]
- 30.Shaw GM, et al. Low birthweight, preterm delivery, and periconceptional vitamin use. The Journal of Pediatrics. 1997;130(6):1013–1014. doi: 10.1016/S0022-3476(97)70303-2. [DOI] [PubMed] [Google Scholar]
- 31.Scholl TO, et al. Use of multivitamin/mineral prenatal supplements: Influence on the outcome of pregnancy. American Journal of Epidemiology. 1997;146(2):134–141. doi: 10.1093/oxfordjournals.aje.a009244. [DOI] [PubMed] [Google Scholar]
- 32.Catov JM, et al. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. American Journal of Epidemiology. 2007;166(3):296–303. doi: 10.1093/aje/kwm071. [DOI] [PubMed] [Google Scholar]
- 33.Li DK, et al. A population-based prospective cohort study of personal exposure to magnetic fields during pregnancy and the risk of miscarriage. Epidemiology. 2002;13(1):9–20. doi: 10.1097/00001648-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Alexander GR, et al. A United States national reference for fetal growth. Obstetrics and Gynecology. 1996;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 35.Ammon Avalos L, et al. Do multivitamin supplements modify the relationship between prenatal alcohol intake and miscarriage? American Journal of Obstetrics and Gynecology. 2009;201(6):563.e561–563.e569. doi: 10.1016/j.ajog.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. Journal of Counseling Psychology. 2004;51(1):115–134. doi: 10.1037/0022-0167.51.1.115. [DOI] [Google Scholar]
- 37.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. American Journal of Public Health. 1992;82(1):85–87. doi: 10.2105/AJPH.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas JD, et al. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000;22(1):703–711. doi: 10.1016/S0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JD, et al. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behavioral Neuroscience. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 40.Wentzel P, Rydberg U, Eriksson UJ. Antioxidative treatment diminishes ethanol-induced congenital malformations in the rat. Alcoholism, Clinical and Experimental Research. 2006;30(10):1752–1760. doi: 10.1111/j.1530-0277.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 41.Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcoholism, Clinical and Experimental Research. 2000;24(4):512–518. doi: 10.1111/j.1530-0277.2000.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka H, Nasu F, Inomata K. Fetal alcohol effects: Decreased synaptic formations in the field CA3 of fetal hippocampus. International Journal of Developmental Neuroscience. 1991;9(5):509–517. doi: 10.1016/0736-5748(91)90037-M. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, et al. The maternal combined supplementation of folic acid and vitamin B(12) suppresses ethanol-induced developmental toxicity in mouse fetuses. Reproductive Toxicology. 2006;22(1):56–61. doi: 10.1016/j.reprotox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Cano MJ, et al. Protective effect of folic acid against oxidative stress produced in 21-day postpartum rats by maternal-ethanol chronic consumption during pregnancy and lactation period. Free Radical Research. 2001;34(1):1–8. doi: 10.1080/10715760100300011. [DOI] [PubMed] [Google Scholar]
- 45.Bâ A, et al. Comparative effects of developmental thiamine deficiencies and ethanol exposure on the morphometry of the CA3 pyramidal cells. Neurotoxicology and Teratology. 1999;21(5):579–586. doi: 10.1016/S0892-0362(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 46.Yeh L-CC, Cerklewski FL. Interaction between ethanol and low dietary zinc during gestation and lactation in the rat. Journal of Nutrition. 1984;114(11):2027–2033. doi: 10.1093/jn/114.11.2027. [DOI] [PubMed] [Google Scholar]
- 47.Zidenberg-Cherr S, et al. Altered mineral metabolism: A mechanical underlying the fetal alcohol syndrome in rats. Drug-Nutrient Interactions. 1988;5(4):257–274. [PubMed] [Google Scholar]
- 48.Mitchell JJ, Paiva M, Heaton MB. The antioxidants vitamin E and beta-carotene protect against ethanol-induced neurotoxicity in embryonic rat hippocampal cultures. Alcohol. 1999;17(2):163–168. doi: 10.1016/S0741-8329(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 49.Heaton MB, et al. Ethanol-induced reduction of neurotrophin secretion in neonatal rat cerebellar granule cells is mitigated by vitamin E. Neuroscience Letters. 2004;370(1):51–54. doi: 10.1016/j.neulet.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 50.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology and Teratology. 2009;31(5):303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summers BL, Rofe AM, Coyle P. Dietary zinc supplementation throughout pregnancy protects against fetal dysmorphology and improves postnatal survival after prenatal ethanol exposure in mice. Alcoholism, Clinical and Experimental Research. 2009;33(4):591–600. doi: 10.1111/j.1530-0277.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 52.Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: A prospective cohort study. American Journal of Obstetrics and Gynecology. 2008;198(3):279.e271–279.e278. doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 53.Li DK, et al. Hot tub use during pregnancy and the risk of miscarriage. American Journal of Epidemiology. 2003;158(10):931–937. doi: 10.1093/aje/kwg243. [DOI] [PubMed] [Google Scholar]
- 54.Kaskutas LA. Understanding drinking during pregnancy among urban American Indians and African Americans: Health messages, risk beliefs, and how we measure consumption. Alcoholism, Clinical and Experimental Research. 2000;24(8):1241–1250. doi: 10.1111/j.1530-0277.2000.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 55.Potdar N, et al. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG. 2009;116(5):637–642. doi: 10.1111/j.1471-0528.2008.02096.x. [DOI] [PubMed] [Google Scholar]
- 56.Zidenberg-Cherr S, et al. Altered mineral metabolism: A mechanism underlying the fetal alcohol syndrome in rats. Drug-Nutrient Interactions. 1988;5(4):257–274. [PubMed] [Google Scholar]
- 57.Thomas JD, Abou EJ, Dominguez HD. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicology and Teratology. 2009;31(5):303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


