Abstract
Earthworm extract has shown anticancer characteristics. In the present study, we examined the effect of chronic treatment with a high dose of earthworm (Eisenia andrei) extract (EE) on cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus (DG) of 3-week-old mice using 5-bromo-2'-deoxyuridine (BrdU) and Ki-67 immunohistochemistry for cell proliferation and doublecortin (DCX) immunohistochemistry for neuroblast differentiation, respectively. BrdU-, Ki-67-, and DCX-immunoreactive cells were easily detected in the subgranular zone of the DG in vehicle (saline)-treated mice. However, BrdU-, Ki-67-, and DCX-immunoreactive cells in the 500 mg/kg EE-treated mice decreased distinctively compared to those in the vehicle-treated mice. In addition, brain-derived neurotrophic factor (BDNF) immunoreactivity and its protein level decreased markedly in the DG of the EE-treated group compared to those in the vehicle-treated group. These results indicate that chronic treatment with high dose EE decreased cell proliferation and neuroblast differentiation, and that BDNF immunoreactivity decreased in the DG of EE-treated mice.
Keywords: Neurogenesis, Subgranular zone, Eisenia andrei extract, Neurotrophic factor
Introduction
Neurogenesis in the adult brain is well defined as a process of functionally generating neural cell types from adult neural precursor cells, and neurogenesis occurs in two discrete areas of the mammalian brain [1]. Adult neural precursor cells undergo cell proliferation, maturation, and migration and eventually integrate into pre-existing neural circuitry in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus [1, 2]. Additionally, neurogenesis plays important roles in physiological, behavioral, and cognitive processes [1, 3, 4]. Recently, many researchers have focused on neurogenesis in relation to brain disorders such as ischemia, Alzheimer's disease, and schizophrenia [5-8].
Earthworms are soil macroinvertebrate oligochaetes that play important roles sustaining soil fertility and productivity [9]. Many tribes in remote villages use earthworms to treat various kinds of ailments. Earthworm extract (EE) has dense nutritional content and is widely used in Chinese traditional medicine [10]. Extraction and use of biologically active compounds from earthworms have been traditionally practiced by indigenous people throughout the world, more particularly in Asia, including China, India, Myanmar, Korea, and Vietnam [11].
EE shows antithrombotic characteristics [12]. However, Cooper et al. [13] reported that EE shows anticancer characteristics. In addition, there are reports on the inhibition of adult neural progenitor cell proliferation by EE [14-16]. Therefore, in the present study, we examined whether chronic treatment with high dose EE affects cell proliferation and neuroblast differentiation in the mouse hippocampal DG using 5-bromo-2'-deoxyuridine (BrdU), Ki-67, and doublecortin (DCX) immunohistochemistry.
Materials and Methods
Preparation of EE
Earthworms, Eisenia andrei, were vermicultured at a breeding center. Briefly, the live earthworms were washed with distilled water to clean attached mud, homogenized, and then centrifuged at 4,500 ×g and 4℃ for 60 minutes. The supernatant was filtered with Celite, and 95% ethanol was added to the filtered solution to make a 40-80% ethanol solution, which was centrifuged at 4,500 ×g and 4℃ for 60 minutes. The precipitate was resuspended in distilled water and filtered with a 0.45 µm membrane filter followed by an ultra filter (NMWC 10,000). The ultra-filtered solution was lyophilized, and the resultant powder was obtained.
Experimental animals
Three-week-old male ICR mice were purchased from Orient Bio Inc. (Seongnam, Korea). They were housed under standard conditions with proper temperature (23℃) and humidity (60%) control and a 12 hour light/12 hour dark cycle with free access to food and water. The procedures for handling and caring for the animals adhered to the guidelines that are in compliance with current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996), and they were approved by the Institutional Animal Care and Use Committee at Hallym's Medical Center. All experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in the present study.
Treatment with EE
Mice were divided into four groups (n=7/group): vehicle (10 ml saline/kg body weight), 50 mg/kg EE, 100 mg/kg EE, and 500 mg/kg EE (50, 100, and 500 mg, respectively, in 10 ml saline/kg body weight)-treated groups. To investigate the effects of EE on cell proliferation and neuroblast differentiation in the SGZ of the DG, vehicle or EE was administered orally using an 18-gauge feeding needle (Harvard Apparatus, Holliston, MA, USA) once per day for 3 months before sacrifice. EE was administrated orally, because EE is always taken orally in traditional medicine. To determine the number of new cells generated in the adult brain, the animals were treated three times with an intraperitoneal injection of 50 mg/kg/day BrdU (Sigma, St. Louis, MO, USA) on days 70 and 80; BrdU-positive cells included both newly-generated cells and those that survived 10 and 20 days later.
Immunohistochemistry
Vehicle- and EE-treated animals were anesthetized with sodium pentobarbital and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4). Brains were removed and fixed in the same fixative for 4 hours. Brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter, frozen tissues were serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 25 µm coronal sections, and the sections were collected into six-well plates containing PBS. The tissue sections were selected according to anatomical landmarks corresponding to bregma -1.46 to -2.46 mm of the mouse brain atlas.
The sections were sequentially treated with 0.3% hydrogen peroxide in PBS for 30 minutes and 10% normal goat serum in 0.05 M PBS for 30 minutes. The sections were then incubated with diluted mouse anti-BrdU (1 : 200, Chemicon International, Temecula, CA, USA), rabbit anti-Ki-67 (1 : 100, Abcam, Cambridge, UK), goat anti-DCX (1 : 500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and rabbit anti-brain derived neurotrophic factor (BDNF; 1 : 500, Abcam) overnight at 4℃. Then, the sections were exposed to biotinylated goat anti-rabbit, goat anti-mouse, or rabbit anti-goat IgG (1 : 200, Vector Laboratories, Burlingame, CA, USA) and streptavidin peroxidase complex (1 : 200, Vector Laboratories). The sections were visualized with 3, 3'-diaminobenzidine tetrahydrochloride in 0.1 M Tris-HCl buffer and mounted on gelatin-coated slides. After dehydration, the sections were mounted in Canada balsam (Kanto Chemical, Tokyo, Japan). To establish immunostaining specificity, a negative control test was performed with preimmune serum rather than primary antibody. The negative control resulted in the absence of immunoreactivity in all structures. To understand the effects of EE on cell proliferation in mice, the total number of Ki-67 positive cells in all groups was counted in the DG in 15 sections/animal using an image analyzing system equipped with a computer-based CCD camera software (Optimas 6.5, CyberMetrics, Scottsdale, AZ, USA). Cell counts were obtained by averaging the counts from the sections taken from each animal.
Western blot analysis
To examine the effects of EE on BDNF level in the DG, the animals (n=5/group) were used for Western blot analysis. After sacrificing the animals and removing the brains, the brains were serially and transversely cut to a thickness of 400 µm on a vibratome (Leica), and the DG was dissected with a surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4) containing EGTA (pH 8.0), 0.2% NP-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthvanadate, 1 mM phenylmethanesulfonylfluoride, and 1 mM dithiothreitol (DTT). After centrifugation, the protein level in the supernatants was determined using a Micro BCA Protein Assay kit with bovine serum albumin as the standard (Pierce Chemical, Rockford, IL, USA). Aliquots containing 50 µg of total protein were boiled in loading buffer containing 250 mM Tris (pH 6.8), 10 mM DTT, 10% sodium dodecyl sulfate, 0.5% bromophenol blue, and 50% glycerol. The aliquots were then loaded onto a suitable polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in TBS containing 0.1% Tween20 for 45 minutes, followed by an incubation with BDNF (1 : 1,000, Abcam) overnight at 4℃ and subsequently exposed to peroxidase-conjugated horse anti-rabbit IgG (Santa Cruz Biotechnology) and an ECL kit (Amersham, Piscataway, NJ, USA). The results of the Western blot analysis were scanned, and densitometric analysis to quantify the bands was performed using Scion Image software (Scion Corp., Frederick, MD, USA), which was used to count relative optical density (ROD). The ratio of the ROD was calibrated as %, with the sham group designated as 100%.
Statistical analysis
Data are presented as means±SEMs. Differences among means were statistically analyzed using a two-tailed Student's t-test to understand the effects of EE on cell proliferation and neuroblast differentiation in mice. A P<0.05 was considered statistically significant.
Results
We administered 50, 100, and 500 mg/kg (n=7/group), and we obtained significant data for cell proliferation (Ki-67) and neuroblast differentiation (DCX) only in the 500 mg/kg EE-treated group. We could not identify any physiological changes, such as changes in body weight, hair condition, or grooming (data not shown).
Cell proliferation
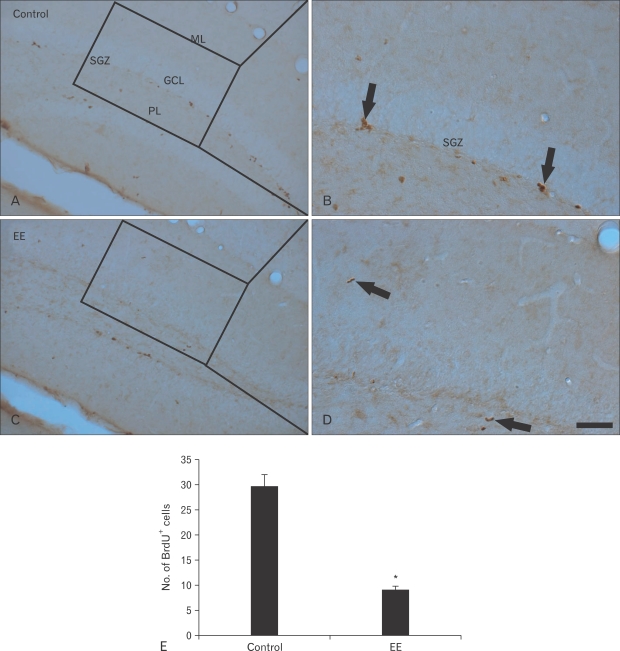
BrdU-immunoreactive (+) cells were mainly detected in the SGZ of the DG. Many BrdU+ cells were observed in the vehicle-treated group (Fig. 1A, B). The mean number of BrdU+ cells was about 30 per section in the DG (Fig. 1E). However, BrdU+ cells decreased significantly in the EE-treated group (Fig. 1C, D). The mean number of BrdU+ cells was approximately nine per section in the DG (Fig. 1E).
Fig. 1.
Immunohistochemistry for 5-bromo-2'-deoxyuridine (BrdU) in the hippocampal dentate gyrus (DG) of the vehicle- (A, B) and earthworm extract (EE)-treated (C, D) groups. BrdU+ cells (arrows) in the DG of the EE-treated group were lower than those in the vehicle-treated group. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SGZ, subgranular layer. Scale bar=100 µm. (E) Mean number of BrdU+ cells per section in the vehicle- and EE-treated groups (n=7/group; *P<0.05, significantly different from the vehicle-treated group).
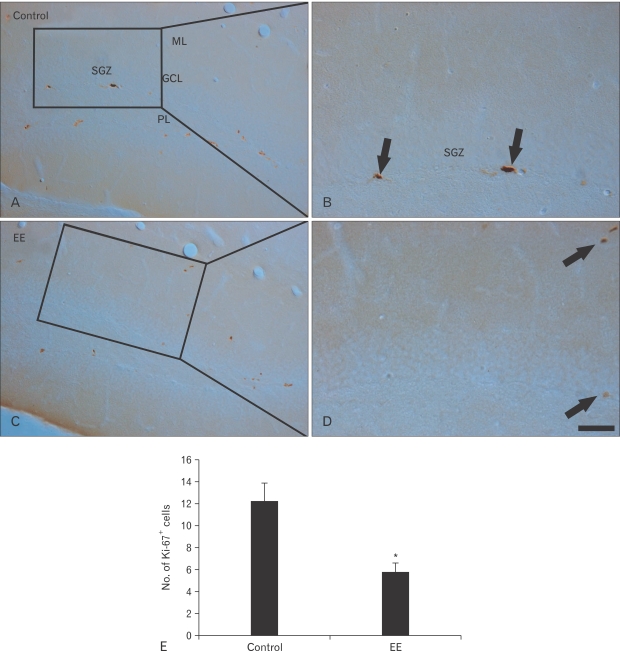
Ki-67+ cells were also mainly detected in the SGZ of the DG. Ki-67+ cells were clearly observed in the vehicle-treated group (Fig. 2A, B). The mean number of Ki-67+ was approximately 12 per DG section (Fig. 2E); however, Ki-67+ cells decreased distinctly in the EE-treated group (Fig. 2C, D). The mean number was about six per section in the DG (Fig. 2E).
Fig. 2.
Immunohistochemistry for Ki-67 in the hippocampal dentate gyrus of the vehicle- (A, B) and earthworm extract (EE)-treated (C, D) groups. Fewer Ki-67+ cells (arrows) were evident in the EE-treated group than those in the vehicle-treated group. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SGZ, subgranular layer. Scale bar=100 µm. (E) Mean number of Ki-67+ cells per section in the vehicle- and EE-treated groups (n=7/group; *P<0.05, significantly different from the vehicle-treated group).
Neuroblast differentiation
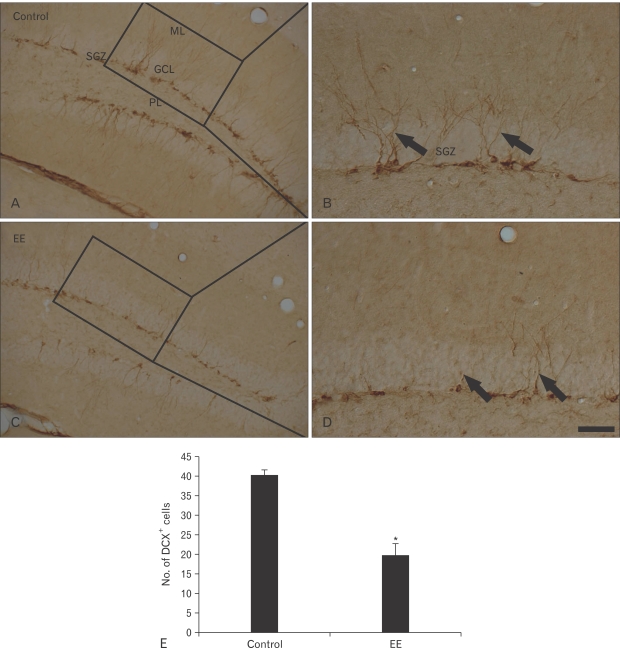
DCX+ neuroblasts were clearly observed in the DG of the vehicle-treated group (Fig. 3A, B). Their cell bodies were located in the SGZ, and their processes projected into the molecular layer of the DG. The mean number of DCX+ neuroblasts was about 40 per section in the DG (Fig. 3E). DCX immunoreactivity decreased significantly in the EE-treated group, and DCX+ processes became very short (Figs. 2D, 3C). The mean number of DCX+ neuroblasts was about 20 per section in the DG (Fig. 3E).
Fig. 3.
Immunohistochemistry for doublecortin (DCX) in the hippocampal dentate gyrus of the vehicle- (A, B) and earthworm extract (EE)-treated (C, D) groups. DCX+ neuroblasts (arrows) were abundant, and DCX+ processes were well stained in the vehicle-treated group. However, DCX immunoreactivity in the EE-treated group decreased markedly. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SGZ, subgranular layer. Scale bar=100 µm. (E) Mean number of DCX+ cells per section in the vehicle- and EE-treated groups (n=7/group; *P<0.05, significantly different from the vehicle-treated group).
BDNF immunoreactivity and its protein level
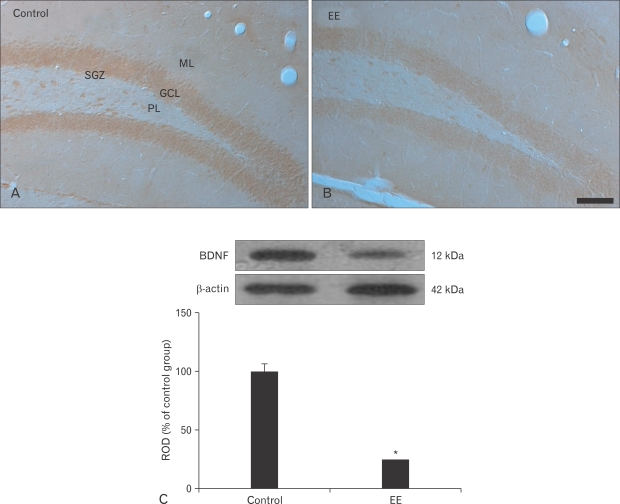
BDNF immunoreactivity was observed in the DG of the vehicle-treated group (Fig. 4A), and immunoreactivity was high in the granules and polymorphic cells. However, weak BDNF immunoreactivity was found in the EE-treated group compared with that in the vehicle-treated group (Fig. 4B).
Fig. 4.
Immunohistochemistry for brain derived neurotrophic factor (BDNF) in the hippocampal dentate gyrus (DG) of the vehicle- (A) and earthworm extract (EE)-treated (B) groups. BDNF immunoreactivity in the EE-treated group was weaker than that in the vehicle-treated group. GCL, granule cell layer; ML, molecular layer; PL, polymorphic layer; SGZ, subgranular layer. Scale bar=100 µm. (C) Western blot analysis of BDNF in the DG of the vehicle- and EE-treated groups. Relative optical density (ROD) as a percentage of the immunoblot band is presented (n=5/group; *P<0.05, significantly different from the vehicle-treated group). Bars indicate mean±SD.
The changes in the BDNF protein levels were similar to the immunohistochemical changes (Fig. 4C). The BDNF level was high in the vehicle-treated group, whereas the BDNF level decreased significantly in the EE-treated group compared to that in the vehicle-treated group.
Discussion
In this study, we examined the effects of chronic EE treatment on cell proliferation and neuroblast differentiation in the SGZ of the DG in mice. EE has been generally used at a dose <300 mg/kg in studies on anti-inflammatory and antioxidant effects [9, 17]. However, in the present study, no obvious changes in the number of BrdU+, Ki-67+, or DCX+ cells were found in the 50 and 100 mg/kg EE-treated groups; only in the high dose (500 mg/kg) EE-treated group did we find distinctive decreases in the number of BrdU+, Ki-67+, and DCX+ cells.
Furthermore, the arborization of DCX+ processes in the DC of the EE-treated group was much worse than that in the vehicle-treated group. These results demonstrate that chronic 500 mg/kg EE treatment decreased cell proliferation and neuroblast differentiation in the SGZ of mice. DG exhibits cytolytic, fibrinolytic, anticoagulant, proteolytic, hemolytic, mitogenic, and antibacterial activities [10, 11, 18]. Furthermore, some researchers have reported that EE has a lethal effect on cancer cells [13, 19].
In this study, we observed a decease in BDNF immunoreactivity in the mouse DG after chronic EE treatment. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor γ, decreases BDNF and glial cell line-derived neurotrophic factor levels and also decreases cell proliferation and neuroblast differentiation in the mouse DG [20]. Many studies have reported that neurotrophic factors including BDNF may act as positive regulators of neurogenesis [21, 22]. Additionally, exogenous BDNF increases the proliferation of hippocampal neural progenitor cells in rats [23, 24]. It has been reported that BDNF-mediated neuronal precursor cell differentiation and survival inside neurospheres occurs by activating signaling pathways that are transduced by AKT, ERK1/2, and STAT-3 [25]. Therefore, in this study, the decreased BDNF immunoreactivity may provide evidence of decreased cell proliferation and neuroblast differentiation in the SGZ after chronic EE treatment.
In conclusion, a chronic 500 mg/kg EE treatment decreased the number of BrdU+, Ki-67+, and DCX+ cells in the mouse DG, and BDNF immunoreactivity and its protein level decreased in mouse DGs chronically treated with 500 mg/kg EE.
Acknowledgements
The authors would like to thank Mr. Seung Uk Lee and Ms. Hyun Sook Kim for their technical help. This research was supported by the Regional Core Research Program funded by the Korea Ministry of Education, Science, and Technology (Medical & Bio-material Research Center).
References
- 1.Ago Y, Yoneyama M, Ishihama T, Kataoka S, Kawada K, Tanaka T, Ogita K, Shintani N, Hashimoto H, Baba A, Takuma K, Matsuda T. Role of endogenous pituitary adenylate cyclase-activating polypeptide in adult hippocampal neurogenesis. Neuroscience. 2011;172:554–561. doi: 10.1016/j.neuroscience.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Zou L, Jin G, Zhang X, Qin J, Zhu H, Tian M, Tan X. Proliferation, migration, and neuronal differentiation of the endogenous neural progenitors in hippocampus after fimbria fornix transection. Int J Neurosci. 2010;120:192–200. doi: 10.3109/00207450903464579. [DOI] [PubMed] [Google Scholar]
- 3.Jaako-Movits K, Zharkovsky T, Pedersen M, Zharkovsky A. Decreased hippocampal neurogenesis following olfactory bulbectomy is reversed by repeated citalopram administration. Cell Mol Neurobiol. 2006;26:1559–1570. doi: 10.1007/s10571-006-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer's disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res. 2006;3:185–190. doi: 10.2174/156720506777632817. [DOI] [PubMed] [Google Scholar]
- 6.Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Cao FY, Wang Q, Wang JZ. Relationship of adult neurogenesis with tau phosphorylation and GSK-3β activity in subventricular zone. Neurochem Res. 2011;36:288–296. doi: 10.1007/s11064-010-0316-y. [DOI] [PubMed] [Google Scholar]
- 7.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 8.Yao RQ, Zhang L, Wang W, Li L. Cornel iridoid glycoside promotes neurogenesis and angiogenesis and improves neurological function after focal cerebral ischemia in rats. Brain Res Bull. 2009;79:69–76. doi: 10.1016/j.brainresbull.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Balamurugan M, Parthasarathi K, Ranganathan LS, Cooper EL. Hypothetical mode of action of earthworm extract with hepatoprotective and antioxidant properties. J Zhejiang Univ Sci B. 2008;9:141–147. doi: 10.1631/jzus.B0720194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper EL. CAM, eCAM, bioprospecting: the 21st century pyramid. Evid Based Complement Alternat Med. 2005;2:125–127. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popović M, Hrcenjak TM, Babić T, Kos J, Grdisa M. Effect of earthworm (G-90) extract on formation and lysis of clots originated from venous blood of dogs with cardiopathies and with malignant tumors. Pathol Oncol Res. 2001;7:197–202. doi: 10.1007/BF03032349. [DOI] [PubMed] [Google Scholar]
- 12.Ryu GH, Park S, Han DK, Kim YH, Min B. Antithrombotic activity of a lumbrokinase immobilized polyurethane surface. ASAIO J. 1993;39:M314–M318. [PubMed] [Google Scholar]
- 13.Cooper EL, Ru B, Weng N. Earthworms: sources of antimicrobial and anticancer molecules. Adv Exp Med Biol. 2004;546:359–389. doi: 10.1007/978-1-4757-4820-8_25. [DOI] [PubMed] [Google Scholar]
- 14.Kalluri HS, Dempsey RJ. D609 inhibits the proliferation of neural progenitor cells. Neuroreport. 2010;21:700–703. [PubMed] [Google Scholar]
- 15.Kim MS, Park HR, Park M, Kim SJ, Kwon M, Yu BP, Chung HY, Kim HS, Kwack SJ, Kang TS, Kim SH, Lee J. Neurotoxic effect of 2,5-hexanedione on neural progenitor cells and hippocampal neurogenesis. Toxicology. 2009;260:97–103. doi: 10.1016/j.tox.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Paizanis E, Kelaï S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: environmental, pharmacological and neurochemical modulations. Neurochem Res. 2007;32:1762–1771. doi: 10.1007/s11064-007-9330-0. [DOI] [PubMed] [Google Scholar]
- 17.Balamurugan M, Parthasarathi K, Cooper EL, Ranganathan LS. Anti-inflammatory and anti-pyretic activities of earthworm extract-Lampito mauritii (Kinberg) J Ethnopharmacol. 2009;121:330–332. doi: 10.1016/j.jep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Hrzenjak T, Popović M, Bozić T, Grdisa M, Kobrehel D, Tiska-Rudman L. Fibrinolytic and anticoagulative activities from the earthworm Eisenia foetida. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:825–832. doi: 10.1016/s0305-0491(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Li X, Liu Y, Ye F, Qiu G. Effects of extract of dilong (pheretima) on the scalded skin in rats. J Tradit Chin Med. 2006;26:68–71. [PubMed] [Google Scholar]
- 20.Lee CH, Choi JH, Yoo KY, Park OK, Moon JB, Sohn Y, Cho JH, Hwang IK, Won MH. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, decreases immunoreactivity of markers for cell proliferation and neuronal differentiation in the mouse hippocampus. Brain Res. 2010;1329:30–35. doi: 10.1016/j.brainres.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Ai Y, Slevin JR, Maley BE, Gash DM. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp Neurol. 2005;196:87–95. doi: 10.1016/j.expneurol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. 2009;6:42–53. doi: 10.2174/156720209787466028. [DOI] [PubMed] [Google Scholar]