Abstract
Isolation of leukocytes from full-thickness excisional wounds has proven to be a difficult process that results in poor cell yield and holds significant limitations for functional assays. Given the increased interest in the isolation, characterization and functional measurements of wound-derived cell populations, herein we describe a method for preparing wound cell suspensions with an improved yield that enables both phenotypic and functional assessments.
Keywords: cell suspensions, excisional wounds, wound healing, flow cytometry, innate immune cells, skin
1. Introduction
The inflammatory cell infiltrate and its associated soluble factors are known to modulate cutaneous wound healing by promoting cell proliferation, angiogenesis and complete wound closure (Brubaker et al., 2011; Schneider et al., 2010, 2011). In efforts to better understand wound healing and the pathophysiologic conditions that can alter this process, many investigators are interested in examining the cellular subsets that reside in or infiltrate cutaneous wounds. Isolation of wound leukocytes, fibroblast and keratinocytes following injury is often achieved by use of the polyvinyl alcohol (PVA) sponge wound models, in which PVA sponges are placed in subcutaneous tissue pockets and infiltrating cells are then isolated from the PVA sponges by compression (Efron and Barbul, 2003; Swift et al., 2001). This procedure relies on implantation of a foreign body previously shown to be reactive in long-term settings (Efron and Barbul, 2003). Moreover, the implantation method is markedly different from models of excisional wound injury often used to study cutaneous healing. In murine models of excisional wound healing, punch biopsies are typically used to make full thickness cutaneous wounds on the dorsum of the mouse and then allowed to heal by secondary intention as in the clinical setting of wound infections or ulcerating diabetic wounds (Swift et al., 2001). Examination of infiltrating cell subsets by flow cytometry, or isolating these cells for further functional analysis such as phagocytosis, has proven to be difficult. Additionally, identification of rare populations like innate lymphocytes proves challenging with immunohistochemical (IHC) staining of wound sections whereas flow cytometry is well-suited for using multiple cell surface markers. Herein, we modified previously described wound cell isolation techniques (Daley et al., 2010; Sepulveda-Merrill et al., 1994; Wilson et al., 2002) to increase yield in wound cell suspensions, allowing characterization of both abundant and infrequent cell populations within the epidermis and dermis of the wound bed by flow cytometry as well as utilization of isolated cells for functional analysis.
2. Materials and Methods
2.1 Time Required
Utilizing 8–10 animals with 2 wounds used per animal, this entire procedure takes approximately 22 hours, broken down in the following steps:
Removal, weighing and preparation of wounds (1 hour)
Overnight incubation in the “Dispase Solution” (16 hours)
Incubation in the “Enzyme Cocktail” (2.5 hours)
Combination and filtration of the “Dispase Solution” and “Enzyme Cocktail” (2.5 hours)
2.2 Materials for Wound Cell Isolation
Cutaneous wounds were created on and removed from the dorsum of 8–10 week old BALB/c mice (with approval from the Institutional Animal Care and Use Committee, Loyola University Medical Center)
3 mm punch biopsy for wounding (Acuderm Inc, Fort Lauderdale, FL)
Scissors and forceps for removal of dorsal skin
Hockey puck
5 mm punch biopsy for removal of wounds and adjacent tissue from dorsal skin pelt (Acuderm Inc, Fort Lauderdale, FL)
Analytical balance and weigh boats
Straight edge razors (Personna America Safety Razor Co, Verona, VA)
24-well culture dishes (Corning Inc, Corning, NY)
-
Dispase Solution, sterile (10mL, sufficient for 8–10 mice, with 2–3 wounds per animal):
5 mL of RPMI 1640 culture media containing 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY),
3 mL of dispase II at 1mg/mL (Roche Diagnostics, Indianapolis, IN)
2 mL of gentamicin sulfate at 10mg/mL (Mediatech Inc, Manassas, VA)
Plate shaker
15 mL polypropylene conical tubes (BD Falcon, Bedford, MA)
-
Enzyme Cocktail, sterile (25 mL, sufficient for 8–10 mice, with 2–3 wounds per animal)
22.5 mL of RPMI 1640 culture media containing 5% FBS (Gibco, Grand Island, NY), 2mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY)
25 mg hyaluronidase from bovine testes, type 1-S (Sigma-Aldrich, St. Louis, MO)
25 mg collagenase from Clostridium histolyticum, type 1A (Sigma-Aldrich, St. Louis, MO)
30 mg DNAse I from bovine pancreas, Grade II (Roche Diagnostics, Indianapolis, IN)
25 mg magnesium chloride hexahydrate (Fisher Scientific, Pittsburg, PA)
2.5 ml of gentamicin sulfate at 5mg/mL (Mediatech Inc, Manassas, VA)
50 mL polypropylene conical tubes (BD Falcon, Bedford, MA)
70 μm cell strainer (BD Falcon, Bedford, MA)
Phosphate buffered saline (PBS) (Gibco, Grand Island, NY)
Accutase (eBioscience, San Diego, CA)
5% Complete Media, sterile: RPMI 1640 culture media containing 5% FBS (Gibco, Grand Island, NY), 2mM L-glutamine (Gibco, Grand Island, NY), 1% penicillin/streptomycin (Gibco, Grand Island, NY)
Plungers from 3 mL syringes (Fisher Scientific, Pittsburg, PA)
2.3 Detailed Procedure for Wound Cell Isolation
Prepare “Dispase Solution.”
Remove dorsal pelt from mice at desired time-point following excisional cutaneous injury.
Lay pelt over a hockey puck (or other firm surface) and excise the wounded tissue (i.e. if wounds were initially created with a 3 mm punch biopsy, excise the wound and the surrounding tissue with a 5 mm punch biopsy). Carefully dissect away any adipose or muscle tissue as these contain cellular components. Be sure to not use any ethanol in washing of the pelt as ethanol may disrupt the cell membrane and promote lysis. If you want to wash the pelt, do so in sterile PBS.
Collect 2–3 wounds per animal and weigh (g).
-
Mince wounds into small pieces (<2 mm × 2 mm) using the straight razor and place into the “Dispase Solution” with 15.4 mL “Dispase Solution”/gram of tissue in a 24 well culture plate (one well per animal).
Note: The purpose of the “Dispase Solution” incubation is to help dissociate the epidermal and dermal layers, further increasing the overall surface area of the subsequent enzymatic digestion.
Note: Depending on the time point examined after injury, 3mm excisional wounds isolated with a 5mm punch biopsy typically weigh between 0.01–0.02g (data not shown). Thus, if three wounds with the combined weight of 0.06g were used in this isolation procedure, 924uL of the “Dispase Solution” would be added to the well in the culture plate.
Incubate plate overnight at 4°C on a rotating shaker at a gentle setting.
The following morning, prepare the “Enzyme Cocktail.”
Remove all remaining solid tissue with sterile forceps from the “Dispase Solution” and weigh. Store the plate with the “Dispase Solution” with suspended and adherent cells at 4°C.
-
Combine solid tissue with 25 ml “Enzyme Cocktail”/gram of tissue in a 15 ml conical tube.
Note: The purpose of the “Enzyme Cocktail” incubation is to help breakdown extracellular matrix proteins, namely collagen and hyaluronan, helping to release cells from the wound tissue into suspension. As cellular lysis during isolation contributes to cellular clumping in single cell suspensions, DNase is added to the solution to prevent cellular aggregates.
Note: Using the same example as above, if the three combined wounds weighed 0.06g then 1.2mL of the “Enzyme Cocktail” would be added to the conical tube with one tube per animal.)
Incubate these tubes at 37°C under constant, light agitation for 2 hours.
Remove tubes from 37°C incubator and remove plate with the “Dispase Solution” containing suspended and adherent cells from 4°C. Place both on ice.
In a 50 mL conical tube, place a 70 μm cell strainer. Pipette the “Dispase Solution” through the cell strainer. Wash the well with PBS, pipetting up and down to remove loosely adherent cells; pipet this through the cell strainer.
Add 250 uL of Accutase to the well and incubate for 3 minutes to remove adherent cells.
Add 5% Complete Media to the well to neutralize the Accutase enzymatic activity. Pipet vigorously to remove cells and add this to the 50 mL tube through the strainer.
Pour the solid tissue and “Enzyme Cocktail” through the cell strainer. Crush remaining solid tissue with plungers from the 3 mL syringes.
Rinse filter with 5% Complete Media.
Centrifuge at 250 x g for 10 minutes at 4°C.
Aspirate supernatant, and resuspend in 5% Complete Media.
Transfer to clean 15 mL conical tubes.
Wash twice with 5% Complete Media (or media preferred for cell counting)
Count cells and resuspend to desired number of cell/mL for flow cytometry staining (i.e. 106 cell/mL as described below).
2.4 Additional Experimental Methods
2.4.1 Flow Cytometry Analysis on Isolated Wound Cells
Flow cytometric analysis was performed as previously described (Schneider et al., 2010, 2011). Wound cells were resuspended to 106 cells/mL in FACS buffer (filtered phosphate buffer saline (PBS) with 1% bovine serum albumin (BSA), 0.01% sodium azide, and 2mM EDTA). Cells were blocked with 1ug/mL rat IgG (Jackson Laboratories, Bar Harbor, Maine) and anti-CD16/32 antibody (eBioscience, San Diego, CA) for 20 minutes. Cells were then stained using rat anti-mouse FITC-conjugated Gr-1 (clone RB6-8C5, eBioscience, San Diego, CA) and rat anti mouse PE-conjugated Cy7-F4/80 (clone BM8, eBioscience, San Diego, CA) to look at macrophage (F4/80+/Gr-1−) and neutrophil (F4/80−/Gr-1+) populations at saturating concentrations. Alternatively, samples were stained with APC-conjugated CD3e (clone 145-2C11, eBioscience, San Diego, CA) and glycolipid loaded dimeric CD1d:Ig Fusion Protein (Dimer) (BD Pharmigen, San Jose, CA) that was subsequently counterstained with a PE-conjugated anti-IgG1 (clone A85-1, BD Pharmigen, San Jose, CA) to determine natural killer T (NKT) cells (CD3+/Dimer+). Unloaded dimeric CD1d/Ig Fusion Protein, which lack the glycolipid and will not bind NKT cells, was used to determine positive staining. After incubation for 30 minutes, cells were washed twice in FACS buffer and resuspended in 0.5 mL FACS buffer. Samples were acquired on FACSCanto I (BD Bioscience, San Jose, CA) and analyzed with FlowJo Software (Tree Star Inc, Ashland, OR).
2.4.2 Ex vivo wound cell phagocytosis
Following isolation, wound cells were resuspended to 106 cells/mL in Phagocytosis Uptake Buffer (Hanks Balanced Salt Solution (HBSS, Gibco, Grand Island, NY) with 20mM HEPES, pH 7.4) per the manufacturer’s instructions (Invitrogen). pHrodo-Staphylococcus (S.) aureus BioParticles (Invitrogen, Carlsbad, CA) were reconstituted in Phagocytosis Uptake Buffer to 1mg/mL. The pHrodo-S. aureus BioParticles were then opsonized with rabbit polyclonal IgG antibodies (Invitrogen, Carlsbad, CA) to enhance their phagocytosis for 1 hour at 37°C. For each animal, a control tube of 2×105 cells was placed on ice and an experimental tube of 2×105 cells were placed at 37°C for 15 minutes. Following temperature equilibration, pHrodo-S. aureus was added so there were approximately 30:1 bacteria particles to cell. Cells were incubated for 60 minutes at 4°C (control) and 37°C (experimental). Phagocytosis was then stopped by addition of 2 mL ice cold Phagocytosis Uptake Buffer and the cells were placed on ice. Samples were washed once, and then subjected to flow cytometry staining as described above.
3. Results
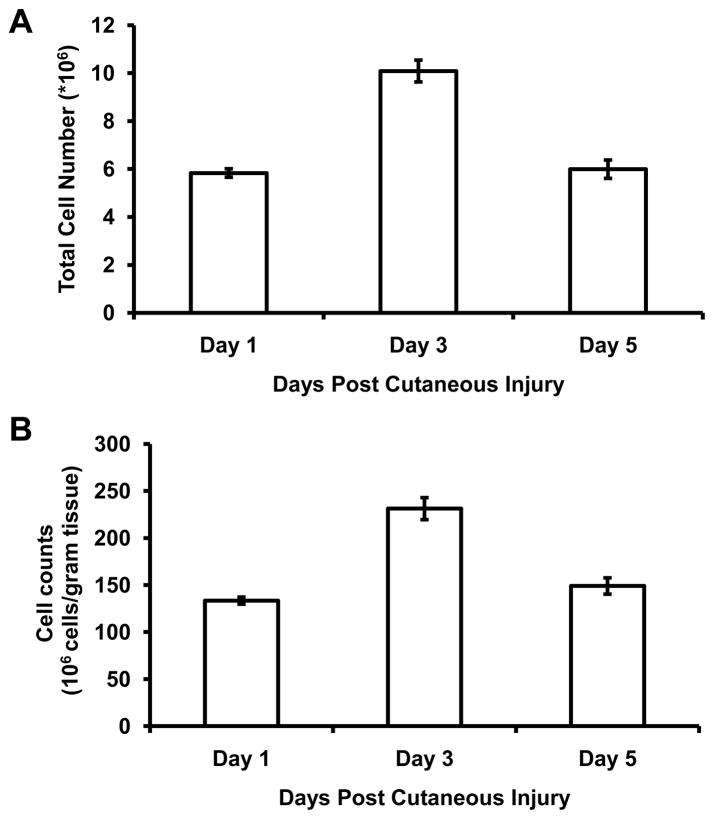
Utilizing the procedure detailed above, we effectively isolated wound cells from cutaneous tissue following excisional cutaneous injury. At days 1, 3 and 5 following wound injury, isolated wound cells were counted and the total cell number (Figure 1A) and cells per gram of tissue (Figure 1B) were determined.
Figure 1. Cell counts following wound cell isolation protocol.
Following excisional injury, wound cells were isolated from excisional cutaneous wounds at days 1, 3 and 5 and cell counts are expressed as total number of cells (A) and cell number per gram of tissue (B). Data are presented as mean ± SEM, N=4–6 animals per time point, with 3 wounds per animal. Data are representative of 8 replicated experiments.
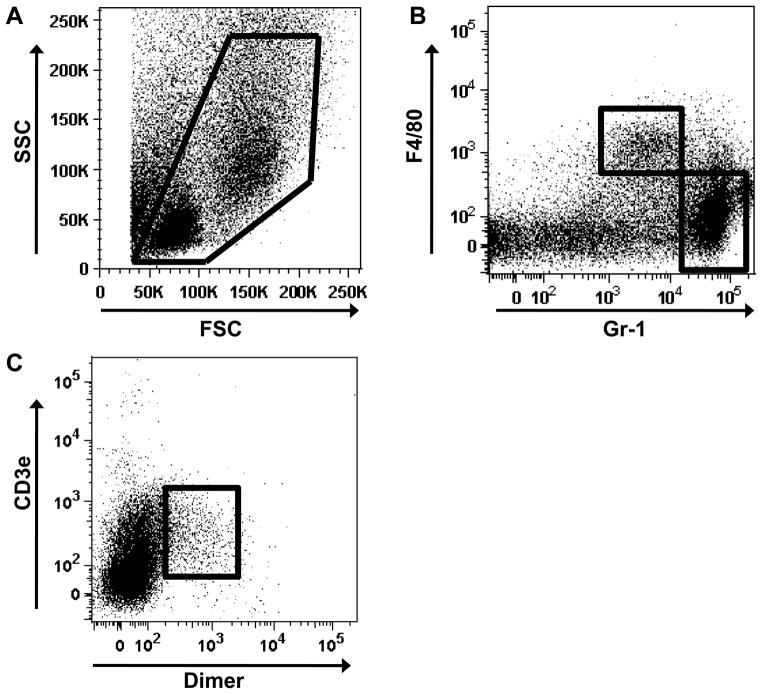
The cell suspensions were then subjected to flow cytometric analysis for examination of cell subpopulations following cutaneous injury (Figure 2). In Figure 2A, an example of the forward scatter (FSC) verse side scatter (SSC) of the wound cell suspension is shown. The wound cell isolates were stained for F4/80, a monocyte and macrophage surface marker, as well as Gr-1, which recognizes neutrophils and myeloid-derived suppressor cells (Figure 2B). This allowed for examination of the abundant early inflammatory mediators of wound healing. We also examined these cell suspensions to determine the composition of less frequent cell populations such as NKT cells, determined by staining with CD3e, present on T lymphocytes and NKT cells, and Dimer (CD1d:IgG1 Fusion Protein), specific for NKT cells (Figure 2C).
Figure 2. Flow cytometric analysis of wound cell suspensions.
Isolated wound cells were subjected to flow cytometry to examine inflammatory composition of the wound sites. (A) Representative dot plot of FSC verse SSC of the wound cell suspension with the Live Gate indicated in red. Wound suspension were stained with F4/80 and Gr-1 to examine macrophage (F4/80+/Gr-1−) and neutrophil (F4/80−/Gr-1+) populations (B). (C) NKT cell (CD3e+/Dimer+) population in wound cell suspensions. Flow cytometry data are representative of cells isolated from Day 1 wounds. Data are representative of 8 replicated experiments with N=4–6 animals and 2–3 wounds per animal.
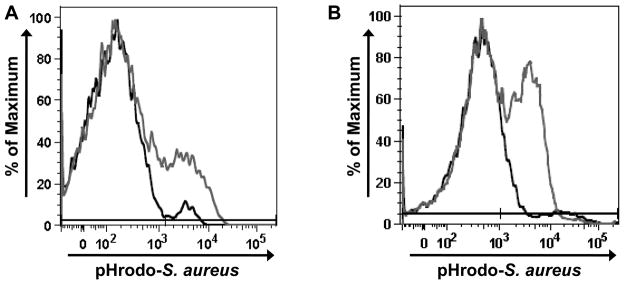
To determine if the isolated cells remained adequately viable for follow up in functional assays, the wound cells were subjected to an ex vivo phagocytosis assay. Following incubation with opsonized pHrodo-S. aureus, both the macrophage and neutrophil populations exhibited uptake and phagosome acidification (Figure 3A and B, respectively), demonstrating that these cells still retained functional capacities for further scientific manipulations.
Figure 3. Phagocytic potential of isolated wound cells.
To determine if isolated wound cells retained functional capacity, wound cells were incubated with opsonized pHrodo-S. aureus at 37°C for 1 hour. The phagocytic reaction was stopped and cells were stained for F4/80 and Gr-1. (A) Representative histogram of F4/80+/Gr-1− cells (macrophages) which have phagocytosed pHrodo-S. aureus (dark gray). Control histogram (black) represents cells incubated with pHrodo-S. aureus maintained at 4°C. (B) Representative histogram of F4/80−/Gr-1+ cells (neutrophils) which have phagocytosed pHrodo-S. aureus (dark gray). Control histogram (black) represents cells incubated with pHrodo-S. aureus maintained at 4°C. Data are representative of N=12 animals, with 2 wounds per animal.
4. Discussion and Conclusions
Described here is an improved method to create wound cell suspensions for examination by flow cytometry, allowing study of immune cell infiltrate, including rare populations such as the NKT cell. The previous isolation techniques provided minimal serum and/or media for cell nutritional support during isolation, lacked proper cofactors for specific enzymatic activity, limited anti-microbial coverage predominately to gram-negative bacteria and did not utilize a method for removal of adherent cells from tissue culture plastic during the isolation procedure (Sepulveda-Merrill et al., 1994; Wilson et al., 2002). Specifically, previous methods cultured cells overnight in HBSS supplemented with 3% FBS (Wilson et al., 2002). In this isolation procedure, cells were cultured in RPMI with 10% FBS to further promote cell survival. In the subsequent enzymatic digestion, magnesium chloride hexahydrate was added to promote DNase I enzymatic activity. In both digests, penicillin was added to provide broader gram-positive organism coverage, as gram-positive organisms are common in the epidermal microflora. By adjusting these factors we have generated a high cell yield and preserved cell viability for functional assays. Additionally, utilization of Accutase to remove cells from tissue culture plastic allows for a more accurate characterization of the adherent cell populations, such as macrophages, neutrophils, fibroblasts or keratinocytes within the wound that may not have been adequately accounted for in past methods. Further, this isolation procedure would also allow for study of non-immune cell subsets such as keratinocytes or fibroblasts by flow cytometry or additional immunological and molecular techniques.
The alternate method for studying immune cell infiltration following cutaneous injury employs the PVA sponge method (Daley et al., 2010; Efron and Barbul, 2003; Gosain et al., 2009; Swift et al., 2001). As mentioned, this does not allow for examination of the cellular milieu in other wound healing models such as the commonly used model of excisional cutaneous wound injury. Additionally, this method requires applied pressure to the sponge to release the wound fluid and cellular components. Pressure and tension are known activators of intracellular signaling cascades in both fibroblast and keratinocytes, and thus this isolation method may alter the phenotype of these cells (Eckes et al., 2006; Tomasek et al., 2002). Our method only applies minimal mechanical force, primarily gentle agitation and washing, which may limit pressure-induced signaling activation. Further, previous reports using the PVA model yield 1×106 cells/animal at day 1 and 3×106 cells/animal at day 3 (Daley et al., 2005, 2010). Using the technique described in our excisional wound model, we have obtained an increased cell yield in comparison to the PVA sponge models (Figure 1). Moreover, we demonstrate that these cells maintain functional capabilities following isolation (Figure 3), allowing further examination of the subpopulations that comprise wound tissue. The ability to isolate cellular components and examine ex vivo functional aspects such as phagocytosis or chemotaxis will provide more relevant information about the impact of the wound environment on cell function. Additionally, examination of keratinocyte functions, such as antimicrobial peptide generation and expression, could potentially be evaluated using this technique. This may be useful in examining how various disease states impact keratinocyte function in response to wound injury. Considering our findings, this technique is ideal for examining the cutaneous cellular composition and can be extended for use in other models of cutaneous injury, such as incisional wound or burn injuries, as well as cutaneous malignancies. Finally, modifications of this protocol may allow for study of other tissues, such as the cornea or gut.
Despite the benefits of this tissue dispersion protocol, limitations do exist. Due to the fibrous nature of skin tissue, excess debris can be observed during cell counting which can ultimately compromise flow cytometric analysis. To combat this, an additional filtration step with a 70uM filter can be added. Additionally, this protocol is designed to allow examination of the combined cellular composition of the epidermis and dermis. Modification of this protocol could allow for examination of cells isolated from a specific tissue layer. Prior to finely dicing the wound tissue, one may use the “Dispase Solution” to carefully dissect the epidermis away from the dermis, proving better characterization of the cell subset present in respective tissue layers. Furthermore, it is difficult to ascertain the impact of this protocol on cell surface markers and cell phenotype of the isolated wound cells. A potential method of comparison is subjecting the spleen, a leukocyte rich lymphoid organ, to a standard dispersion protocol and the procedure described above. However, this method of comparison is not adequate as the splenocytes are more directly exposed to the enzymes used using this protocol as compared to cell subsets present in the fibrous skin matrix. Alternatively, validating the flow cytomtery results with IHC is difficult for rare cellular subsets and complex phenotypic characterization. Overall, this procedure allows for relative comparison of isolated cells between experimental groups. Though these limitations exist, use and further modifications of this protocol will allow for enhanced understanding of wound and skin biology in a variety of experimental and clinical settings.
Highlights.
Improved isolation of wound cells from excisional cutaneous wounds
Method allows for phenotypic characterization of abundant and rare cellular subsets at the wound site
Isolated cells retain functional capabilities and are suitable for use in functional assays
Acknowledgments
The authors would like to thank Patricia Simms in the Loyola University FACS Core for her insight and thoughtful discussion and Juan Rendon for critical review of this manuscript. This work was supported by NIH R21 AI073987 (EJK), R01 AG018859 (EJK), T32 AG031780 (PWL) and Dr. Ralph and Marian C. Falk Medical Research Trust (EJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brubaker AL, Schneider DF, Kovacs EJ. Neutrophils and natural killer T cells as negative regulators of wound healing. Expert Rev Dermatol. 2011;6:5–8. doi: 10.1586/edm.10.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Reichner JS, Mahoney EJ, Manfield L, Henry WL, Jr, Mastrofrancesco B, Albina JE. Modulation of macrophage phenotype by soluble product(s) released from neutrophils. J Immunol. 2005;174:2265–2272. doi: 10.4049/jimmunol.174.4.2265. [DOI] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, Krieg T. Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006;11:66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- Efron DT, Barbul A. Subcutaneous Sponge Models. In: DiPietro LA, Burns AL, editors. Wound Healing: Methods and Protocols. 2003. pp. 83–10. [DOI] [PubMed] [Google Scholar]
- Gosain A, Gamelli RL, DiPietro LA. Norepinephrine-mediated suppression of phagocytosis by wound neutrophils. J Surg Res. 2009;152:311–318. doi: 10.1016/j.jss.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DF, Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Faunce DE. Prevention of NKT Cell Activation Accelerates Cutaneous Wound Closure and Alters Local Inflammatory Signals. J Surg Res. 2010 doi: 10.1016/j.jss.2010.03.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DF, Palmer JL, Tulley JM, Speicher JT, Kovacs EJ, Gamelli RL, Faunce DE. A Novel Role for NKT Cells in Cutaneous Wound Repair. J Surg Res. 2011;168:325–333. doi: 10.1016/j.jss.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Merrill C, Mayall S, Hamblin AS, Breathnach SM. Antigen-presenting capacity in normal human dermis is mainly subserved by CD1a+ cells. Br J Dermatol. 1994;131:15–22. doi: 10.1111/j.1365-2133.1994.tb08451.x. [DOI] [PubMed] [Google Scholar]
- Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- Tomasek JT, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechanoregulation of connective tissue remodelling. Nat Reviews. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Wilson L, Fathke C, Isik F. Tissue dispersion and flow cytometry for the cellular analysis of wound healing. Biotechniques. 2002;32:548–551. doi: 10.2144/02323st07. [DOI] [PubMed] [Google Scholar]