Abstract
Fluoroquinolones are broad spectrum antibiotics widely indicated in the treatment of both human and animal diseases. The primary objective of this study was to assess short and long term affinity of gemifloxacin towards efflux transporters (P-gp, MRP2) and nuclear hormone receptor (PXR). Uptake and dose dependent inhibition studies were performed with [14C] erythromycin (0.25μCi/ml) on MDCKII-MDR1 and MDCKII-MRP2 cells. Cellular accumulation of calcein-AM was further determined to confirm the affinity of gemifloxacin towards P-gp and MRP2. Transport studies were conducted to determine bi-directional permeability and to assess efflux ratio of gemifloxacin. LS-180 cells were treated with three different concentrations of gemifloxacin for 72hrs and real-time PCR analysis was performed to study the quantitative gene expression levels of PXR, MDR1 and MRP2. Further, [14C] erythromycin uptake was also performed on LS-180 treated cells to better delineate the functional activity of efflux transporters. Results from our study suggest that gemifloxacin may be a substrate of both the efflux transporters studied. This compound inhibited both P-gp and MRP2 mediated efflux of [14C] erythromycin in a dose dependent manner with IC50 values of 123 ± 2μM and 16 ± 2μM, respectively. The efflux ratio of [14C] erythromycin lowered from 3.56 to 1.63 on MDCKII-MDR1 cells and 4.93 to 1.26 on MDCKII-MRP2 cells. This significant reduction in efflux ratio further confirmed the substrate specificity of gemifloxacin towards P-gp and MRP2. Long term exposure significantly induced the expression of PXR (18 fold), MDR1 (6 fold) and MRP2 (6 fold). A decrease (20%) in [14C] erythromycin uptake further confirmed the elevated functional activity of P-gp and MRP2. In conclusion, our studies demonstrated that gemifloxacin is effluxed by both P-gp and MRP2. Long term exposure induced their gene expression and functional activity. This substrate specificity of gemifloxacin towards these efflux transporters may be one of the major factors accounting for low oral bioavailability (71%). Better understanding of these mechanistic interactions may aid in the development of newer strategies to achieve adequate therapeutic levels and higher bioavailability.
Keywords: gemifloxacin, efflux, cellular translocation, P-glycoprotein, multidrug resistance associated protein-2, pregnane X receptor
1. INTRODUCTION
Fluoroquinolones are broad spectrum antibiotics widely indicated in both human and animal diseases. These compounds demonstrate excellent bactericidal activity and concentration dependent killing effect (Andriole, 2005; Hooper, 1999b; Turnidge, 1999; Zhanel and Noreddin, 2001). Nalidixic acid and oxolinic acid were the earliest quinolone antibacterial drugs to be introduced. Emergence of resistance coupled with undesirable side effects has driven researchers to develop newer analogs, leading to the discovery of second, third and fourth generation fluoroquinolones. These agents have been widely indicated in the treatment of bacterial diseases including systemic infections (Alvarez et al., 2008; Appelbaum and Hunter, 2000; Goldstein et al., 1999; Saravolatz and Leggett, 2003). The newer fluoroquinolone, gemifloxacin was approved by the United States Federal Drug Administration (US FDA) in 2003. It is recommended for the treatment of mild to moderate community acquired pneumonia (CAP), acute bacterial exacerbations of chronic bronchitis (ABECB) and urinary tract infections (Ramji et al., 2001; Yoo et al., 2004).
The structure of gemifloxacin is depicted in Fig. 1. It is a fluoronaphthyridine molecule having a side chain with cyclopropanyl group at position-1 and 3-aminomethyl-4-syn-methoxyimino-1-pyrrolidinyl substituent at C-7 position. Substitution of the carbon at C-8 position with nitrogen enhanced antimicrobial activity. Gemifloxacin acts by forming a ternary complex with both DNA gyrase and topoisomerase IV. Formation of this complex is responsible for the blockade of DNA replication and transcription, resulting in chromosomal disruption and bacterial cell death (Saravolatz and Leggett, 2003; Yoo et al., 2004). Most fluoroquinolones acquire resistance due to two chromosomal mutations in the quinolone resistance determining regions (QRDR). However, gemifloxacin has often shown retained activity despite two-step mutations in the QRDR (Heaton et al., 2000; Hooper, 1999a). Unlike ciprofloxacin and levofloxacin, reduced photosensitivity and phototoxicity was observed with gemifloxacin (Blondeau, 1999; Domagala, 1994; Lipsky and Baker, 1999; Vousden et al., 1999).
Fig. 1.
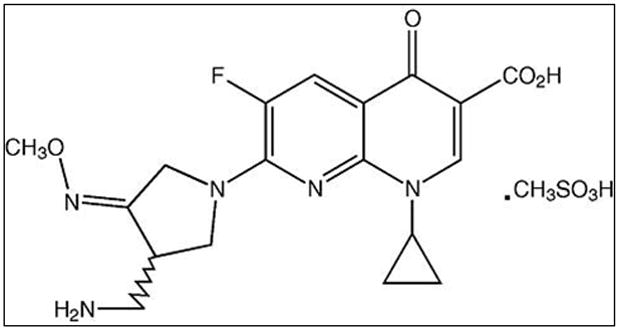
Structure of Gemifloxacin mesylate
Gemifloxacin exhibits a broad spectrum of activity against gram-positive and gram-negative bacteria (Cormican and Jones, 1997; Hohl et al., 1998; Johnson et al., 1999; Oh et al., 1996). The compound showed excellent antimicrobial activity with low minimum inhibitory concentrations (MIC90) of 0.03, 0.06 and 0.008μg/ml against Streptococcus pneumoniae, S. pyrogenes and Haemophilus influenzae, respectively (Fuchs et al., 2000; Goldstein et al., 2002; King et al., 2000; Koeth et al., 2002; Rittenhouse et al., 2000). Potent antibacterial activity against clinical isolates and reference strains was observed with gemifloxacin in both in vitro as well as in vivo infectious animal models (Berry et al., 2000; Erwin and Jones, 1999; Johnson et al., 1999; Ramji et al., 2001). Upon oral administration, gemifloxacin is rapidly absorbed with peak concentration reaching within 0.5–2hrs. The absolute bioavailability (71%) was found to be lower than that of gatifloxacin (96%) and levofloxacin (99%) (Allen et al., 2000; Zhanel and Noreddin, 2001). This limitation could be due to efflux of fluoroquinolones by ATP-binding cassette (ABC) transporters (Alvarez et al., 2008).
ABC transporters i.e. P-glycoprotein (P-gp), multidrug resistance associated protein-2 (MRP2) and breast cancer resistant protein (BCRP) are responsible for the efflux of several drugs, altering their absorption, distribution and excretion (Glavinas et al., 2004; Kwatra et al., 2010; Pal et al., 2010; Pal and Mitra, 2006; Sikri et al., 2004). These efflux transporters are one of the leading membrane bound protein families in both prokaryotes and eukaryotes. P-gp, a 170 KDa transmembrane protein, is expressed on the apical membrane of many epithelial and endothelial cells. It acts as a biological barrier by extruding toxins and xenobiotics into extracellular environment (Katragadda et al., 2005). MRP family consists of 190 kDa proteins responsible for the transport of drugs across lipid membranes. These proteins are similar to P-gp with regard to function and localization, but may differ in substrate specificity. These efflux pumps derive their energy from ATP hydrolysis and expel antimicrobial drugs out of cell, thus reducing intracellular drug accumulation. This process may eventually lead to suboptimal eradication of microorganisms. Expression of efflux transporters is regulated by the ligand activated transcription factor, pregnane X receptor (PXR, NR1I2) (Dussault and Forman, 2002; Geick et al., 2001; Kast et al., 2002; Raucy and Lasker, 2010). PXR is considered to play an important role in regulating response to various drugs, thereby regulating their physiological disposition.
Interaction of gemifloxacin with efflux transporters in short and long term could possibly reduce bioavailability and consequently drug efficacy, which may also augment the risk of resistance development. Better understanding of these mechanistic interactions may aid in the development of newer strategies to achieve adequate therapeutic levels and higher bioavailability. Therefore, the primary objective of this study was to assess the short term affinity of gemifloxacin towards efflux transporters using polarized canine MDCKII-MDR1, MDCKII-MRP2 cells and to evaluate the changes in differential expression and functional activity of efflux transporters upon long term treatment in human intestinal cells (LS-180).
2. MATERIALS AND METHODS
2.1 Materials
Gemifloxacin mesylate was obtained from Bosche Scientific LLC (New Brunswick, NJ). Madin-Darby Canine Kidney (MDCK) type II cells over expressing human P-gp and MRP2 proteins (MDCKII-MDR1, MDCKII-MRP2) were a generous gift from Drs. A. Schinkel and P. Borst (The Netherlands Cancer Institute, Amsterdam). LS-180 cells were obtained from American Type Culture Collection (ATCC) (Manassas, VA). [14C] Erythromycin (specific activity: 51.3 mCi/mMol) was procured from Moravek Biochemicals (Brea, CA, USA). Dulbecco’s modified eagle’s medium (DMEM), trypsin replacement (TrypLE™ Express), non-essential amino acids, TRIzol® and ATP determination kit were obtained from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA, USA). Culture flasks (75cm2 and 25cm2growth area), 12-well plates (3.8cm2 growth area per well), polyester transwells (pore size of 0.4μm) and 96-well plates (0.32cm2 growth area per well) were procured from Corning Costar Corp. (Cambridge, MA, USA). OligodT, dNTP, M-MLV reverse transcriptase and CellTiter 96® Aqueous Non-Radioactive cell proliferation assay were obtained from Promega Corporation (Madison, WI, USA). Light Cycler 480® SYBR I green master mix was obtained from Roche Applied Science (Indianapolis, IN, USA). All other chemicals were purchased from Sigma Chemicals (St. Louis, MO, USA) and used without further purification.
2.2 Cell culture
MDCK cells are the most studied epithelial cells with respect to genetics, lipid and protein composition (Braun et al., 2000). MDCK type-II cells transfected with human MDR1 and MRP2 genes have been considered the best models to study various substrates for their efflux mechanisms (Evers et al., 1997; Evers et al., 1998). MDCKII-MDR1, MDCKII-MRP2 cells of passage numbers 5–10, 5–15 respectively were used for the studies. LS-180 cells are considered as high throughput models to study PXR mediated induction of efflux transporters on long term exposure to various xenobiotics (Gupta et al., 2008; Harmsen et al., 2010). LS-180 cells of passage numbers 65–70 were selected for long term studies. These cells were maintained with DMEM medium supplemented with 10% FBS (heat inactivated), 1% non essential amino acids, 29mM sodium bicarbonate, 100μg/ml of penicillin and streptomycin each at 37°C. Cells were grown in T-75 flasks, passaged using TrypLE™ Express and plated at a density of 250,000cells/well for uptake and transport studies. Medium was changed every alternate day and studies were conducted 5–7 days post seeding, unless specified.
2.3 Uptake studies
Uptake studies were performed on cells grown on 12-well plates. [14C] erythromycin (0.25μCi/ml) was employed to study P-gp and MRP2 mediated efflux on MDCKII-MDR1 and MDCKII-MRP2 cells (Dey et al., 2004; Hariharan et al., 2009). Cellular accumulation of [14C] erythromycin was determined alone, and in the presence of gemifloxacin as well as quinidine and MK-571, specific inhibitors for P-gp and MRP2 respectively. After reaching confluency, the medium was removed and cells were washed thrice with 1ml of Dulbecco’s phosphate buffered saline (DPBS) (130mM NaCl, 0.03mM KCl, 7.5mM Na2HPO4, 1.5mM KH2PO4, 1mM CaCl2, 0.5mM MgSO4, 20mM HEPES and 5mM glucose). Later 500μL of the test solution was added. Cells were then incubated for 30min at 37°C. Later, uptake was arrested with ice cold PBS and 1ml of lysis solution (0.1% (v/v) Triton-X in 0.3N NaOH) was added. Cells were lysed overnight and the cell lysate was then quantified for radioactivity with scintillation cocktail (Fisher Scientific, Fair Lawn, NJ) in scintillation counter (Model LS-6500; BeckmanCounter, Fullerton, CA). The protein concentration in each well was estimated by Bio-Rad protein estimation kit (Bio-Rad, Hercules, CA) and used to normalize the radioactivity.
Following a similar procedure, dose dependent inhibition studies were performed. [14C] erythromycin was spiked with various concentrations of gemifloxacin ranging from 1μM to 1000μM. Studies were performed in a similar way and the data was fitted to calculate the half maximal inhibitory concentration (IC50) according to a published method (Anand et al., 2003).
2.4 Cellular accumulation of calcein-AM
Calcein-AM is an acetoxymethyl ester derivative of calcein, a cell impermeable compound. This ester derivative is cell permeable and upon translocation is metabolized by intracellular esterases into calcein. Calcein-AM is a substrate of P-gp and calcein is a substrate of MRP2 (Essodaigui et al., 1998; Evers et al., 2000). Inhibition of these efflux proteins causes an increase in intracellular accumulation of calcein, which produces fluorescence. This assay was performed on cells cultured in 96-well plates at a density of 10,000cells/well. After washing, cells were exposed to 100μL of calcein-AM (0.05μM) alone, in the presence of specific inhibitor and gemifloxacin for 15min. The test solution was immediately removed and replaced with 100μL of DPBS. Fluorescence associated with the cells was quantified with a 96-well micro titer plate reader (SpectraFluor Plus, Tecan, Maennedorf, Switzerland) at an excitation and emission wavelengths of 495 and 515nm respectively.
2.5 Permeability studies
Transport studies were performed on cells grown on transwells. The donor chamber contained test solution where as the receiver chamber contained DPBS. Apparent permeability of [14C] erythromycin was determined from apical to basolateral (AP-BL) and basolateral to apical (BL-AP) directions across MDCKII-MDR1 and MDCKII-MRP2 cells. At predetermined intervals (0, 15, 30, 45, 60, 90, 120, 150min); samples were withdrawn from the receiver chamber and replaced with same amount of DPBS to maintain sink conditions. The samples were then quantified for [14C] erythromycin as described previously. Cumulative amount transported was plotted with time to determine apparent permeability.
2.6 ATP assay
The efflux proteins require ATP to extrude xenobiotics out of the cell (Boumendjel et al., 2002). Any change in the intrinsic levels of ATP may alter the functional activity of efflux proteins. Hence ATP activity assay was performed to examine if gemifloxacin alters ATP activity in cells. MDCKII-MDR1 and MRP2 cells grown in 96 well plates were treated with 100μL of various concentrations of gemifloxacin for 150min. Later 100μL of lysis solution was added and cell lysate was used for the quantification of ATP with a kit (Molecular Probes, Invitrogen) following manufacturer’s protocol.
2.7 Cell proliferative assay
MTS assay is used to determine if concentrations of gemifloxacin used are toxic to the cells. Cells were exposed to various concentrations of gemifloxacin for 150min. The solutions were aspirated and 100μL of serum free media was added. Twenty microliters of MTS and PMS (CellTiter 96® Aqueous Non-Radioactive cell proliferation assay) reagent were then added to wells. After incubating for 4hrs, the quantity of formazan formed from MTS, is measured at 490nm with a plate reader. The amount of formazan is directly proportional to the number of viable cells.
2.8 Quantitative gene expression and functional activity studies
LS-180 cells were treated with three different concentrations of gemifloxacin (2.5, 5, 7.5μM) and rifampin (10μM). The concentrations of gemifloxacin were determined from multiple dose pharmacokinetics in human volunteers (Allen et al., 2001). Rifampin, a potential inducer drug is used as a positive control (Gupta et al., 2008). After subsequent treatment for 72 hours, mRNA was extracted with Trizol® reagent. The mRNA obtained was dissolved in DNase RNase-free water and concentration was determined by Nanodrop (Thermo Fisher Scientific, Wilmington, DE, USA). M-MLV reverse transcriptase and oligodT were added to reverse transcribe mRNA into complementary DNA (cDNA). Conditions for reverse transcription are initial denaturation at 70°C for 5 min, reverse transcription at 42°C for 1hour and final extension at 72°C for 5 min. Finally, the cDNA was amplified for GAPDH (internal control), PXR, MDR1 and MRP2 genes using Light cycler SYBR-green master mix on ABI Prism 5700 Sequence Detection System (Applied Biosystems). Primers for these genes were designed using Primer Blast tool from Pubmed. The sequences of the primers are as follows: GAPDH-forward 5′-ATCCCTCCAAAATCAAGTGG-3′ and reverse 5′-GTTGTCATGGATGACCTTGG-3′; PXR-forward 5′-GCAGGTGGCTTCCAGCAACT-3′ and reverse 5′-GGGCGGTCTGGGGAGAAGAG-3′; MDR1-forward 5′-CTTATGCTCTGGCCTTCTGG-3′ and reverse 5′-TGCTTCAATGCTTGGAGATG-3′; MRP2-forward 5′-AAATATTTTGCCTGGGAACC-3′ and reverse 5′-TGTGACCACAGATACCAGGA-3′. Pfaffl’s method was used to calculate relative fold induction of each gene in control relative to treated sample (Pfaffl, 2001). Further, cellular accumulation of [14C] erythromycin was also performed on LS-180 cells to delineate the functional activity upon treatment with gemifloxacin.
2.9 Data analysis
Cellular accumulation of [14C] erythromycin was determined with Eq. 1.
| Eq. 1 |
Disintegrations per minute (DPM) of sample and donor are represented as DPMsample and DPMdonor respectively. The concentration of donor used is represented Cdonor and Csample represents the concentration of sample thus obtained.
The half maximal inhibitory concentration (IC50) of [14C] erythromycin by gemifloxacin was calculated with Eq. 2.
| Eq. 2 |
Y is the cellular accumulation of [14C] erythromycin and X represents the logarithm of the gemifloxacin concentration used. Data was fitted to Eq. 2 with a transformed nonlinear regression curve analysis program (GraphPad Prism Version 4.0; GraphPad Software, Inc., San Diego, CA) to calculate IC50.
The amount of [14C] erythromycin transported across the cell monolayers over a specific interval (dM/dt) divided by the cross-sectional area of the transwell (A) generates the flux (J). Flux is thus estimated by Eq. 3.
| Eq. 3 |
Apparent permeability (P) was then obtained by normalizing flux to donor concentration, as described in Eq. 4.
| Eq. 4 |
The efflux ratio was then obtained by dividing the BL–AP permeability by AP–BL permeability as described in Eq.5.
| Eq. 5 |
2.10 Statistical analysis
All experiments were performed atleast in quadruplicate (n=4). The results were represented as mean ± standard deviation (SD). Student’s t-test was performed to determine statistical difference from the control. A P-value of <0.05 is considered to be statistically significant.
3. RESULTS
3.1 Uptake studies
Accumulation of [14C] erythromycin on MDCKII-MDR1 cells was significantly enhanced (1.7 fold) in the presence of quinidine (100μM), suggesting the inhibition of MDR1 efflux protein. A similar increase was also observed in the presence of gemifloxacin (250μM) (Fig. 2). Cellular accumulation of [14C] erythromycin was also performed on MDCKII-MRP2 cells. Uptake of [14C] erythromycin was elevated by almost 1.8 fold in the presence of MK-571 (100μM). A similar rise was also observed in the presence of gemifloxacin (250μM) (Fig. 3).
Fig. 2.
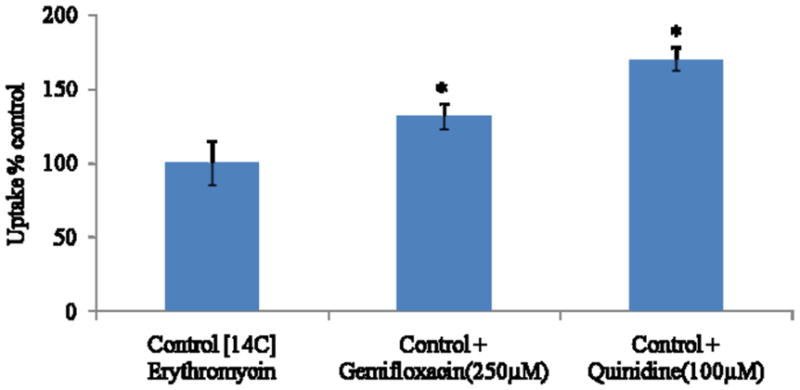
Cellular accumulation of [14C] erythromycin alone, in presence of gemifloxacin (250μM) and quinidine (100μM) in MDCKII-MDR1 cells. Values represent mean ± SD (n=4). * represents statistically significant from control at a P-value of < 0.05.
Fig. 3.
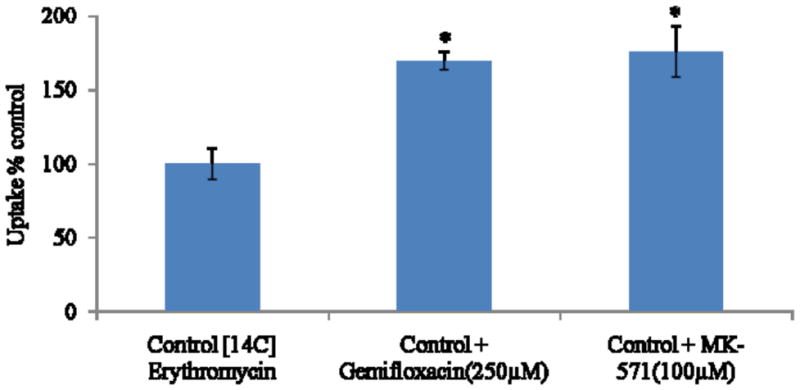
Cellular accumulation of [14C] erythromycin alone, in presence of gemifloxacin (250μM) and MK-571 (100μM) in MDCKII-MRP2 cells. Values represent mean ± SD (n=4). * represents statistically significant from control at a P-value of < 0.05.
3.2 Dose dependent inhibition studies
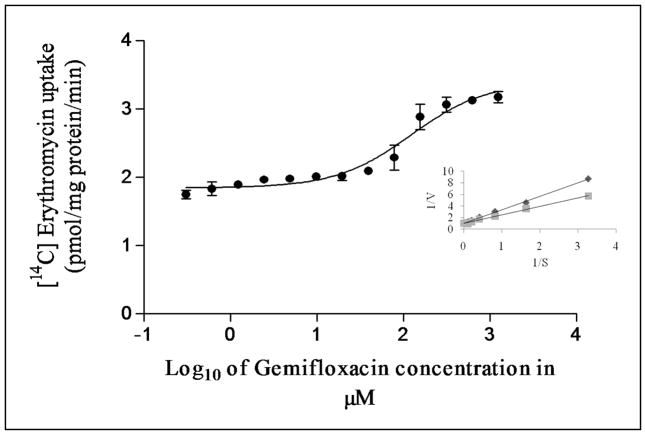
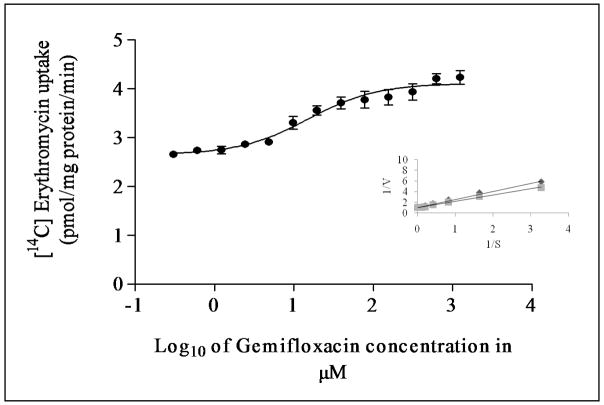
Dose dependent inhibition of [14C] erythromycin excretion was performed on MDCKII-MDR1 cells with varying concentrations of gemifloxacin (1μM to 1000μM). IC50 value of gemifloxacin from the dose–response curve was calculated to be 123 ± 2μM (Fig. 4). Similar inhibition studies were also performed on MDCKII-MRP2 cells to delineate the inhibitory potential of gemifloxacin on [14C] erythromycin uptake. IC50 value of gemifloxacin from the dose–response curve was calculated to be 16 ± 2μM (Fig. 5).
Fig. 4.
Dose dependent inhibition of [14C] erythromycin uptake in MDCKII-MDR1 cells in presence of varying concentrations of gemifloxacin (1μM to1000μM). Values represent mean ± SD (n=6). Inset shows the Lineweaver-Burk transformation of the data; ◆ represents gemifloxacin and ■ represents erythromycin.
Fig. 5.
Dose dependent inhibition of [14C] erythromycin uptake in MDCKII-MRP2 cells in presence of varying concentrations of gemifloxacin (1μM to1000μM). Values represent mean ± SD (n=6). Inset shows the Lineweaver-Burk transformation of the data; ◆ represents gemifloxacin and ■ represents erythromycin.
3.3 Cellular calcein fluorescence
Intracellular fluorescence in MDCKII-MDR1 cells is doubled in the presence of quinidine (100μM), suggesting increased accumulation of calcein-AM through inhibition of MDR1. Similar increase in fluorescence was also observed in the presence of gemifloxacin (250μM) (Fig. 6). This study was also performed on MDCKII-MRP2 cells to delineate calcein efflux by MRP2. Intracellular calcein fluorescence did significantly rise (2.5 fold) in presence of MK-571 (100μM), probably through reduction in calcein efflux by MRP2. Accumulation of calcein was also significantly higher in the presence of gemifloxacin (250μM) (Fig. 7A). Such enhanced fluorescence in MDCKII-MRP2 cells in presence of gemifloxacin and MK-571 was also confirmed by fluorescent microscopic images (Fig. 7B).
Fig. 6.
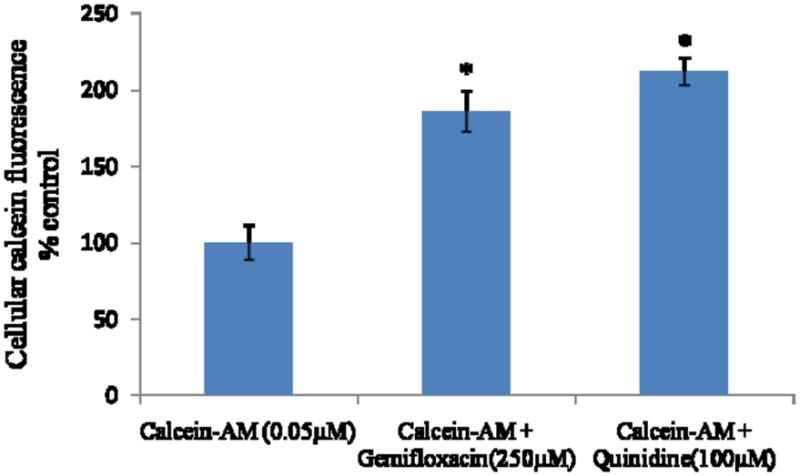
Cellular accumulation of calcein-AM alone, in presence of gemifloxacin (250μM) and quinidine (100μM) in MDCKII-MDR1 cells, as measued by intrinsic calcein flourescence. Values represent mean ± SD (n=6). * represents statistically significant from control at a P-value of < 0.05.
Fig. 7.
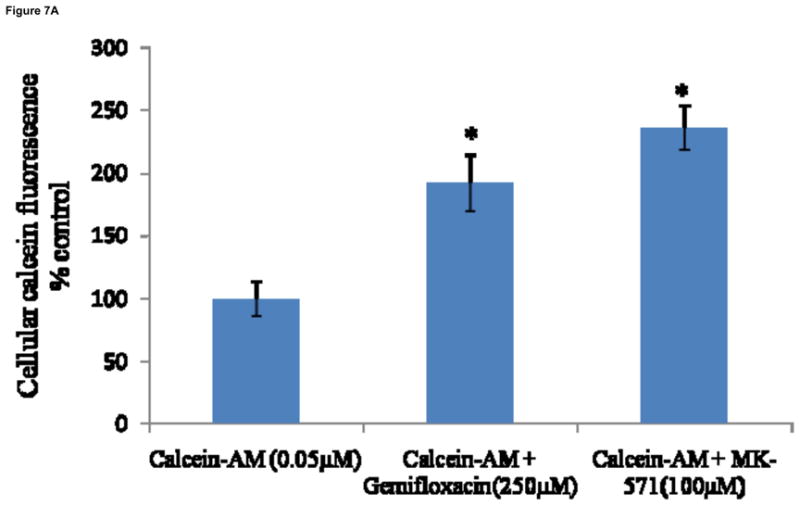
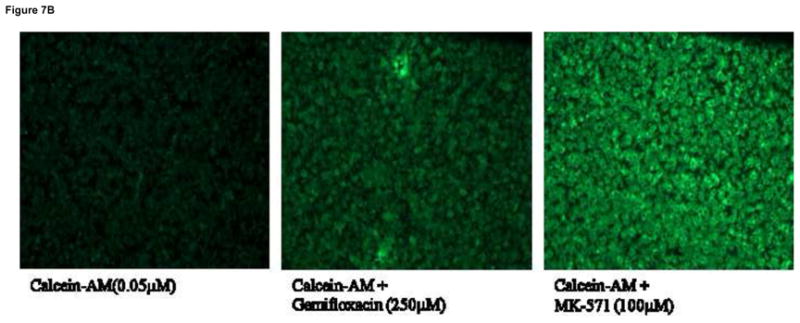
Fig. 7A: Cellular accumulation of calcein-AM alone, in presence of gemifloxacin (250μM) and MK-571 (100μM) in MDCKII-MRP2 cells, as measued by intrinsic calcein flourescence. Values represent mean ± SD (n=6). * represents statistically significant from control at a P-value of < 0.05.
Fig. 7B: Cellular images representing efflux of fluorescent calcein alone, in presence of gemifloxacin (250μM) and MK-571 (100μM) in MDCKII-MRP2 cells. Pictures are representative of two independent experiments.
3.4 Permeability studies
Transport of [14C] erythromycin across MDCKII-MDR1 cells was assessed in both AP-BL and BL-AP directions. Since these efflux transporters are polarized on the apical side, permeability of [14C] erythromycin in AP-BL is lower than BL-AP direction. Apparent permeability of [14C] erythromycin from AP-BL direction was observed to be 2.96 ± 0.37 × 10-6 cm/sec. This permeability significantly ascended to 6.37 ± 0.74 × 10-6 cm/sec in the presence of quinidine (100μM), probably due to inhibition of MDR1. A similar rise in the permeability of [14C] erythromycin to 5.24 ± 0.52 × 10-6 cm/sec was also observed in the presence of gemifloxacin (250μM). Permeability of [14C] erythromycin was obtained as 10.56 ± 0.86 × 10-6 cm/sec from BL-AP direction. Such BL-AP permeability was significantly reduced in the presence of quinidine (100μM) and gemifloxacin (250μM) to 8.56 ± 0.43 and 7.38 ± 0.72 × 10-6 cm/sec, respectively (Fig. 8). Efflux ratio of [14C] erythromycin on MDCKII-MDR1 cells as calculated by ratio of permeability in BL-AP to AP-BL direction was found to significantly diminish in the presence of quinidine (100μM) and gemifloxacin (250μM).
Fig. 8.
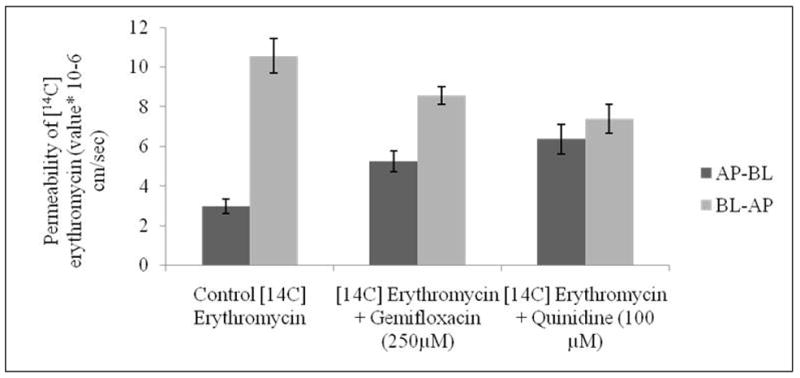
Transepithelial transport of [14C] erythromycin alone, in presence of gemifloxacin (250μM) and quinidine (100μM) in MDCKII-MDR1 cells from AP-BL and BL-AP directions. Values represent mean ± SD (n=6).
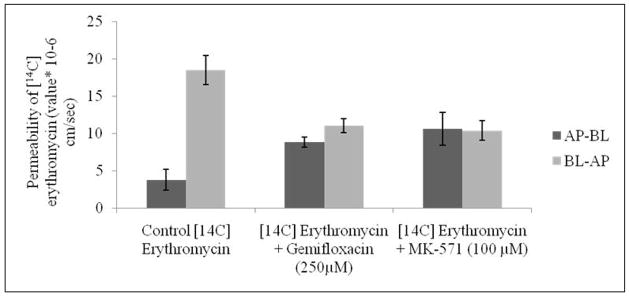
Transport of [14C] erythromycin across MDCKII-MRP2 cells was similarly assessed in both AP-BL and BL-AP directions. Apparent permeability of [14C] erythromycin in AP-BL direction was found to be 3.76 ± 1.37 × 10-6 cm/sec. This permeability significantly rises in the presence of MK-571 (100μM) to 10.61 ± 2.20 × 10-6 cm/sec, probably indicating the inhibition of MRP2 efflux pump. In a similar manner, permeability was also enhanced in the presence of gemifloxacin (250μM) to 8.80 ± 0.68 × 10-6 cm/sec. In a similar manner, permeability of [14C] erythromycin decreased from BL-AP in the presence of MK-571 and gemifloxacin to 10.38 ± 1.34 and 11.05 ± 0.93 × 10-6 cm/sec respectively, relative to control with 18.53 ± 1.94 × 10-6 cm/sec (Fig. 9). Efflux ratio of [14C] erythromycin also diminished significantly in the presence of MK-571 (100μM) and gemifloxacin (250μM) on MDCKII-MRP2 cells.
Fig. 9.
Transepithelial transport of [14C] erythromycin alone, in presence of gemifloxacin (250μM) and MK-571 (100μM) in MDCKII-MRP2 cells from AP-BL and BL-AP directions. Values represent mean ± SD (n=6).
3.5 ATP activity assay
ATP activity assay was performed post treatment with gemifloxacin for 150min on MDCKII-MDR1 and MRP2 cells. All concentrations of gemifloxacin used (1μM to 1000μM) except 1000μM, did not significantly alter ATP levels compared to control. Gemifloxacin (1000μM) increased ATP level by almost 15% and 10% in MDCKII-MDR1 and MRP2 cells, respectively (data not shown).
3.6 Cell proliferative assay
MTS assay was performed to examine the cytotoxicity of various concentrations of gemifloxacin (1μM to 1000μM) post treatment over 150min. Gemifloxacin (1000μM) decreased cell viability by almost 25% and 20% on MDCKII-MDR1 and MRP2 cells. All the other concentrations used were found to be non-cytotoxic (data not shown).
3.7 Quantitative gene expression and functional activity studies
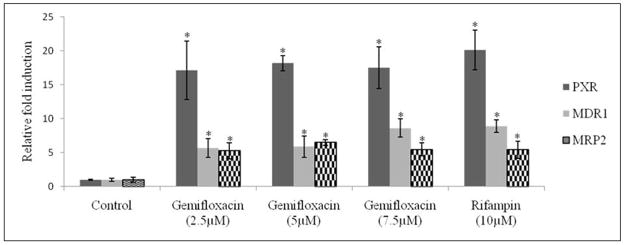
LS-180 cells were treated for 72hrs with three different concentrations of gemifloxacin (2.5, 5, 7.5μM) and rifampin (10μM). Real-time PCR analysis was performed to determine the quantitative gene expression levels of PXR, MDR1 and MRP2. All concentrations of gemifloxacin significantly induced PXR by almost 18 fold. The same concentrations induced MDR1 and MRP2 genes by almost 6 fold. Rifampin induced PXR by 20 fold, MDR1 by 9 fold and MRP2 by 6 fold (Fig. 10A). Cellular accumulation of [14C] erythromycin was decreased almost by 20% in LS-180 cells post treatment with gemifloxacin and rifampin (Fig. 10B).
Fig 10.
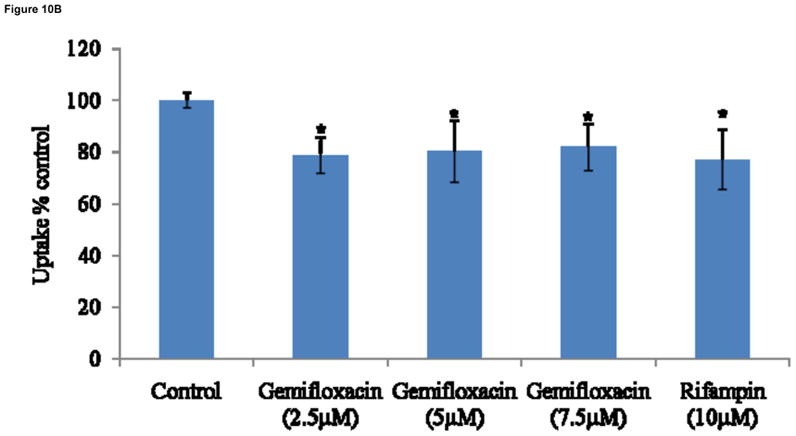
Fig. 10A: Relative fold induction of PXR, MDR1 and MRP2 in LS-180 cells treated with three concentrations of gemifloxacin (2.5, 5, 7.5μM) and rifampin (10 μM). Data represent relative fold induction (n=4) of three different experiments. * represents statistically significant from control at a P-value of < 0.05.
Fig. 10B: Cellular accumulation of [14C] erythromycin in LS-180 cells treated with three concentrations of gemifloxacin (2.5, 5, 7.5μM) and rifampin (10 μM). Values represent mean ± SD (n=4). * represents statistically significant from control at a P-value of < 0.05.
4. DISCUSSION
Gemifloxacin is a new fluoroquinolone antibiotic approved by US FDA for treatment of both gram positive and negative bacterial infections. It has broad spectrum of activity and inhibits both bacterial DNA gyrase and topoisomerase IV. Most fluoroquinolones, being substrates of efflux transporters, are generally extruded out from cellular matrix, significantly reducing intracellular accumulation and thereby altering drug bioavailability (Alvarez et al., 2008). The primary objective of this study was to evaluate the effect of efflux transporters on the cellular translocation of gemifloxacin, which may account for its low oral bioavailability (71%). Moreover mechanism behind differential expressions of efflux transporters upon long term exposure to gemifloxacin was also investigated.
Cellular accumulation of [14C] erythromycin in the presence of quinidine (a known substrate and/or inhibitor) appears to be significantly higher than the control, confirming the presence of P-gp in MDCKII-MDR1 cells (Kwatra et al., 2010; Sikri et al., 2004). A significantly elevated cellular accumulation of [14C] erythromycin in the presence of gemifloxacin is also observed (Fig. 2). This result indicates that this fluoroquinolone interacts with P-gp, suggesting gemifloxacin may be a substrate. Similarly, cellular accumulation of [14C] erythromycin in the presence of MK-571 (a known inhibitor of MRPs) (Kwatra et al., 2010; Luders et al., 2009) was found to be significantly higher than the control, confirming the presence of MRP2 in MDCKII-MRP2 cells. A significantly elevated cellular accumulation of [14C] erythromycin in the presence of gemifloxacin (Fig. 3) suggests that this fluoroquinolone may also be a substrate of MRP2. A significant rise in [14C] erythromycin uptake in the presence of quinidine and MK571 is consistent with our earlier results that both P-gp and MRP2 are functionally active in MDCKII-MDR1 and MDCKII-MRP2 cells, respectively (Agarwal et al., 2007; Kwatra et al., 2010). Dose-dependent inhibition studies suggest high affinity of gemifloxacin towards both P-gp and MRP2. Gemifloxacin inhibited both P-gp and MRP2 mediated efflux of [14C] erythromycin in a dose dependent manner with an IC50 value of 123 ± 2μM (Fig. 4) and 16 ± 2μM (Fig. 5), respectively. A comparison of IC50 values indicates that gemifloxacin probably has a much higher affinity towards MRP2 relative to P-gp. IC50 value of gemifloxacin was also found to be significantly lower than another fourth generation fluoroquinolone, gatifloxacin previously reported from our laboratory (Kwatra et al., 2010). These lower IC50 values represent that gemifloxacin is a better substrate of efflux transporters as compared to gatifloxacin. This further explains the difference in their bioavailability values (gatifloxacin-97% as compared to gemifloxacin- 71%). Lineweaver–Burk transformation of the above data revealed that the inhibitory mode of gemifloxacin for P-gp and MRP2 mediated excretion of [14C] erythromycin was a competitive type. This analysis suggests that gemifloxacin and erythromycin may share a common binding site in both the efflux transporters (Figs. 4 and 5 insets).
Further evidence of functional activity of P-gp and MRP2 on the cellular translocation of gemifloxacin has been studied with calcein-AM. Calcein-AM is a substrate of P-gp and calcein is a substrate of MRP2 (Eneroth et al., 2001; Essodaigui et al., 1998; Evers et al., 2000). A rise in intracellular calcein fluorescence in presence of quinidine and MK-571 confirms inhibition of both P-gp (Fig. 6) and MRP2 (Fig. 7A) functional activities. A similar elevation in calcein fluorescence in presence of gemifloxacin in MDCKII-MDR1 and MRP2 cells demonstrates its substrate specificity for both P-gp and MRP2. Such increase in calcein accumulation in MDCKII-MRP2 cells is further confirmed by fluorescent microscopic images (Fig. 7B).
The efflux ratio (ratio of basolateral to apical versus apical to basolateral permeability) is considered as one of the indicators for identifying P-gp and MRP2 substrates (Polli et al., 2001). Since these efflux transporters (P-gp and MRP2) are localized on the apical direction, [14C] erythromycin alone exhibited much higher transport in the BL-AP direction relative to AP-BL direction (Figs. 8 and 9). When this ratio of apparent permeability approaches 1.0 transport equals in both the directions. Efflux ratios of [14C] erythromycin were found to be 3.56 and 4.93 in MDCKII-MDR1 and MRP2 cells, respectively. A significant reduction in efflux ratio to 1 (complete inhibition) was evident in the presence of quinidine and MK-571 confirming inhibition of P-gp and MRP2 functional activities. Furthermore, the presence of gemifloxacin also affected bi-directional permeability of [14C] erythromycin. The efflux ratio is lowered to 1.63 and 1.26 in MDCKII-MDR1 and MRP2 cells. This significant reduction further confirms the substrate specificity of gemifloxacin towards P-gp and MRP2.
Since the efflux pumps require ATP for their activation, it is postulated that any change in the amount or activity of ATP may signify sensitivity of the substrate for the transporter. ATP determination assay was performed in MDCKII-MDR1 and MRP2 cells to examine ATP involvement in cellular translocation of gemifloxacin. No significant change in the ATP activity was observed at various gemifloxacin concentrations. This result suggests that P-gp and MRP2 mediated efflux by gemifloxacin is not affected by ATP activity. Though not to a large extent, a minor increase in ATP levels was observed in the presence of 1000μM concentration of gemifloxacin. This aberration may be due to stress response generated with the usage of a very high concentration. A cell proliferation assay is carried out to determine if the concentrations used are cytotoxic. Results obtained from this assay indicated that gemifloxacin tested at various concentrations do not elicit any cytotoxic effects in MDCKII-MDR1 and MRP2 cells. However, the highest concentration (1000μM) tested was found to reduce the number of viable cells. This high concentration leads to cytotoxicity which may be one of the factors responsible for elevated ATP levels (Fairchild and Cowan, 1991; Luo et al., 2010).
Development of multidrug resistance in response to fluoroquinolones may be in part mediated by efflux transporters (Alvarez et al., 2008). Since P-gp and MRP2 are well known efflux transporters, involved in the extrusion of a wide variety of intracellular substrates, relative expression of these two transporters was evaluated following treatment with three concentrations of gemifloxacin. Pretreatment of LS-180 cells with gemifloxacin caused a substantial rise in the mRNA expression of MDR1 (6 fold) and MRP2 (6 fold). Several drugs can induce the expression of efflux proteins through activation of nuclear factors. Nuclear hormone receptors are a super family of transcription factors activated by a plethora of endogenous stimuli such as steroids, retinoids, bile acids and oxysterols (Kliewer et al., 1998; Kullak-Ublick and Becker, 2003). These activated receptors such as PXR heterodimerize with retinoid X receptor (RXR) and then bind to the promoter of target genes. Activation of PXR may affect not only its own pharmacokinetics, but also of other concomitantly administered drugs. Therefore, PXR levels were also determined post treatment with gemifloxacin. Results indicated that gemifloxacin can also induce PXR to a higher extent (18 fold) (Fig. 10A). The reduced intracellular accumulation of [14C] erythromycin in LS-180 treated cells further confirmed the elevated functional activity of transcribed efflux transporters (Fig. 10B).
In conclusion, this report provides direct evidence that gemifloxacin is effluxed by both P-gp and MRP2. Prolonged administration of this fluoroquinolone induced PXR, P-gp and MRP2, which may contribute to the development of drug resistance. This induction in the expression of nuclear hormone receptor and efflux transporters may affect intracellular drug accumulation, limiting its oral bioavailability. This report provides an insight onto the mechanism of gemifloxacin interaction with efflux transporters. It also offers strategy for the development of new therapeutic delivery systems. This study also indicates that simultaneous administration of macrolides and fluoroquinolones may be more effective against various strains of Streptococci (Sikri et al., 2004). A combination of erythromycin and gemifloxacin apart from inhibiting P-gp and MRP2 mediated efflux can improve the bioavailability of erythromycin and possibly lower incidence of drug resistance. However, further clinical studies are required to confirm this hypothesis.
Acknowledgments
This work has been supported by NIH grant RO1EY009171-16
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Pal D, Mitra AK. Both P-gp and MRP2 mediate transport of Lopinavir, a protease inhibitor. Int J Pharm. 2007;339:139–147. doi: 10.1016/j.ijpharm.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Bygate E, Oliver S, Johnson M, Ward C, Cheon AJ, Choo YS, Kim IC. Pharmacokinetics and tolerability of gemifloxacin (SB-265805) after administration of single oral doses to healthy volunteers. Antimicrob Agents Chemother. 2000;44:1604–1608. doi: 10.1128/aac.44.6.1604-1608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Bygate E, Vousden M, Oliver S, Johnson M, Ward C, Cheon A, Choo YS, Kim I. Multiple-dose pharmacokinetics and tolerability of gemifloxacin administered orally to healthy volunteers. Antimicrob Agents Chemother. 2001;45:540–545. doi: 10.1128/AAC.45.2.540-545.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez AI, Perez M, Prieto JG, Molina AJ, Real R, Merino G. Fluoroquinolone efflux mediated by ABC transporters. J Pharm Sci. 2008;97:3483–3493. doi: 10.1002/jps.21233. [DOI] [PubMed] [Google Scholar]
- Anand BS, Patel J, Mitra AK. Interactions of the dipeptide ester prodrugs of acyclovir with the intestinal oligopeptide transporter: competitive inhibition of glycylsarcosine transport in human intestinal cell line-Caco-2. J Pharmacol Exp Ther. 2003;304:781–791. doi: 10.1124/jpet.102.044313. [DOI] [PubMed] [Google Scholar]
- Andriole VT. The quinolones: past, present, and future. Clin Infect Dis. 2005;41(Suppl 2):S113–119. doi: 10.1086/428051. [DOI] [PubMed] [Google Scholar]
- Appelbaum PC, Hunter PA. The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents. 2000;16:5–15. doi: 10.1016/s0924-8579(00)00192-8. [DOI] [PubMed] [Google Scholar]
- Berry V, Page R, Satterfield J, Singley C, Straub R, Woodnutt G. Comparative in vivo activity of gemifloxacin in a rat model of respiratory tract infection. J Antimicrob Chemother. 2000;45(Suppl 1):79–85. doi: 10.1093/jac/45.suppl_3.79. [DOI] [PubMed] [Google Scholar]
- Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther. 1999;21:3–40. doi: 10.1016/s0149-2918(00)88266-1. discussion 41–42. [DOI] [PubMed] [Google Scholar]
- Boumendjel A, Di Pietro A, Dumontet C, Barron D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med Res Rev. 2002;22:512–529. doi: 10.1002/med.10015. [DOI] [PubMed] [Google Scholar]
- Braun A, Hammerle S, Suda K, Rothen-Rutishauser B, Gunthert M, Kramer SD, Wunderli-Allenspach H. Cell cultures as tools in biopharmacy. Eur J Pharm Sci. 2000;11(Suppl 2):S51–60. doi: 10.1016/s0928-0987(00)00164-0. [DOI] [PubMed] [Google Scholar]
- Cormican MG, Jones RN. Antimicrobial activity and spectrum of LB20304, a novel fluoronaphthyridone. Antimicrob Agents Chemother. 1997;41:204–211. doi: 10.1128/aac.41.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Gunda S, Mitra AK. Pharmacokinetics of erythromycin in rabbit corneas after single-dose infusion: role of P-glycoprotein as a barrier to in vivo ocular drug absorption. J Pharmacol Exp Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- Dussault I, Forman BM. The nuclear receptor PXR: a master regulator of “homeland” defense. Crit Rev Eukaryot Gene Expr. 2002;12:53–64. doi: 10.1615/critreveukaryotgeneexpr.v12.i1.30. [DOI] [PubMed] [Google Scholar]
- Eneroth A, Astrom E, Hoogstraate J, Schrenk D, Conrad S, Kauffmann HM, Gjellan K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur J Pharm Sci. 2001;12:205–214. doi: 10.1016/s0928-0987(00)00117-2. [DOI] [PubMed] [Google Scholar]
- Erwin ME, Jones RN. Studies to establish quality control ranges for SB-265805 (LB2030) when using National Committee for Laboratory Standards antimicrobial susceptibility test methods. Quality Control Study Group. J Clin Microbiol. 1999;37:279–280. doi: 10.1128/jcm.37.1.279-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essodaigui M, Broxterman HJ, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug resistance protein and P-glycoprotein. Biochemistry. 1998;37:2243–2250. doi: 10.1021/bi9718043. [DOI] [PubMed] [Google Scholar]
- Evers R, Cnubben NH, Wijnholds J, van Deemter L, van Bladeren PJ, Borst P. Transport of glutathione prostaglandin A conjugates by the multidrug resistance protein 1. FEBS Lett. 1997;419:112–116. doi: 10.1016/s0014-5793(97)01442-7. [DOI] [PubMed] [Google Scholar]
- Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101:1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild CR, Cowan KH. Keynote address: multidrug resistance: a pleiotropic response to cytotoxic drugs. Int J Radiat Oncol Biol Phys. 1991;20:361–367. doi: 10.1016/0360-3016(91)90121-j. [DOI] [PubMed] [Google Scholar]
- Fuchs PC, Barry AL, Brown SD. In vitro activity of gemifloxacin against contemporary clinical bacterial isolates from eleven North American medical centers, and assessment of disk diffusion test interpretive criteria. Diagn Microbiol Infect Dis. 2000;38:243–253. doi: 10.1016/s0732-8893(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- Goldstein EJ, Conrads G, Citron DM, Merriam CV, Warren Y, Tyrrell K. In vitro activity of gemifloxacin compared to seven other oral antimicrobial agents against aerobic and anaerobic pathogens isolated from antral sinus puncture specimens from patients with sinusitis. Diagn Microbiol Infect Dis. 2002;42:113–118. doi: 10.1016/s0732-8893(01)00341-8. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology. 1999;106:1313–1318. [PubMed] [Google Scholar]
- Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36:1172–1180. doi: 10.1124/dmd.107.018689. [DOI] [PubMed] [Google Scholar]
- Hariharan S, Gunda S, Mishra GP, Pal D, Mitra AK. Enhanced corneal absorption of erythromycin by modulating P-glycoprotein and MRP mediated efflux with corticosteroids. Pharm Res. 2009;26:1270–1282. doi: 10.1007/s11095-008-9741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Febus CL, Maas-Bakker RF, Beijnen JH, Schellens JH. PXR-mediated induction of P-glycoprotein by anticancer drugs in a human colon adenocarcinoma-derived cell line. Cancer Chemother Pharmacol. 2010;66:765–771. doi: 10.1007/s00280-009-1221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton VJ, Ambler JE, Fisher LM. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitro. Antimicrob Agents Chemother. 2000;44:3112–3117. doi: 10.1128/aac.44.11.3112-3117.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl AF, Frei R, Punter V, von Graevenitz A, Knapp C, Washington J, Johnson D, Jones RN. International multicenter investigation of LB20304, a new fluoronaphthyridone. Clin Microbiol Infect. 1998;4:280–284. doi: 10.1111/j.1469-0691.1998.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Hooper DC. Mechanisms of fluoroquinolone resistance. Drug Resist Updat. 1999a;2:38–55. doi: 10.1054/drup.1998.0068. [DOI] [PubMed] [Google Scholar]
- Hooper DC. Mode of action of fluoroquinolones. Drugs. 1999b;58(Suppl 2):6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Jones RN, Erwin ME. Anti-streptococcal activity of SB-265805 (LB20304), a novel fluoronaphthyridone, compared with five other compounds, including quality control guidelines. Diagn Microbiol Infect Dis. 1999;33:87–91. doi: 10.1016/s0732-8893(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Budda B, Anand BS, Mitra AK. Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin Drug Deliv. 2005;2:683–705. doi: 10.1517/17425247.2.4.683. [DOI] [PubMed] [Google Scholar]
- King A, May J, French G, Phillips I. Comparative in vitro activity of gemifloxacin. J Antimicrob Chemother. 2000;45(Suppl 1):1–12. doi: 10.1093/jac/45.suppl_3.1. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Koeth LM, Jacobs MR, Bajaksouzian S, Zilles A, Lin G, Appelbaum PC. Comparative in vitro activity of gemifloxacin to other fluoroquinolones and non-quinolone agents against Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in the United States in 1999–2000. Int J Antimicrob Agents. 2002;19:33–37. doi: 10.1016/s0924-8579(01)00431-9. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35:305–317. doi: 10.1081/dmr-120026398. [DOI] [PubMed] [Google Scholar]
- Kwatra D, Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Interaction of gatifloxacin with efflux transporters: a possible mechanism for drug resistance. Int J Pharm. 2010;395:114–121. doi: 10.1016/j.ijpharm.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28:352–364. doi: 10.1086/515104. [DOI] [PubMed] [Google Scholar]
- Luders AK, Saborowski R, Bickmeyer U. Inhibition of multidrug/xenobiotic resistance transporter by MK571 improves dye (Fura 2) accumulation in crustacean tissues from lobster, shrimp, and isopod. Comp Biochem Physiol C Toxicol Pharmacol. 2009;150:368–371. doi: 10.1016/j.cbpc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Luo S, Pal D, Shah SJ, Kwatra D, Paturi KD, Mitra AK. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Mol Pharm. 2010;7:412–420. doi: 10.1021/mp900193e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JI, Paek KS, Ahn MJ, Kim MY, Hong CY, Kim IC, Kwak JH. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob Agents Chemother. 1996;40:1564–1568. doi: 10.1128/aac.40.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 2010 doi: 10.1016/j.lfs.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-drug interactions. J Neuroimmune Pharmacol. 2006;1:323–339. doi: 10.1007/s11481-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- Ramji JV, Austin NE, Boyle GW, Chalker MH, Duncan G, Fairless AJ, Hollis FJ, McDonnell DF, Musick TJ, Shardlow PC. The disposition of gemifloxacin, a new fluoroquinolone antibiotic, in rats and dogs. Drug Metab Dispos. 2001;29:435–442. [PubMed] [Google Scholar]
- Raucy JL, Lasker JM. Current in vitro high throughput screening approaches to assess nuclear receptor activation. Curr Drug Metab. 2010;11:806–814. doi: 10.2174/138920010794328896. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S, McCloskey L, Broskey J, Niconovich N, Jakielaszek C, Poupard J, Coleman K. In vitro antibacterial activity of gemifloxacin and comparator compounds against common respiratory pathogens. J Antimicrob Chemother. 2000;45(Suppl 1):23–27. doi: 10.1093/jac/45.suppl_3.23. [DOI] [PubMed] [Google Scholar]
- Saravolatz LD, Leggett J. Gatifloxacin, gemifloxacin, and moxifloxacin: the role of 3 newer fluoroquinolones. Clin Infect Dis. 2003;37:1210–1215. doi: 10.1086/378809. [DOI] [PubMed] [Google Scholar]
- Sikri V, Pal D, Jain R, Kalyani D, Mitra AK. Cotransport of macrolide and fluoroquinolones, a beneficial interaction reversing P-glycoprotein efflux. Am J Ther. 2004;11:433–442. doi: 10.1097/01.mjt.0000132643.69143.64. [DOI] [PubMed] [Google Scholar]
- Turnidge J. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs. 1999;58(Suppl 2):29–36. doi: 10.2165/00003495-199958002-00006. [DOI] [PubMed] [Google Scholar]
- Vousden M, Ferguson J, Richards J, Bird N, Allen A. Evaluation of phototoxic potential of gemifloxacin in healthy volunteers compared with ciprofloxacin. Chemotherapy. 1999;45:512–520. doi: 10.1159/000007246. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Triller DM, Yong CS, Lodise TP. Gemifloxacin: a new fluoroquinolone approved for treatment of respiratory infections. Ann Pharmacother. 2004;38:1226–1235. doi: 10.1345/aph.1E003. [DOI] [PubMed] [Google Scholar]
- Zhanel GG, Noreddin AM. Pharmacokinetics and pharmacodynamics of the new fluoroquinolones: focus on respiratory infections. Curr Opin Pharmacol. 2001;1:459–463. doi: 10.1016/s1471-4892(01)00080-7. [DOI] [PubMed] [Google Scholar]