Abstract
Psychological stress is a major risk factor for mood and anxiety disorders. However, the phenotypic manifestation of stress effects varies across individuals, likely due, in part, to genetic variation. Modeling the behavioral and neural consequences of stress across genetically diverse inbred mouse strains is a valuable approach to studying gene x stress interactions. Recent work has shown that C57BL/6J mice exposed to ten daily sessions of restraint stress exhibited increased exploration of the aversive light compartment in the light/dark exploration (LDE) test. Here we sought to clarify the nature of this stress-induced phenotype by testing the ability of treatment with various clinically efficacious drugs of different therapeutic classes to rescue it. Ten days of restraint increased light compartment exploration, reduced body weight and sensitized the corticosterone response to swim stress. Subchronic administration (during stress and LDE testing) of fluoxetine, and to a lesser extent, lithium chloride, rescued stress-induced LDE behavior. Chronic fluoxetine treatment prior to (plus during stress and testing) failed to block the LDE stress effect. Acute administration of antipsychotic haloperidol, anti-ADHD medication methylphenidate or anxiolytic drug chlordiazepoxide, prior to LDE testing, was also unable to normalize the LDE stress effect. Collectively, these data demonstrate a treatment-selective prophylactic rescue of a restraint stress-induced behavioral abnormality in the C57BL/6J inbred strain. Further work with this novel model could help elucidate genetic and neural mechanisms mediating stress-induced changes in mouse ‘emotion-relevant’ behaviors and, ultimately, further understanding of the pathophysiology of stress-related neuropsychiatric disorders.
Keywords: fluoxetine, lithium, mood, depression, animal model
Introduction
Exposure to psychological stress and trauma is a major risk factor for many neuropsychiatric disorders. In addition, genetic factors are estimated to contribute a significant proportion to risk for these conditions (Kendler et al., 2004). Thus, there has been an intense research effort aimed at identifying sources of genetic variation that confer susceptibility or, conversely, resilience to the deleterious effects of exposure to stress (Caspi et al., 2010; Rutter, 2008). An issue that has received less attention is why individuals differ not just in their relative sensitivity to stress, but in the manner in which such stress effects manifest phenotypically (Yehuda and LeDoux, 2007). What are the mechanistic factors, including genetic background, that influence whether exposure to traumatic stress leads to an anxiety disorder in one person, but depression in another?
Animal models, and mouse models in particular, are a valuable research tool for parsing the relative influence of genes and stress on risk for neuropsychiatric disease (Cryan and Holmes, 2005). In this context, previous studies have described differences between mouse strains in basal anxiety- and ‘depression-related’ behaviors and stress-induced changes in these behaviors (e.g., Anisman et al., 2001; Hefner et al., 2008; Holmes et al., 2005; Ibarguen-Vargas et al., 2008; Jacobson and Cryan, 2007; Mineur et al., 2006; Trullas and Skolnick, 1993; Uchida et al., 2011; Yang et al., 2008). Building upon this literature, a recent study screened a panel of inbred mouse strains for anxiety- and ‘depression-related’ phenotypes following repeated exposure to restraint stress (Mozhui et al., 2010). One of the main findings of this study was that two inbred strains, C57BL/6J and DBA/2J, showed different behavioral profiles at baseline and opposite profiles in response to this stress regimen in the light/dark exploration test (LDE) for anxiety-like behavior. These behavioral differences were associated with divergent stress-induced alterations in gene expression and excitatory neurotransmission in the basolateral amygdala, a brain region mediating stress and strongly implicated in stress-related neuropsychiatric disorders (Holmes and Hariri, 2003; Ressler and Mayberg, 2007).
The specific observation made by Mozhui et al. was that DBA/2J had higher anxiety-like behavior at baseline, relative to C57BL/6J, and that repeated restraint stress produced an additional change indicative of a further heightening of anxiety-like behavior in DBA/2J (i.e., reduced time spent exploring the aversive light compartment). By contrast, C57BL/6J responded to stress with what would be conventionally interpreted as decreased anxiety-like behavior (i.e., increased time spent exploring the aversive light compartment). Thus, these data might indicate a stress-vulnerable phenotype in DBA/2J and a stress-resilient phenotype in C57BL/6J. This would be generally consistent with the conclusions of earlier studies (cited above), although these strain differences are clearly dependent upon the stressor and endpoint measure employed.
There are, however, alternative interpretations of these strain differences, and the C57BL/6J profile in particular. Thus, as discussed by Mozhui et al., increased light compartment exploration in stressed C57BL/6J could reflect an active, possibly escape-motivated, response that contrasts with the passive, withdrawal-from-aversion response in DBA/2J. That is, rather than being an indication of stress-resiliency, the response of the C57BL/6J strain may be a different manifestation of a stress-induced effect. For example, this strain might show an active ‘coping strategy’ following stress, driven primarily by attempts to escape from an anxiety-producing situation. This type of response is seen in wild-caught mice exposed to anxiety-related assays (Holmes et al., 2000).
One approach to try and disentangle these alternative possibilities is to pharmacologically probe the behavior with clinically efficacious anxiolytics. If the C57BL/6J stress response reflects a decrease in anxiety-like behavior and stress-resiliency, then anxiolytic drugs should be without effect. Conversely, rescue of the behavior by anxiolytic treatments would be consistent with an atypical stress-sensitive response that ‘paradoxically’ manifests as an active exploratory behavior.
The main objective of the present study was to examine stress-induced changes in LDE behavior in C57BL/6J mice after chronic treatment with fluoxetine (Prozac), a front-line anxiolytic and antidepressant treatment, or the mood stabilizer, lithium (Cibalith). This treatment regimen was employed as these drugs are clinically efficacious after chronic treatment and it has been argued, therefore, that rodent models of anxiety- and stress-related behaviors should be tested for sensitivity to chronic treatment (Borsini et al., 2002; Dulawa et al., 2004). A secondary aim was to assess the effects of treatment with the classic benzodiazepine anxiolytic chlordiazepoxide (Librium), the anti-Attention Deficit Hyperactivity Disorder (ADHD) medication methylphenidate (Ritalin) or the antipsychotic haloperidol (Haldol). Although limiting the comparability with the chronically treated drugs, these three compounds were administered acutely instead of chronically because they typically produce effects on rodent behaviors after a single administration (Fitzgerald et al., 2010; Holmes et al., 2001; Karlsson et al., 2008b).
Methods
Subjects
Mice (8-9 wks at the start of experiments) were male C57BL/6J obtained from The Jackson Laboratory (Bar Harbor, ME) and housed 2/cage in a temperature-(22 ±3°C) and humidity-(45 ±15%) controlled vivarium under a 12 hr light/dark cycle (lights on 0600 h). Both mice in a cage were assigned to the same stress and treatment condition and stressed, treated and tested at the same time. Cages contained substrate, a cotton nestlet and a single toilet roll tube, as per local Animal Care and Use Committee (ACUC) requirements. The number of mice used for each experiment is given in the figure legends. All experimental procedures were approved by the NIAAA ACUC and followed the NIH guidelines outlined in ‘Using Animals in Intramural Research’ and the local Animal Care and Use Committees.
Stress and light/dark exploration test procedures
Repeated restraint stress
Previous studies have shown that 10 days of immobilization in ‘immobilization bags’ produces significant changes in neuronal morphology in the medial prefrontal cortex, basolateral amygdala and CA3 region of the hippocampus in rats and mice (Holmes and Wellman, 2009). As in our previous study (Mozhui et al., 2010), we adopted a modified version of this protocol in which mice were placed in ventilated 50 mL Falcon tubes for 2 hr/day (1000-1200 hr) for 10 consecutive days. To be consistent with earlier studies using this stressor in rats and mice (Govindarajan et al., 2006; Shansky et al., 2009; Vyas et al., 2002), non-restrained mice remained in the home cage.
As a measure of the efficacy of repeated restraint as a stressor, we measured restraint-induced reductions in body weight (Krishnan et al., 2007; Pothion et al., 2004; Surget et al., 2009; Willner et al., 1996). Changes in body weight over the 10-day restraint period were compared between the restrained and non-restrained groups.
Light/dark exploration test
The light/dark exploration (LDE) test for anxiety-related behavior (Crawley, 1981) was conducted as previously described (Mozhui et al., 2010). Mice began the test in an opaque black Plexiglas shelter (dark compartment; 39 × 13 × 16 cm) with a 13 × 8 cm aperture at floor level that opened onto a large white Plexiglas square arena (light compartment; 39 × 39 × 35 cm) illuminated by ceiling room lights to ~90 lux (room illuminated tô90 lux). Latency to first enter the light compartment, the number of light compartment entries (defined as all 4 paws out of the shelter) and time spent inside the light compartment over a 15 min session were scored by an observer using the Hindsight software program (Scientific Programming Services, Wokingham, UK) (for the fluoxetine experiments; note: distance traveled could not be calculated using this scoring method), or automatically by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA). Mice that did not enter the light compartment were given a 900 sec latency to enter. Analysis was based on the first 5 min of the test, when behavior is most strongly influenced by novelty and approach (Mozhui et al., 2010).
Experiment 1: Effects of subchronic fluoxetine treatment during stress
Treatment and LDE test
Mice were treated with fluoxetine by providing 120 mg/L fluoxetine hydrochloride (LKT Laboratories Inc, St. Paul, MN) in their only source of drinking water, as previously described (Norcross et al., 2008). This concentration was chosen to attain an average self-administered daily dose of 10-15 mg/kg and plasma levels in the human therapeutic range (Dulawa et al., 2004). Previous studies have confirmed this treatment regimen is sufficient to produce effects on behavior and stimulate hippocampal neurogenesis (Brigman et al., 2010; Karlsson et al., 2008a). Controls received tap water as only source of fluids. To allow the drug to achieve steady state levels prior to stress, mice were fluoxetine treated for 4 days prior to the start of stress (days 1-4 of treatment). They were then maintained on drug through 10 days of stress (days 5-14 of treatment) and the 2 days of behavioral testing (days 15-16 of treatment). On day 7 of treatment, solutions were refreshed. Fluid consumption was measured via bottle weights (corrected for evaporation and spillage) on day 7 and day 16 of treatment. For mice receiving fluoxetine, daily dose was calculated as mg fluoxetine per kg body weight.
Mice were tested on the LDE 1 day after the final stressor (for schematic, see Figure 1a).
Figure 1. Effects of subchronic fluoxetine treatment during stress.
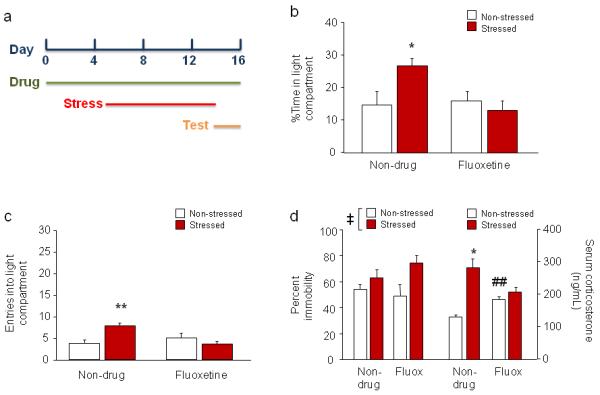
(a) Schematic of experimental timeline. Mice were fluoxetine treated on days 1-16, stressed on days 5-14, and tested in LDE and FST on days 15 and 16, respectively. (b) Non-drug-treated stressed mice spent more time inside the light compartment than non-stressed mice. These differences were absent in fluoxetine-treated mice. (c) Stressed mice made more entries into the light compartment than non-stressed mice after water, but not fluoxetine, treatment. (d) In the FST, stressed mice had higher immobility than non-stressed mice (left). Serum corticosterone levels were higher after the FST in stressed mice than non-stressed mice after non-drug treatment but not fluoxetine treatment (right). Fluoxetine treatment produced higher corticosterone levels than non-drug treatment in non-stressed mice. n=8/treatment/stress condition. Data are means ±SEM. **p<.01, *p<.05 vs. non-drug-treated non-stressed; ‡p<.01 stressed vs. non-stressed; ##p<.01 vs. non-drug/non-stressed
Forced swim test and swim-induced corticosterone
In addition to its anxiolytic-like effects, fluoxetine has antidepressant-like activity in various rodent assays (Cryan and Holmes, 2005). We tested the effects of fluoxetine in stressed mice using the forced swim test (FST) for depression-related behavior (Porsolt et al., 1977). All mice were assayed in the FST the day after LDE testing to avoid confounding effects of the swim stress on LDE behavior and to permit collection of corticosterone. Mice were gently lowered into a transparent Plexiglas cylinder (26 cm high, 20 cm diameter) filled halfway with water (24 ±1°C) for a 6 min session (under room illumination at ~500 lux). The presence/absence of immobility (cessation of limb movements except minor involuntary movements of the hind limbs) was scored by an observer using an instantaneous sampling technique every 5 sec during min 3-6 (125-360 sec) and expressed as percent total observations, as previously described (Baganz et al., 2011; Carroll et al., 2007).
To assessed HPA-axis activation to swim stress, mice were placed in a clean cage (with fresh bedding) after testing and, 30 min later, sacrificed via rapid cervical dislocation and decapitation to collect trunk blood as previously described (Boyce-Rustay et al., 2007). Blood samples were centrifuged at 13,000 rpm for 30 sec. Serum was extracted and assayed for total corticosterone (bound and free) using the Coat-a-Count RIA TKRC1 kit (limit of detection: 5.7 ng/ml; Diagnostic Products Corp, Los Angeles).
Open field test
In order to test for potential changes in locomotor activity (e.g., hyperlocomotion) as a result of stress, and the effects of fluoxetine on this behavior, we examined a naïve cohort of mice in the open field test. Mice were treated and stressed as above and then tested in the open field 24 hr after the final stressor. The open field test was conducted as previously described (Karlsson et al., 2008b). The apparatus was a square arena (39 × 39 × 35 cm) with opaque white Plexiglas walls and floor, evenly-illuminated to ~60 lux (room illuminated to ~20 lux). Mice were placed in a corner and allowed to freely explore for 5 min. Total distance traveled and time spent in the (20 × 20 cm) center was measured using the Ethovision videotracking system.
Experiment 2: Effects of chronic fluoxetine treatment prior to and during stress
Mice were fluoxetine treated as above for 28 days prior to the start of stress, and maintained on drug through 10 days of stress and behavioral testing (for schematic, see Figure 2a). Fluoxetine and water consumption was measured via bottle weights (corrected for evaporation and spillage) weekly and at the completion of testing (i.e., 40 days), and converted to a mg/kg body weight daily fluoxetine dose. Mice were tested on the LDE 1 day after the final stressor and the FST 2 days after the final stressor. Fluoxetine dose was measured weekly as described for experiment 1 and expressed as a value for the entire treatment period.
Figure 2. Effects of chronic fluoxetine treatment prior to and during stress.
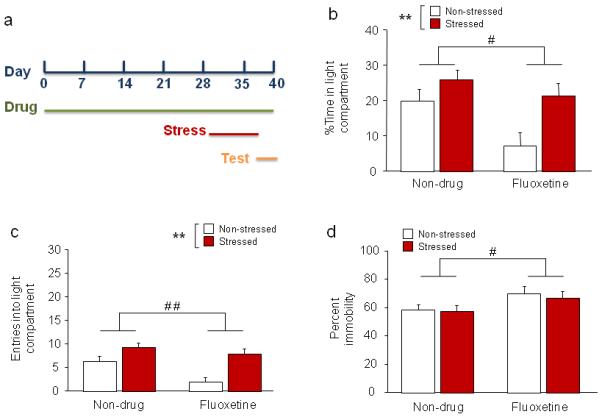
(a) Schematic of experimental timeline. Mice were fluoxetine treated on days 1-40, stressed on days 29-38, and tested in LDE and FST on days 39 and 40, respectively. (b) Stressed mice spent more time inside the light compartment than non-stressed mice, regardless of fluoxetine treatment. Fluoxetine-treated mice spent less time inside the light compartment than non-drug-treated controls. (c) Stressed mice made more entries into the light compartment than non-stressed mice, regardless of fluoxetine treatment. Fluoxetine treated mice made fewer light compartment entries than non-drug-treated controls. (d) In the FST, fluoxetine-treated mice had higher immobility than non-drug-treated controls. n=7-8/treatment/stress condition. Data are means ±SEM. **p<.01 stressed vs. non-stressed, # #p<.01, #p<.05 non-drug vs. fluoxetine
Experiment 3: Effects of subchronic lithium treatment during stress
Mice were treated with lithium by providing 4 g/kg drug-containing pellets as their only source of chow, as previously described (Shaltiel et al., 2008). We have previously shown that this dose produces blood levels in the therapeutic range (approximately 1.1 mEq/L) (Fitzgerald et al., 2010). To allow the drug to achieve steady state levels prior to stress, mice were lithium treated for 4 days prior to the start of stress. They were then maintained on drug through 10 days of stress and behavioral testing (for schematic, see Figure 3a). Mice were tested on the LDE 1 day after the final stressor. Because lithium has antidepressant-related effects in rodents (Shaltiel et al., 2008), we also tested this drug in the FST (the day after the LDE).
Figure 3. Effects of subchronic lithium treatment during stress.
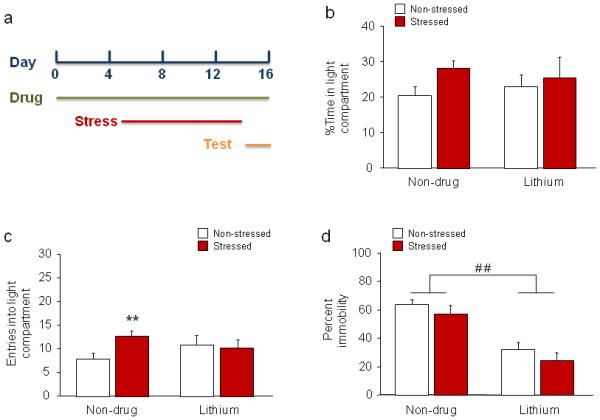
(a) Schematic of experimental timeline. Mice were lithium treated on days 1-16, stressed on days 5-14, and tested in LDE and FST on days 15 and 16, respectively. (b) Neither stress nor lithium treatment significantly affected percent time inside the light compartment. (c) Non-drug-treated stressed mice made more entries into the light compartment than non-stressed mice. These differences were absent in lithium-treated mice. (d) In the FST, lithium treatment decreased immobility, irrespective of stress. n=10/treatment/stress condition. Data are means ±SEM. **p<.01 vs. non-stressed/non-drug treatment, # #p<.01 non-drug vs. lithium
Experiment 4: Effects of acute haloperidol treatment after stress
Mice were subjected to stress as above and, 1 day after the final stress, tested on the LDE. Immediately before LDE testing, mice were injected intraperitoneally (i.p., in a volume of 10 mL/kg body weight) with either 0.25 mg/kg haloperidol (Sigma, St. Louis, MO, dissolved in 0.9% saline) or saline vehicle. The drug was administered acutely and this dose chosen based upon previous studies showing that acute treatment is sufficient to reverse ‘schizophrenia-related’ phenotypes in mice (Karlsson et al., 2008b; Wiedholz et al., 2008).
We did not test the effects of haloperidol, methylphenidate or chlordiazepoxide (see next 2 experiments) in the FST because these drugs are not typically associated with antidepressant-related effects.
Experiment 5: Effects of acute methylphenidate treatment after stress
Mice were subjected to stress as above and, 1 day after the final stress, tested on the LDE. Thirty min before LDE testing, mice were injected i.p. (in a volume of 10 mL/kg body weight) with either 7.5 mg/kg methylphenidate (Sigma, St. Louis, MO, dissolved in 0.9% saline) or saline vehicle. The drug was administered acutely and this dose chosen based upon previous studies showing that acute treatment affected ‘schizophrenia-related’ phenotypes in mice, and to avoid potential locomotor hyperactivity effects (Fitzgerald et al., 2010).
Experiment 6: Effects of acute chlordiazepoxide treatment after stress
Mice were subjected to stress as above and, 1 day after the final stress, tested on the LDE. Thirty min before LDE testing, mice were injected i.p. (in a volume of 10 mL/kg body weight) with either 10 mg/kg chlordiazepoxide (Sigma, St. Louis, MO, dissolved in 0.9% saline) or saline vehicle. The drug was administered acutely and this dose was chosen based upon previous studies showing that acute treatment is sufficient to produce anxiolytic-like effects in mice (Holmes et al., 2001). Given unexpected negative effects of chlordiazepoxide (see Results), we conducted follow-up validation experiments in the LDE test and other anxiety-related tests that are described in the Results.
Statistical analysis
The effects of drug and stress were analyzed using 2-factor analysis of variance (ANOVA) followed by Newman Keuls post hoc tests in the presence of significant interaction effects. Student’s t-test was used to examine the effect of stress on fluoxetine consumption and effects of chlordiazepoxide in the C57BL/6J validation experiments. The threshold for statistical significance was set at p<.05.
Results
Experiment 1: Effects of subchronic fluoxetine treatment during stress
Body weight
Demonstrating the efficacy of the repeated restraint stress procedure, there was a significant decrease in body weight over the stress period in stressed mice relative to non-stressed controls, irrespective of fluoxetine treatment (main effect of stress: F1,28=25.23, p<.01) (Table 1).
Table 1. Effects of stress on body weight.
Change in body weight (g) represents difference in weight before and after 10 days of restraint stress. Stress reliably reduced body weight relative to non-stressed controls, irrespective of drug treatment. One exception was in the case of lithium, where a drug-induced loss of weight precluded detection of a further decrease with stress. Data are means ± SEM.
| Non-drug | Drug | |||
|---|---|---|---|---|
| Non-stressed | Stressed | Non-stressed | Stressed | |
| Stress-induced change in weight (g) | ||||
| EXPT 1: Subchronic fluoxetine** | +1.51 ±0.15 | -2.00 ±1.02 | +1.55 ±0.34 | -0.43 ±0.13 |
| EXPT 2: Chronic fluoxetine** | +0.44 ±0.25 | -1.73 ±0.34 | +1.70 ±0.51 | -1.54 ±0.40 |
| EXPT 3: Subchronic lithium‡ | +0.64 ±0.18 | -0.96 ±0.20# | -1.24 ±0.11 | -1.30 ±0.14 |
| EXPT 4: Acute haloperidol** | +1.11 ±0.18 | -0.45 ±0.12 | +1.54 ±0.27 | -0.03 ±0.22 |
| EXPT 5: Acute methylphenidate** | +1.33 ±0.26 | +0.61 ±0.34 | +1.15 ±0.45 | -0.22 ±0.26 |
| EXPT 6: Acute chlordiazepoxide* | +1.28 ±0.34 | +0.48 ±0.26 | +1.25 ±0.21 | +0.65 ±0.23 |
p<.01
p<.05 stressed vs. non-stressed
p<.01 drug vs. non-drug
p<.01 vs. non-stressed/non-drug
LDE test
There was a significant stress x drug treatment interaction for percent time in the light compartment (F1,28=5.65, p<.05) and number of light compartment entries (F1,28=11.56, p<.01), as well as in latency to first enter the light compartment (F1,28=5.19, p<.05). Post hoc analyses showed that, in non-drug-treated mice, percent time inside light compartment (Figure 1b) and number of light compartment entries (Figure 1c) was significantly higher, and latency to enter the compartment (non-drug/non-stressed=174.0 ±30.7, non-drug/stressed=73.9 ±11.8, fluoxetine/non-stressed=149.6 ±29.3, fluoxetine/stressed=157.3 ±17.3) significantly lower, in stressed mice as compared to non-stressed controls. There was no significant difference between stressed and non-stressed mice in time spent inside light compartment, entries, or latency following fluoxetine treatment. Fluoxetine treatment per se did not affect any measure, as determined from comparisons between non-stressed fluoxetine- and non-stressed non-drug-treated mice. Thus, subchronic fluoxetine treatment selectively blocked the effects of stress on LDE behavior.
FST and corticosterone
Stress increased percent immobility relative to non-stressed controls (F1,26=8.14, p<.01), but there was no effect of fluoxetine treatment and no stress x drug treatment interaction (Figure 1d). In this experiment, 2 mice with 0 immobility scores (the only such occurrences in the current study) were omitted from the analysis. There was a significant stress x drug interaction (F1,12=16.90, p<.01) for post-FST corticosterone levels. Post hoc tests showed that stressed mice had higher corticosterone levels than non-stressed controls after non-drug treatment but not after fluoxetine treatment (Figure 1d). Fluoxetine treatment itself increased corticosterone levels, as indicated by comparison of drug- and non-drug-treated non-stressed mice. These data show that subchronic fluoxetine treatment prevented stress-induced changes in the HPA-axis but not the behavioral response to the FST.
Fluoxetine consumption
In mice given fluoxetine as their only drinking source, we found that fluoxetine consumption was altered during the latter part of the stress regimen. Specifically, fluoxetine consumption did not differ between stressed and non-stressed mice during the first week of treatment, which comprised 4 days of pre-stress baseline + 3 days of stressed (non-stressed=10.58±1.12 mg/kg/day, stressed=12.94±1.51). However, fluoxetine consumption was significantly greater in stressed mice than non-stressed mice during last 9 days of treatment, comprising 7 days of stress and 2 days of behavioral testing (non-stressed=11.07±1.64, stressed=16.91±.54, t=3.39, df=14, p<.01). In mice given water as their only drinking source, water consumption showed non-significant trends for a stress-induced increase during the first 7 days of treatment (non-stressed=164.3±24.3 g/kg/day, stressed=193.4±26.7) and the latter 9 days of treatment (non-stressed=169.8±34.0, stressed=223.7±16.0) weeks.
The unexpected increase in fluoxetine consumption in stressed mice raised the question of whether mice were ‘self-medicating.’ To address this possibility more directly, an additional cohort was given a 2-bottle, fluoxetine-solution versus water, preference test. Mice were presented with 2-bottles, side-by-side in the home cage for 22 days. One bottle contained a 120 mg/L fluoxetine-containing solution and the other bottle contained tap water. Mimicking the design of experiment 1, half of the mice were stressed on days 5-14 and half were undisturbed. The volume of consumption was measured for each bottle every 2 days, at which time the left versus right position of the bottles was switched to avoid side-bias. Results showed that mice strongly preferred water over the fluoxetine solution and this did not differ between stressed and non-stressed mice either during the stress period (non-stressed=95.67 ±0.86% preference for water, stressed=96.12 ±0.82%, n=6 per stress group). Therefore, the increase in fluoxetine consumption that was evident in experiment 1, when stressed mice had access to only this solution, may be an artifact of a general increase in drinking rather than stress-induced ‘self-medication.’
Open field
In a naïve cohort of mice, neither stress nor subchronic fluoxetine treatment affected locomotor activity in an open field test (non-drug/non-stressed=17.2 ±2.5 mean ±SEM total meters traveled, non-drug/stressed=23.1 ±1.3, fluoxetine/non-stressed=18.4 ±2.1, fluoxetine/stressed=19.7 ±1.4) or percent center time (non-drug/non-stressed=8.4 ±2.5, non-drug/stressed=11.0 ±2.5, fluoxetine/non-stressed=8.9 ±2.6, fluoxetine/stressed=4.5 ±1.0).
Experiment 2: Effects of chronic fluoxetine treatment prior to and during stress
Body weight
Chronic fluoxetine treatment did not prevent stress from decreasing body weight, relative to non-stressed controls (main effect of stress: F1,26=46.06, p<.01) (Table 1). There was a borderline main effect for fluoxetine treatment to increase weight relative to non-drug-treated controls (F1,26=3.30, p=.081).
LDE test
Stress increased percent time in the light compartment (main effect of stress: F1,26=9.11, p<.01) (Figure 2b) as well as light compartment entries (main effect of stress: F1,26=19.23, p<.01) (Figure 2c), relative to non-stressed controls, irrespective of fluoxetine treatment. Fluoxetine itself decreased light compartment time (main effect of drug: F1,26=6.54, p<.05) and entries (main effect of drug: F1,26=19.23, p<.01) relative to non-drug-treated controls. Latency to enter the light compartment was lower in stressed than non-stressed mice after fluoxetine, but not non-drug, treatment (stress x drug treatment interaction: F1,26=4.31, p<.05, followed by post hoc tests; non-drug/non-stressed=86.4 ±18.5, non-drug/stressed=47.0 ±21.9, fluoxetine/non-stressed=312.2 ±95.1, fluoxetine/stressed=52.0 ±12.0). Thus, in contrast to subchronic fluoxetine treatment, chronic treatment with this drug failed to block stress-induced changes in LDE behavior. In addition, this extended regimen of fluoxetine treatment appeared to produce either an anxiogenic-like effect or, more likely, non-specific sedation-related inhibition of behavior.
Fluoxetine consumption
There was a non-significant trend toward an increase in fluoxetine consumption in stressed mice as compared to non-stressed controls (stressed=19.11 ±1.10 (mean ±SEM) mg/kg/day, non-stressed=16.48 ±0.76; t(14)=1.96, p=.070). Water consumption was not different between groups (stressed=223.9 ±21.4 g/kg/day, non-stressed=203.7 ±11.8).
Experiment 3: Effects of subchronic lithium treatment during stress
Body weight
Both stress and drug led to reduced body weight in this experiment (stress x drug treatment interaction: F1,36=21.98, p<.01). Post hoc tests showed that stress produced significant weight loss relative to non-stressed mice in non-drug, but not lithium-treated mice, due to lithium treatment itself reducing weight relative to non-drug treatment (as we have previously: Fitzgerald et al., 2010) (Table 1).
LDE test
While neither stress nor lithium treatment significantly affected percent time in the light compartment (Figure 3b), there was also a trend for a stress x lithium interaction for number of light compartment entries (F1,36=3.07, p=.088). Although there was only a statistical trend, an effect of stress on this measure was repeatedly observed across experiments, and we therefore proceeded with post hoc analyses and this showed that stress significantly increased light compartment entries in non-drug-treated, but not lithium-treated, mice (Figure 3c). On the other hand, stress decreased latency to enter the light compartment, irrespective of lithium treatment (main effect of stress: F1,36=7.17, p<.05; non-drug/non-stressed=99.8 ±18.5, non-drug/stressed=43.2 ±11.9, fluoxetine/non-stressed=101.5 ±26.2, fluoxetine/stressed=54.7 ±17.9), while stress increased total distance traveled in control-treated mice but decreased distance traveled in lithium-treated mice (stress x lithium interaction F1,36=13.02, p<.01, followed by post hoc tests) (Table 2). These data suggest that lithium treatment partially prevented stress-induced changes in LDE behavior.
Table 2. Effects of stress on total distance traveled in the LDE test.
Neither stress nor drug treatment altered total distance traveled in the haloperidol, methylphenidate or chlordiazepoxide experiments. In the lithium experiment, stressed mice had showed more distance traveled than non-stressed mice under non-drug conditions, and less distance traveled than non-stressed mice under drug-treatment. n/a= data not available because videotracking not performed. Data are means ± SEM.
| Non-drug | Drug | |||
|---|---|---|---|---|
| Non-stressed | Stressed | Non-stressed | Stressed | |
| Total distance traveled (m) | ||||
| EXPT 1: Subchronic fluoxetine | n/a | n/a | n/a | n/a |
| EXPT 2: Chronic fluoxetine | n/a | n/a | n/a | n/a |
| EXPT 3: Subchronic lithium | 16.96 ±0.87 | 20.90 ±0.56* | 20.09 ±1.19 | 17.48 ±0.90* |
| EXPT 4: Acute haloperidol | 16.45 ±1.16 | 19.46 ±0.94 | 18.18 ±0.83 | 18.76 ±0.83 |
| EXPT 5: Acute methylphenidate | 21.02 ±1.10 | 22.18 ±0.51 | 23.10 ±1.78 | 25.30 ±1.56 |
| EXPT 6: Acute chlordiazepoxide | 20.94 ±0.67 | 22.33 ±0.92 | 23.73 ±1.46 | 24.95 ±2.59 |
p<.05 stressed vs. non-stressed
FST
Lithium treatment significantly decreased percent immobility, irrespective of stress (main effect of drug: F1,36=36.26, p<.01), consistent with an antidepressant-like effect. As in the chronic fluoxetine experiment, stress itself was without effect (Figure 3d).
Experiment 4: Effects of acute haloperidol treatment after stress
Body weight
Stress significantly reduced body weight relative to non-stressed controls (main effect of stress: F1,40=57.18, p<.01) (Table 1).
LDE test
Stress increased percent time in the light compartment (main effect of stress: F1,40=11.98, p<.01) (Figure 4a) and light compartment entries (main effect of stress: F1,40=4.24, p<.05) (Figure 4b), and decreased latency to enter the light compartment (main effect of stress: F1,40=6.55, p<.05) (non-drug/non-stressed=118.4 ±28.4, non-drug/stressed=48.1 ±11.3, fluoxetine/non-stressed=89.7 ±28.3, fluoxetine/stressed=48.5 ±12.7) but not total distance traveled (Table 2), relative to non-stressed controls, irrespective of haloperidol treatment. Haloperidol did not modify these stress effects and, per se, had no effects on any measure of behavior.
Figure 4. Effects of acute haloperidol, methylphenidate or chlordiazepoxide treatment after stress.
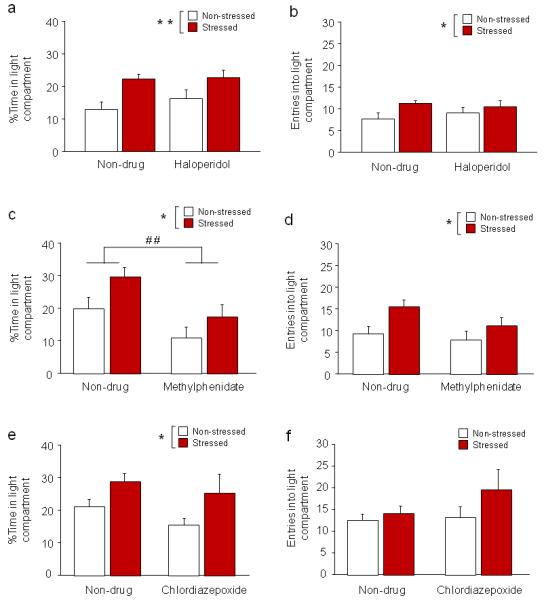
(a) Stressed mice spent more time inside the light compartment than non-stressed mice, regardless of haloperidol treatment (n=11/treatment/stress condition). (b) Irrespective of haloperidol treatment, stressed mice made more entries into the light compartment than non-stressed mice. (c) Stressed mice spent more time inside the light compartment than non-stressed mice, regardless of methylphenidate treatment. Methylphenidate treatment decreased time inside the light compartment, irrespective of stress (n=7-8/treatment/stress condition). (d) Stressed mice made more entries into the light compartment than non-stressed mice, regardless of methylphenidate treatment. (e) Stressed mice spent more time inside the light compartment than non-stressed mice, regardless of chlordiazepoxide treatment (n=8/treatment/stress condition). (f) Neither stress nor drug treatment significantly affected the number of entries into the light compartment. Data are means ±SEM. **p<.01, *p<.05 stressed vs. non-stressed, # #p<.01 non-drug vs. methylphenidate
Experiment 5: Effects of acute methylphenidate treatment after stress
Body weight
Stress significantly reduced body weight relative to non-stressed controls (main effect of stress: F1,26=9.22, p<.01) (Table 1).
LDE test
Methylphenidate treatment failed to reverse stress-induced changes in the LDE test. Stress increased percent time inside the light compartment (main effect of stress: F1,26=5.60, p<.05) (Figure 4c) and increased light compartment entries (main effect of stress: F1,26=6.76, p<.05) (Figure 4d), irrespective of methylphenidate treatment. Stress also produced a non-significant trend for decreased latency to enter the light compartment (main effect of stress: F1,26=3.53, p=.072; non-drug/non-stressed=111.0 ±39.7, non-drug/stressed=45.1 ±15.1, fluoxetine/non-stressed=200.8 ±83.8, fluoxetine/stressed=77.3 ±20.6) but did not affect total distance traveled (Table 2), irrespective of drug treatment. Methylphenidate also reduced percent light compartment time (but not entries) relative to non-drug-treated controls (main effect of drug: F1,26=9.54, p<.01) (Figure 4c). These data show that this drug was unable to block the effects of stress on LDE behavior, but may have produced an anxiogenic-like effect per se.
Experiment 6: Effects of acute chlordiazepoxide treatment after stress
Body weight
Stress significantly reduced body weight relative to non-stressed controls (main effect of stress: F1,28=7.15, p<.05) (Table 1).
LDE test
The ability of stress to increase percent light compartment time during the LDE test, relative to non-stressed controls, was intact regardless of chlordiazepoxide treatment (main effect of stress: F1,28=6.22, p<.05) (Figure 4e). Stress did not significantly increase light compartment entries (Figure 4f) or decrease latency to enter the light compartment (non-drug/non-stressed=66.9 ±15.1, non-drug/stressed=44.4 ±7.7, fluoxetine/non-stressed=33.9 ±9.8, fluoxetine/stressed=138.1 ±109.5) or total distance traveled (Table 2) in this experiment. Chlordiazepoxide per se was without effects.
Validation experiments
The absence of anxiolytic-like effects of chlordiazepoxide contrasts with some earlier studies showing the drug’s anxiolytic-like effects in the LDE in outbred mouse strains (Holmes et al., 2001), but is consistent with other reports testing the C57BL/6J strain in the LDE and other anxiety-related tests (e.g., Mathiasen et al., 2008). In order to allow us to better interpret the failure of chlordiazepoxide to prevent stress-induced changes in LDE behavior, we conducted an additional set of experiments examining the effects of this drug in stress-naïve C57BL/6J mice in the LDE test, as well as two other common tests for anxiety-like behavior: the elevated plus-maze and novel open field test (Cryan and Holmes, 2005). Mice were injected with 10 mg/kg chlordiazepoxide or saline vehicle 30 min before testing in either 1) another version of the LDE, in which the light:dark compartment scaling is 50:50 and mice begin the test in the light compartment (apparatus and procedure as previously described, Karlsson et al., 2008a), 2) the elevated plus-maze test (apparatus and procedure as previously described, Mozhui et al., 2010), or 3) a novel open field test (apparatus and procedure as above). The results of these experiments are summarized (in Table S1) and showed that, with the exception of a significant reduction in percent open field center time relative to vehicle (t=2.20, df=14, p<.05), chlordiazepoxide had no significant effects on any behavioral measures in these 3 test assays. This supports our negative findings in the LDE stress experiment as being a true negative.
Discussion
The main finding of the current study is that subchronic treatment with fluoxetine prevented stress-induced changes in LDE test behavior in male C57BL/6J mice. A similar subchronic treatment regimen with the mood stabilizer, lithium, had similar, although somewhat less clear-cut, effects. By contrast, acute pre-test treatment with the antipsychotic haloperidol, the anti-ADHD drug methylphenidate, or the benzodiazepine anxiolytic chlordiazepoxide, failed to reverse the behavioral effects of stress.
The principal behavioral readout of the ten-day restraint stress procedure we employed was a significant increase in exploration (time spent in and/or entries) of the aversive light compartment of the LDE test. This profile was consistently seen across six separate experiments in the current study and replicates one of the main findings of Mozhui et al. (Mozhui et al., 2010). Mozhui et al. also reported that this stress regimen increased time spent in the aversive open arms of the elevated plus-maze (EPM) in C57BL/6J mice, but did not affect immobility behavior in the FST (as also replicated here). Collectively, this pattern of data suggests that restraint stress produced behavioral alterations that generalize across anxiety-related but not ‘depression-related’ tests. A dissociation between anxiety- and depression-related measures is not unexpected considering examples in the literature where other repeated stressors, such as social defeat, produce assay- or domain-specific behavioral effects in C57BL/6J mice and other strains (e.g., Krishnan et al., 2007; Strekalova et al., 2004).
It can sometimes be difficult to parse a change in anxiety-related behavior from a change in locomotor activity in exploration-based assays for anxiety-like behavior, including the LDE test because more or less exploration of the (larger) light compartment typically drives up the overall amount of distance traveled in tandem (Holmes, 2001). However, a number of observations argue that the stress-induced profile of C57BL/6J mice is unlikely to be a generalized hyperactivity effect. First, stress failed to alter measures of locomotor activity in the open field (this study) or the elevated plus-maze (Mozhui et al., 2010). Second, a generalized hyperactivity effect of stress would have been expected to decrease forced swim test immobility, but this was not found either in the current study or in Mozhui et al. (Mozhui et al., 2010). Third, of the four experiments where total distance traveled in the LDE could be measured via videotracking, there an effect of stress on this measure only in one experiment (lithium). Given the increase in distance traveled in the lithium experiment, and despite the arguments to the contrary above, the possibility that stress-induced increase in light compartment exploration in this experiment was confounded by hyperactivity cannot be discounted.
Another interpretation of the current results is that the stress-induced increase in the aversive light compartment of the LDE test reflect a decrease in anxiety-like behavior. This would be logical given a similar profile is produced by anxiolytic drugs in non-stressed subjects (Crawley, 1981; Cryan and Holmes, 2005), but paradoxical in the sense that stress is a risk factor for anxiety. However, the stress-induced profile of C57BL/6J mice cannot simply be explained by stress-resilience in this strain because stressed mice reliably showed a significant loss of body weight - a measure typically used to confirm the efficacy of restraint stress in rats and mice (Surget et al., 2009; Willner, 1997). Furthermore, stressed C57BL/6J mice showed significantly elevated serum corticosterone response to a novel (swim) stressor, demonstrating that the HPA-axis had been sensitized by restraint. Similar HPA-axis sensitization is seen with other repeated stressors in C57BL/6J mice (e.g., Krishnan et al., 2007). Thus, increased LDE light compartment exploration occurred in the context of peripheral and neuroendocrine alterations classically associated with increased stress. We therefore suggest that C57BL/6J mice respond to restraint stress with an ‘active coping strategy’ that contrasts with inhibited behavioral responses exhibited by other strains such as DBA/2J and BALB/cJ (Mozhui et al., 2010; Uchida et al., 2011). Clearly, further studies will be needed to elucidate the precise nature of this ‘paradoxical’ response to stress in the C57BL/6J strain.
The major finding of the current study, which was that stress-induced LDE behavior and corticosterone sensitization (although not body weight loss) was ‘rescued’ by subchronic treatment with the clinically efficacious anxiolytic and antidepressant fluoxetine, may also be in line with an increase, rather than resilience, to stress. Subchronic treatment with the prototypical mood stabilizer lithium chloride also partially reversed stress-induced alterations in the LDE test, although this effect was less robust than that produced fluoxetine. Because both drugs were administered during stress exposure as well as LDE testing, it is unclear whether they prophylactically prevented a stress effect from developing, or blocked the expression of the behavioral effect. It is difficult to design an experiment to parse these possibilities because pre-test subchronic administration of fluoxetine is needed to mimic their efficacy in the clinic, and it is unclear whether the effect of stress in the LDE would be robustly manifest if a two-week treatment interval was interposed between stress and testing. Notwithstanding, the main conclusion from these data is stress-induced LDE behavioral is reversible by these clinically relevant drugs.
A stress-preventing effect of fluoxetine on LDE behavior in C57BL/6J extends similar findings examining other monoaminergic antidepressants, mouse strains and stress paradigms. For example, imipramine treatment five weeks into a nine-week chronic unpredictable mild stress (UCMS) regimen prevented anxiety-related and HPA-axis alterations in a number of strains including BALB/c and C57BL/6 (Ibarguen-Vargas et al., 2008). More recently, Isingrini and colleagues reported that BALB/c mice exposed to UCMS for seven weeks showed reduced grooming, coat degradation and anhedonia that was blocked by fluoxetine treatment from the second week onward (Isingrini et al., 2010) (see also Yalcin et al., 2008). Similarly, fluoxetine concomitantly administered with low dose corticosterone over four weeks blocked corticosterone-induced coat degradation and anxiety-like behavior in C57BL/6NTac mice (David et al., 2009). Taken together with the current findings, these data demonstrate that fluoxetine is effective in preventing effects of various chronic stressors.
In contrast to the effectiveness of fluoxetine and lithium, acute treatment with the antipsychotic haloperidol, the anti-ADHD medication methylphenidate, and the benzodiazepine chlordiazepoxide all failed to normalize stress-induced LDE behavior. Methylphenidate reduced light compartment time regardless of stress, while haloperidol and chlordiazepoxide were inactive in both stressed and non-stressed mice. The haloperidol dose used is known to be effective in other behavioral settings (Karlsson et al., 2008b). The null chlordiazepoxide profile was also likely a true negative, as we showed in a series of additional experiments that, in non-stressed C57BL/6J mice, chlordiazepoxide was without effect in the EPM, novel open field and another variant of the LDE test. Moreover, a previous study also found no anxiolytic-like effects in these tests in C57BL/6JOIaHsd mice (Rodgers et al., 2002). Thus, our negative data with these three drugs indicate that the stress-rescuing effects of fluoxetine (and to a lesser extent, lithium) are not reproduced by an antipsychotic, anti-ADHD or benzodiazepine anxiolytic medication. An important caveat in making this comparison across drugs is that fluoxetine and lithium were administered chronically during stress and LDE testing, whereas haloperidol, methylphenidate and chlordiazepoxide were given acutely prior to testing. Thus, whether chronic treatment with these drugs would rescue the effect of stress remains to be tested.
Another issue to be resolved stems from our observation that chronic fluoxetine treatment produced a general decrease in LDE behavior and increased FST immobility in non-stressed mice, and to a lesser degree, stressed mice. This was unexpected given similar regimens exert therapeutic efficacy in human patients, and prior studies demonstrate that from three injections to four weeks of treatment produces anxiolytic-like and antidepressant-related effects (Dulawa et al., 2004; Holick et al., 2008; Isingrini et al., 2010; Karlsson et al., 2008a; Mombereau et al., 2010; Oh et al., 2009; Richardson-Jones et al., 2010). The apparent inconsistency between these studies and our current data may be explained by genetic background. Chronic treatments typically produce anxiolytic-like and antidepressant-related effects in BALB/cJ or ′129′ strains, or mixed genetic backgrounds, whereas, similar to our finding, C57BL/6J are often either unresponsive or found to exhibit non-specific sedation (Balu et al., 2009; David et al., 2009; Dulawa et al., 2004) (but see Chen et al., 2006; Oh et al., 2009). These data suggest an atypical behavioral response to prolonged fluoxetine treatment in the C57BL/6J strain, and confound interpretation of stress-related effects of this treatment in the current study.
In summary, the current study found that repeated restraint stress produces behavioral and HPA-axis alterations that are prevented by subchronic fluoxetine treatment, and to some extent, subchronic lithium. Acute treatment with haloperidol methylphenidate or chlordiazepoxide prior to LDE testing failed to block the effect of stress. Together, these experiments demonstrate treatment-selective rescue of stress-induced behavior in C57BL/6J mice. The precise nature of this stress-induced phenotype and its potential relevance to specific human disease classes remains to be determined, but could prove useful for studying the genetic and neural mechanisms underlying individual differences in responses to stress. In this context, we and others have shown that various stressors have convergent effects on corticolimbic glutamatergic neurotransmission and expression of glutamate-related plasticity genes (Krishnan et al., 2007; Mombereau et al., 2010; Mozhui et al., 2010; Surget et al., 2009). Future work will seek to extend these findings by elucidating the role of the glutamate system in the current stress model.
Supplementary Material
*Research Highlights.
Psychological stress is a major risk factor for mood and anxiety disorders.
Chronic stress increases C57BL/6J shelter exiting in light/dark exploration test.
Subchronic fluoxetine and lithium “rescues” this anxiety-related behavior.
Chronic fluoxetine and acute haloperidol, methylphenidate, chlordiazepoxide do not.
These results indicate selective pharmacological modulation of this stress effect.
Acknowledgements
We are very grateful to Carolyn Graybeal and Heather Cameron for performing the corticosterone analyses, and to Etienne Sibille and Cara Wellman for valuable discussions.
Research was supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest
None.
References
- Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci. 2001;115:443–454. [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2011;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Boyce-Rustay JM, Millstein R, Yang R, Wiedholz LM, Murphy DL, Holmes A. Effects of Mild Early Life Stress on Abnormal Emotion-related Behaviors in 5-HTT Knockout Mice. Behav Genet. 2007;37:214–222. doi: 10.1007/s10519-006-9129-9. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM, Machado-Vieira R, Graybeal C, Sharp T, Zarate C, Harvey-White J, Du J, Sprengel R, Gass P, Bannerman D, Holmes A. Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis. 2010;40:608–621. doi: 10.1016/j.nbd.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Holmes A. Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev. 2001;25:261–273. doi: 10.1016/s0149-7634(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ, Rodgers RJ. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res. 2001;122:159–167. doi: 10.1016/s0166-4328(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol Behav. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarguen-Vargas Y, Surget A, Touma C, Palme R, Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33:1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S, Belzung C. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS ONE. 2010;5:e10404. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, Heilig M. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2008a;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008b;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Mathiasen LS, Mirza NR, Rodgers RJ. Strain- and model-dependent effects of chlordiazepoxide, L-838,417 and zolpidem on anxiety-like behaviours in laboratory mice. Pharmacol Biochem Behav. 2008;90:19–36. doi: 10.1016/j.pbb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Gur TL, Onksen J, Blendy JA. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int J Neuropsychopharmacol. 2010;13:321–334. doi: 10.1017/S1461145709990630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA, Harvey-White J, Holmes A. Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008;200:413–424. doi: 10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JE, Zupan B, Gross S, Toth M. Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology. 2009;34:2197–2207. doi: 10.1038/npp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Davies B, Shore R. Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav. 2002;1:242–251. doi: 10.1034/j.1601-183x.2002.10406.x. [DOI] [PubMed] [Google Scholar]
- Rutter M. Biological implications of gene-environment interaction. J Abnorm Child Psychol. 2008;36:969–975. doi: 10.1007/s10802-008-9256-2. [DOI] [PubMed] [Google Scholar]
- Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, Rogawski M, Gasior M, Luckenbaugh D, Chen G, Manji HK. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology. 2004;29:2007–2017. doi: 10.1038/sj.npp.1300532. [DOI] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, Belzung C, Sibille E. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R, Celikel T, Daws LC, Holmes A. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Belzung C, Surget A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res. 2008;193:140–143. doi: 10.1016/j.bbr.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


