Abstract
Background
Previously, cytotoxic T lymphocyte antigen 4 (CTLA4)-Ig has been shown to allow sustained engraftment in dog leukocyte antigen (DLA)-identical hematopoietic cell transplant (HCT) after nonmyeloablative conditioning with 100 cGy total body irradiation (TBI). In the current study, we investigated the efficacy of pre-transplant CTLA4-Ig in promoting engraftment across a DLA-mismatched barrier after nonmyeloablative conditioning.
Methods
Eight dogs were treated with CTLA4-Ig and donor peripheral blood mononuclear cells (PBMC) prior to receiving 200 cGy TBI followed by transplantation of granulocyte colony stimulating factor mobilized peripheral blood stem cells from DLA-haploidentical littermates with post-grafting immunosuppression. A control group of 6 dogs was conditioned with 200 cGy only and transplanted with grafts from DLA-haploidentical littermates followed by post-grafting immunosuppression.
Results
In vitro and in vivo donor-specific hyporesponsiveness was demonstrated on day 0 before TBI in 8 dogs that received CTLA4-Ig combined with donor PBMC infusions. Four of 5 dogs treated with increased doses of CTLA4-Ig achieved initial engraftment but eventually rejected with duration of mixed chimerism ranging from 12 to 22 weeks. CTLA4-Ig did not show any effect on host natural killer (NK) cell function in vitro or in vivo. No graft-versus-host disease (GVHD) was observed in dogs receiving CTLA4-Ig treatment.
Discussion
Nonmyeloablative conditioning with 200 cGy TBI and CTLA4-Ig combined with donor PBMC infusion was able to overcome the T-cell barrier to achieve initial engraftment without GVHD in dogs receiving DLA-haploidentical grafts. However, rejection eventually occurred, which we hypothesize could be due to the inability of CTLA4-Ig to abate NK-cell function.
Keywords: Animal model, CTLA4 Ig, haploidentical transplantation, nonmyeloablative transplantation
INTRODUCTION
The clonal expansion and activation of naïve T-cells is not only dependent on antigen-specific binding of the T-cell receptor (TCR) to peptide: major histocompatibility complex (MHC) and ligation of either the CD4 or CD8 co-receptors, but also requires a second co-stimulatory signal. One of the best characterized co-stimulatory signals is through the CD28:B7 pathway [1]. CD28, a glycoprotein found on most mature T-cells, serves as a costimulation receptor via B7 (CD80 and CD86) on antigen-presenting cells [2–4]. Without CD28 costimulation, T-cells fail to up-regulate interleukin-2 expression and progress through the cell cycle [4,5]. The interaction between CD28 and B7 molecules can be blocked with antibodies or with cytotoxic T lymphocyte antigen 4 (CTLA4)-Ig, a fusion protein of CTLA4 and human immunoglobulin that competitively binds CD80 and CD86 [6–8].
In vitro studies of T-cell activation through the CD3/TCR complex have shown that absence of co-stimulatory signals abates T-cell proliferation and induces functional unresponsiveness [2]. Consistent with in vitro findings, in vivo studies have shown that CTLA4-Ig can induce prolonged, antigen-specific T-cell hyporesponsiveness promoting graft acceptance and prevention of graft-versus-host disease (GVHD) in mouse, rat, and primate studies [9–18]. In the canine hematopoietic cell transplantation (HCT) model, it has not previously been possible to establish sustained engraftment with 100 cGy alone [19]. However, with the addition of CTLA4-Ig treatment to 100 cGy total body irradiation (TBI), stable mixed chimerism was observed in dog leukocyte antigen (DLA)-identical HCT [20]. In this study, the recipients’ T-cells were activated by intravenous injection of donor antigen in the form of donor peripheral blood mononuclear cells (PBMC) before administration of 100 cGy TBI followed by mycophenolate mofetil (MMF) plus cyclosporine (CSP) immunosuppression. Thus, this study suggested that the 100 cGy engraftment barrier in MHC-matched HCT could be abrogated if recipients’ T-cells were initially activated by donor antigens prior to CTLA4-Ig blockade. In MHC-haploidentical HCT, there are greater histocompatibility barriers that must be overcome. Previously, we have demonstrated that nonmyeloablative conditioning with 200 cGy TBI and anti-CD44 mAb (S5) was sufficient to allow initial uniform engraftment across DLA haplotype-mismatched barriers, with approximately half of the dogs achieving long-term engraftment [21]. Although the in vivo mechanism by which S5 facilitated engraftment still remains unclear [22,23], we previously demonstrated that S5 treatment, both in vitro and in vivo, led to activation of natural killer (NK) cells, hereby possibly making the cells more sensitive to irradiation [24–26]. Currently, it is unknown whether CTLA4-Ig therapy is sufficient to overcome both T-cell and NK cell barriers to achieve sustained engraftment in an MHC-mismatched setting. Here, we present the results of our study, which suggest that donor specific T-cell immune tolerance could be induced sufficiently by pre-transplant CTLA4-Ig treatment and donor PBMC infusions to achieve initial short term engraftment without GVHD, in dogs receiving DLA-haploidentical HCT after conditioning with 200 cGy TBI and MMF/CSP based post-grafting immunosuppression.
MATERIALS AND METHODS
Dogs and DLA typing
Litters of beagles, beagle-crossbreeds, and other mixed breeds were either raised at the Fred Hutchinson Cancer Research Center (Seattle, WA) or purchased from commercial kennels. They were observed for disease for at least 1 month before study. All were immunized for papillomavirus, leptospirosis, distemper, hepatitis, and parvovirus. The dogs were a median of 15 (range, 8 to 21) months old and weighed a median of 10.8 (range, 8.1 to 14.2) kg. Dogs G053 (recipient), G052 (haploidentical donor) and E816 (unrelated dog) which were in our previous study using S5 and 200 cGy TBI as conditioning for DLA-haploidentical HCT [21] were used for in vitro assays of CTLA4-Ig and S5 on T-cell and NK cell functions. The data presented here were not published in the previous study [21]. The research protocols were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. Research was conducted according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Research Council. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care, International.
DLA-haploidentical littermates were selected based on family studies showing haplo-disparty for highly polymorphic MHC class I and class II microsatellite makers [27–29] and for DLA-DRB1 alleles determined by direct sequencing [29]. Histocompatibility studies routinely include sire and dam of each respective litter. There was no coincidental sharing of DLA antigens on the mismatched haplotypes among the recipient/donor pairs. Additionally, mutual lymphocyte reactivity was found in mixed leukocyte cultures (MLC) [30]. Unrelated pairs were from different breeding colonies or different pedigrees for at least five generations.
CTLA4-Ig and plasma concentration
Recombinant human CTLA4 linked to human IgG1 heavy chain (RG2077) was kindly provided by Richard Boismenu, Ph.D., Repligen Corporation, Needham, MA. Blood samples were drawn daily before and after CTLA4-Ig injection (within 30min) and sent to Repligen Corporation for plasma CTLA4-Ig concentration detection.
Hematopoietic stem cell transplantation
We designed three schemes of CTLA4-Ig and donor PBMC infusions (shown in Table 1). Briefly, Group 1’s design was based on the previous DLA-identical transplant study [20]. In Group 2, the dose of CTLA4-Ig was increased and both CTLA4-Ig and PBMC infusions were moved earlier before HCT. In Group 3, two injections of CTLA4-Ig were added on day 0 and day +1 aiming to reach a sustained immunosuppressive effect post-transplant. For collection of the non-mobilized PBMC given prior to HCT, DLA-haploidentical littermate donors were leukapheresed and 200 mL of blood was collected. PBMC were frozen and kept in liquid nitrogen 3 weeks before HCT or earlier. Donor PBMC (1×107/kg/day i.v.) were given on days -7 and -3 in Group 1 and on days -9 and -4 in Groups 2 and 3. On day 0, all dogs were conditioned with 200 cGy TBI delivered at 7 cGy/min from two opposing 60Co sources or a linear accelerator (Varian CLINAC 4, Palo Alto, CA) [31,32] followed by donor peripheral blood stem cell (PBSC infusion). The procedures of PBSC collection are described in our previous publications [32–34]. Briefly, dogs were given recombinant canine granulocyte colony-stimulating factor (G-CSF) (kindly provided by Amgen, Thousand Oaks, CA, USA) subcutaneously at a dose of 5 μg/kg twice a day from day -5 to day 0. PBSC were collected from the donor through percutaneous dual lumen catheters using the COBE SPECTRA Version 6 (COBE BCT, Lakewood, CO, USA) on day 0. Proportions of PBMC and CD34+ cells were determined by May-Giemsa stain and flow cytometry using mAb directed toward canine CD34, respectively. G-CSF mobilized PBSC were infused intravenously in the recipient within 4 hours after 200 cGy TBI.
Table 1.
Results of HCT from DLA-haploidentical donor after CTLA4-Ig/donor PBMC with 200 cGy TBI
| Group | Recipient Dog ID# | Cells Subsets of PBSC Grafts
|
Rejection | Maximum Chimerism Achieved
|
Final Donor Chimerism in MNC (%) | Duration of Mixed Chimerism (weeks) | GVHD | Survival Weeks | Cause of Death | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD34+ (×106/kg) | CD3+ (×107/kg) | CD4+ (×107/kg) | CD8+ (×107/kg) | % in MNC | Weeks | ||||||||
| 1a | E923 | 1.9 | 9.8 | 7.1 | 0.5 | Yes | 0 | / | 0 | 0 | None | 8 | ET2-Rejection |
| E879 | 2.3 | 3.7 | 8.4 | 0.7 | Yes | 0 | / | 0 | 0 | None | 6 | ET2-Rejection | |
| E871 | 10.7 | 24.9 | 22.7 | 5.9 | NE | 78 | 4 | 47 | >5 | None | 7 | ET2 | |
| 2b | E934 | 1.8 | 5.8 | 3.6 | 0.3 | Yes | 43 | 3 | 0 | 15 | None | 19 | ET2-Rejection |
| E932 | 15.1 | 27.6 | 26.2 | 1.0 | Yes | 79 | 4 | 0 | 12 | None | 23 | ET2-Rejection | |
| G024 | 4.4 | 17.3 | 10.7 | 2.3 | Yes | 0 | / | 0 | 0 | None | 6 | ET2-Rejection | |
| 3c | G096 | 2.7 | 36.2 | 24.7 | 0.9 | Yes | 96 | 4 | 0 | 22 | None | 27 | ET2-Rejection |
| G067 | 1.2 | 10.0 | 6.5 | 1.1 | Yes | 47 | 5 | 0 | 12 | None | 13 | ET2-Rejection | |
| 4d | G891 | 1.2 | 32.3 | 2.1 | 6.1 | No | 77 | 12 | 77 | 12 | None | 12 | ET1-PTLD |
| G888 | 0.7 | 40.8 | 27.6 | 4.4 | Yes | 43 | 5 | 0 | 17 | None | 18 | ET2-Rejection | |
| G889 | 1.7 | 43.7 | 27.2 | 5.7 | No | 100 | 19 | 100 | 34 | Yes | 34 | ET1-GVHD | |
| H122 | 3.3 | 18.4 | 10.5 | 3.9 | Yes | 6 | 4 | 0 | 5 | None | 5 | ET1-Intussusception | |
| H141 | 12.0 | 44.4 | 32.6 | 12.9 | Yes | 46 | 7 | 0 | 17 | None | 18 | ET2-Rejection | |
| H211 | 23.0 | 25.0 | 198.0 | 5.4 | Yes | 57 | 3 | 0 | 13 | None | 13 | ET2-Rejection | |
CTLA4-Ig 4 mg/kg i.v. days -7 to -1; Donor PBMC 1×107/kg i.v. days -7 and -3; 200 cGy TBI on day 0 followed by donor PBSC infusion; CSP 30 mg/kg per day from days -1 to day +100; MMF 20 mg/kg per day from day 0 to +40, 10 mg/kg per day from day+41 to +100.
CTLA4-Ig 5 mg/kg i.v. days -10, -8 to -5; 10 mg/kg on days -9 and -4; Donor PBMC 1×107/kg i.v. on days -10 and -5; 200 cGy TBI on day 0 followed by donor PBSC infusion; and same regimen of CSP and MMF as Group 1.
CTLA4-Ig 5 mg/kg i.v. days -10, -8 to -5; 10 mg/kg on days -9, -4, 0 and +1; Donor PBMC 1×107/kg i.v. on days -10 and -5; 200 cGy TBI on day 0 followed by donor PBSC infusion; and same regimen of CSP and MMF as Group 1.
200 cGy TBI on day 0 followed by donor PBSC infusion; CSP 30 mg/kg per day from day -3 to day +180, 15 mg/kg per day from day +181 to day +190, 7.5 mg/kg per day from day +191 to day +200, 3 mg/kg per day from day +200 to day +210; MMF 20 mg/kg per day from day 0 to day+100, 10 mg/kg per day from day +101 to day +130, 5 mg/kg per day from day +131 to day +160, 2.5 mg/kg per day from day +161 to day +180.
Abbreviations: CSP, cyclosporine; DLA, dog leukocyte antigen; ET1, euthanized due to poor conditions; ET2, euthanized in good condition due to the end of study; GVHD, graft-versus-host disease; HCT, hematopoietic cells transplantation; ID#, identifying number; MMF, mycophenolate mofetil; MNC, mononuclear cells; PBMC, peripheral blood mononuclear cells; PBSC, peripheral blood stem cells; PTLD, post-transplant lymphoproliferative disease; NE, not evaluable; TBI, total body irradiation.
Recipient dogs treated with CTLA4-Ig received the same post-grafting immunosuppression which consisted of MMF at 20 mg/kg/day from day 0 through +40, 10 mg/kg/day from day +41 through +100; and CSP 30 mg/kg/day from days -1 through +100. Control dogs in Group 4 were condition with TBI 200 cGy only, and the immunosuppressive regimen was MMF 20 mg/kg/day, day 0 through +100, then tapered and discontinued at day +180, and CSP 30 mg/kg/day, from day -3 through +180, then tapered and discontinued at day +210.
Standard supportive care was given to recipient dogs as described in previous studies [35,36]. Recipient dogs were evaluated for hematologic recovery, GVHD, and infections as described in previous studies [22].
Monoclonal antibodies
Monoclonal antibodies (mAbs) recognizing canine CD3 (CA17.6B3, IgG2b kindly provided by Dr. Peter Moore, School of Veterinary Medicine, University of California, Davis), CD4 (CA13.1.E4, IgG1), CD8 (CA9.JD3, IgG2a) [37], TCRαβ (CA15.9D5, IgG1) [38] and myeloid cells (DM5, IgG1) [39] were used for flow cytometry to assay the cell subsets of PBSC grafts. As isotype control, mAb 31A (IgG1), directed at the mouse Thy-1 receptor (does not cross-react with canine cells) was used [40]. Anti-CD44 mAb S5 (subclass IgG1) [22] was used in MLC and natural killer (NK)-cell cytotoxicity assays. All mAbs were produced and purified at the Biologics Production Facilities of the Fred Hutchinson Cancer Research Center (Seattle, WA). The mAbs were fluorescein conjugated according to standard protocols. In addition, the commercially available anti-human CD14 mAb (DAKO Corporation, Carpenteria, CA), which cross-reacts with canine CD14, was used [41].
Chimerism analysis
Genomic DNA of the cells of interest was extracted. Genetic marker studies using microsatellite polymorphisms were done to document donor and host (CA)n dinucleotide repeats in PBMC [42]. Percent donor chimerism was analyzed with image analyzing software (Image Quant, Molecular Dynamics, Sunnyvale, CA, USA). The densities of the donor-specific and host-specific bands were added as total events (T=D+H). The percentage of donor origin DNA was calculated as donor-specific density / (donor-specific density+host-specific density) ×100. This technique allowed us to detect between 2.5% to 97.5% donor cell chimerism. Graft rejection was defined as mononuclear cell chimerism (MNC) of < 5%.
Mixed lymphocyte culture (MLC)
MLC [30] were performed as described previously. Briefly, PBMC were resuspended in Waymouth medium supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 1% L-glutamine and 20% heat-inactivated, pooled normal dog serum. Responder cells (1×105/well) and irradiated (2200 cGy) stimulator cells (1×105/well) were cocultured in triplicate in 96-well plates for 6 days at 37°C in a humidified 5% carbon dioxide air atmosphere. In triplicates used as positive controls, 4 μg concanavalin A (ConA)_was added to responder cells on day 3. On day 6, cultures were pulsed with 1μCi (37 kBq) tritium-thymidine for 18 hours before harvesting. Tritium-thymidine uptake was measured as mean counts per minute (CPM) for three replicates by using a β-scintillation counter (Packard BioScience Company, Meriden, CT). CTLA4-Ig MLC inhibition experiments were performed in triplicates on pre-treatment blood samples for each recipient (responder cells)/donor (stimulator cells) pairs in Group 1. MLC with or without CTLA4-Ig (0–10 μg/ml) added on the first day of culture were cultured on the same 96-well plate. Inhibition of the MLC by CTLA4-Ig was calculated as [1 − (CPM in the presence of CTLA4-Ig / CPM in the abscence of CTLA4-Ig)] × 100%.
NK cell cytotoxicity assay
To evaluate NK cell activity before and after CTLA4-Ig treatment, chromium release assays were performed as described previously [8]. Effector cells were PBMC prepared by Ficoll-Hypaque density-gradient centrifugation (density, 1.074) and targets were cells from a canine thyroid adenocarcinoma (CTAC) cell line. Effector-to-target ratios of 60:1, 30:1, and 15:1 in triplicate wells were used. The percentage of cytotoxicity (percentage of specific lysis) was calculated by using the mean value of triplicate cultures: % specific lysis=[(experimental release-spontaneous release)/(maximum release-spontaneous release)]×100. Spontaneous release was determined in wells with target cells and medium alone. Maximum release was determined in wells with target cells and 2% Triton X.
In vitro effects of CTLA4-Ig and S5 alone or in combination on MLC and NK-cell activity
In vitro effects of CTLA4-Ig and S5 alone or in combination in MLC was tested on dogs G053, G052 and E816 which were enrolled in a previous study [21] but in vitro data provided here was not part of the publication. MLC immunoreactivity of G053, G052 and E816 towards autologous cells, DLA haploidentical cells, or cells from an unrelated dog (URD) were tested in the absence or presence of CTLA4-Ig 10 μg/ml and S5 10 μg/ml alone or in combination. Similarly, NK-cell cytotoxicity of G052, G053 and E816 were tested in the presence or absence of CTLA4-Ig 10 μg/ml and S5 10 μg/ml.
Statistical analysis
For each dog in Group 1 and Group 2, the average 7 peaks or 7 troughs of CTLA4-Ig plasma levels during treatment were calculated after log-transformation. The averages in Group 1 were compared to the averages in Group 2 by two-sample t-test. Graft composition in the four groups was compared using by Mann-Whitney U test.
RESULTS
CTLA4-Ig plasma levels
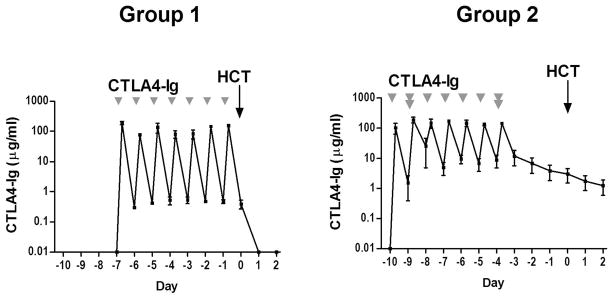
Data are shown in Figure 1. During the period of CTLA4-Ig treatment (day -7 to day -1 in Group 1, and day -10 to day -4 in Group 2), the average peak plasma levels of the three dogs in Group 1 or Group 2 reached a similar level [mean 124.09 (range, 76.03–189.30 ) vs. 148.45 (range, 103.39–190.00 ) μg/mL, respectively, P=0.44]. However, the average daily trough level of CTLA4-Ig was significantly lower in Group 1 compared to Group 2 [mean 0.45 (range, 0.30–0.52) vs. 11.81 (range, 4.61–25.49) μg/mL, respectively, P=0.03]. CTLA4-Ig concentration returned to baseline on day +1 in Group 1, but was maintained above 1μg/mL until day +2 in Group 2.
Figure 1. CTLA4-Ig plasma levels in dogs before and after CTLA4-Ig injections.
Plasma samples were obtained just before CTLA4-Ig injection and within 30 minutes post-injection. The data for each time point are presented as the mean and range of 3 dogs from Group 1 and Group 2. Group 1: 3 dogs treated with 4 mg/kg CTLA4-Ig on day -7 to -1 and 1 × 107cells/kg donor peripheral blood mononuclear cells (PBMC) on days -7 and -3. Group 2: 3 dogs treated with 5 mg/kg on days -10, -8 to -5, 10 mg/kg on days -9 and -4 and 1 × 107cells/kg donor PBMC on days -10 and -5.
Results of hematopoietic stem cell transplantation
The results of the HCT studies are summarized in Table 1. Graft composition was similar across all 4 groups, with no statistical differences (P>0.05, Mann-Whitney U test) in median numbers of infused CD34+ and CD3+ cells (data not shown). Overall 7 out of 8 dogs (87.5%) treated with CTLA4-Ig and donor PBMC infusions rejected their allografts. In Group 1, where dogs were treated with lower doses of CTLA4-Ig (4 mg/kg), 2 dogs rejected (66%) without showing signs of engraftment, while one dog had initial engraftment with maximal donor MNC chimerism of 78% on day +28, and final donor MNC chimerism of 47%, when the dog was euthanized in good condition on day +39. In Group 2 and 3, where dogs received increased CTLA4-Ig doses (5 and 10 mg/kg), 1 dog (20%) did not show initial engraftment, while 4 dogs (80%) achieved initial engraftment, with the maximum donor MNC chimerisms ranging from 43% to 96%. However, all 4 dogs rejected their allografts with subsequent autologous recovery after a median duration of mixed chimerism for 13.5 weeks (range, 12–22). No clinical or histological signs of GVHD were observed in the CTLA4-Ig treated dogs and all were euthanized in good condition. In comparison, dogs in Group 4, which were not treated with CTLA4-Ig, all had initial engraftment, with sustained engraftment in 2 out of 6 (40%). Both dogs with sustained engraftment were euthanized in poor condition due to GVHD and post-transplant lymphoproliferative disease (PTLD). Of the 4 dogs with rejection in Group 4, 3 dogs were euthanized in good condition while 1 was euthanized in poor condition due to intussusception.
Effects of CTLA4-Ig on in vitro and in vivo T-cell function
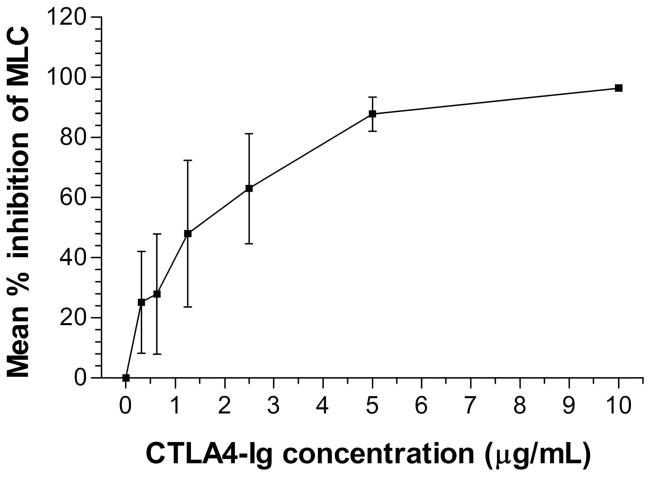
The immunosuppressive effect of CTLA4-Ig in vitro was studied in MLC from Group 1. PBMC from the DLA-haploidentical recipient/donor pairs were co-cultured in the absence or presence of CTLA4-Ig added on the first day of culture. A dose-dependent inhibition of recipient cell proliferation was observed as the concentration of CTLA4-Ig increased from 0 to10 μg/mL. At a CTLA4-Ig concentration of 10 μg/mL the MLC reactivity was inhibited by a mean of 96% (range 95%–98%), while at concentrations below 1 μg/mL the MLC reactivity was inhibited by 18% to 57% (Fig. 2).
Figure 2. In vitro immunosuppressive effects of CTLA4-Ig in MLC.
The MLC data from three pairs in Group 1 at different time points is presented as the mean and range of the percent inhibition of counts per minute (CPM) from triplicate experiments. MLCs were performed on pre-treatment blood samples. Percent inhibition was calculated as [1 − (CPM in the presence of CTLA4-Ig / CPM in the absence of CTLA4-Ig)] × 100%.
Abbreviation: MLC, mixed leukocyte culture.
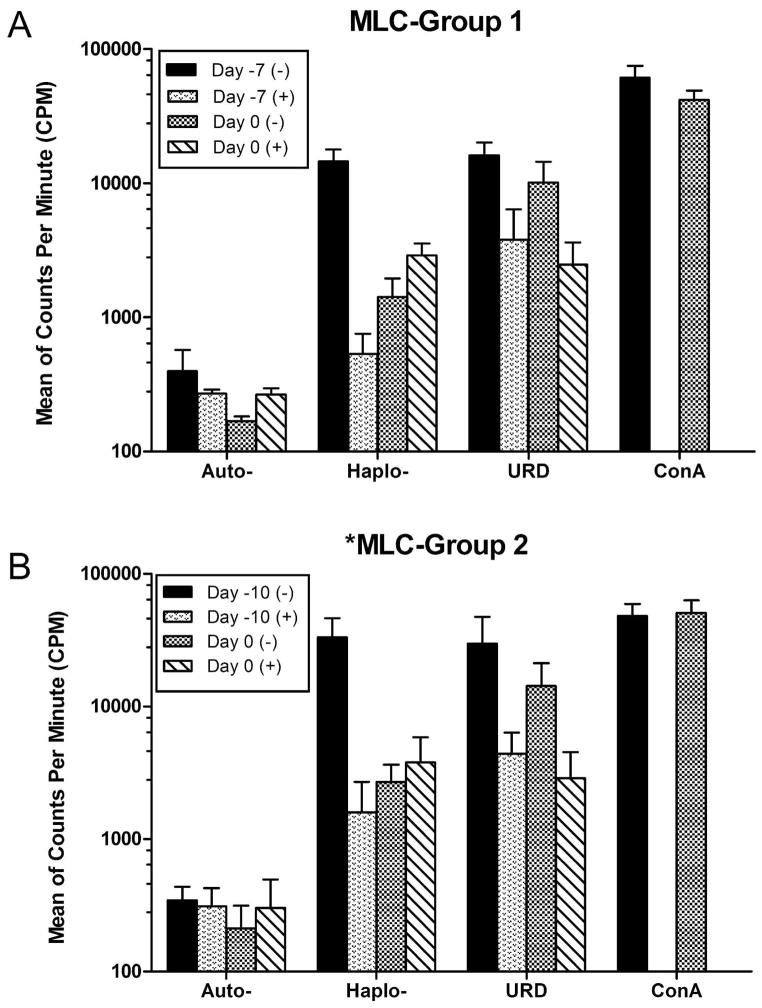
The in vivo immunosuppressive effects of CTLA4-Ig were studied before and after CTLA4-Ig treatment. Figure 3A and 3B shows MLC data from dogs in Group 1 and Group 2 at days -7 and -10 respectively, before CTLA4-Ig treatment and day 0 before TBI, compared to parallel MLC assays where 10 μg/mL CTLA4-Ig was added in vitro to the culture medium. Both in Group 1 and 2, prior to the dogs receiving CTLA4-Ig in vivo, the recipients reactivity against DLA-haploidentical donors and unrelated dogs could be suppressed by the CTLA4-Ig added in vitro (Fig. 3A and 3B). On day 0 after 7 days of in vivo CTLA4-Ig administration and before TBI, the recipient dogs were hyporesponsive to their DLA-haploidentical donors but still responsive to the unrelated dogs. However, unlike what was observed prior to CTLA4-Ig treatment, in vitro addition of CTLA4-Ig did not result in additional effects on hyporesponsiveness of MLC shown on day 0 (Fig. 3A and 3B).
Figure 3. MLC reactivity before CTLA4-Ig treatment and at day 0.
Recipient MLC reactivity from three recipient/donor pairs in Group 1 (A) or Group 2 (B) against autologous (auto), DLA-haploidentical donors (haplo), or unrelated dogs (URD), presented as mean and range of counts per minute (CPM). Day -7 and day -10 represent MLC reactivity prior to treatment of the dogs with CTLA4-Ig in Group 1 and Group 2 respectively, while day 0 represents the reactivity after 7 days of treatment with CTLA4-Ig and prior to TBI. Dogs in both groups are maximally immunosuppressed after receiving a pre-transplant conditioning course containing CTLA4-Ig.
Abbreviations: (+) represents MLC assays where CTLA4-Ig (10μg/ml) has been added to the culture medium at the first day of assay, while (−) represents assays without CTLA4-Ig in the medium. ConA, concanavalin-A; MLC, mixed leukocyte culture.
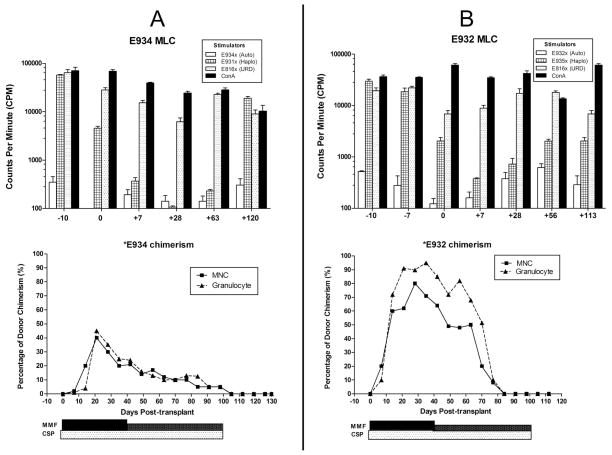
Figure 4 shows MLC data and donor MNC chimerism from dogs E934 and E932 in Group 2 at different timepoints of transplantation. On day 0, after 7 treatments with CTLA4-Ig and before TBI, both E934 and E932 were hyporesponsive to their DLA-haploidentical donors, while still responsive towards the unrelated dogs. Maximal hyporesponsiveness towards the DLA-haploidentical donors was observed at day +28 in E934 and day +7 in E932 after immunosuppression of MMF/CSP. Immunoreactivity was increased in both dogs subsequently, almost reaching pre-transplant levels in E934 at day +120 (Fig. 4A and 4B). Although a relatively lower initial donor MNC chimerism was observed in E934 (maximum of 43% at day +21), MNC chimerism was not lost until day 100 when MMF and CSP were discontinued per protocol (Fig. 4A). The dog survived with autologous recovery and was euthanized in good condition at day +131. In dog E932, high maximum donor chimerism was initially achieved in both MNC and granulocytes (80% and 95%, at days +28 to +35, respectively). However, chimerism in TCR+ cells was only 13% at day+42 with complete graft rejection evident by day +84 with autologous recovery.
Figure 4. Mixed leukocyte culture (MLC) and donor chimerism results for recipients E934 (A) and E932 (B) (Group 2).
MLC data is presented as count per minute (CPM) of E934 (A) or E932 (B), which is the reactivity against autologous (auto), DLA-haploidentical (haplo) or unrelated donor cells (URD) at different time points of transplantation. Chimerism plots represent donor chimerism in E934 (A) and E932 (B) at different time points post-transplantation. Bars below the chimerism plots represent duration and dose of immunosuppression which consisted of cyclosporine (CSP, 30mg/kg/day from day -1 to +100) and mycophenolate mofetil (MMF, 20 mg/kg/day from day 0 to +40 and 10mg/kg/day from day+41 to +100). Abbreviations: ConA, concanavalin-A; DM5+, donor chimerism in canine myeloid cells marker positive cells; MNC, mononuclear cells; TCR, donor chimerism in αβ-T-cell receptor positive cells.
Effects of CTLA4-Ig on in vitro and in vivo NK cell function
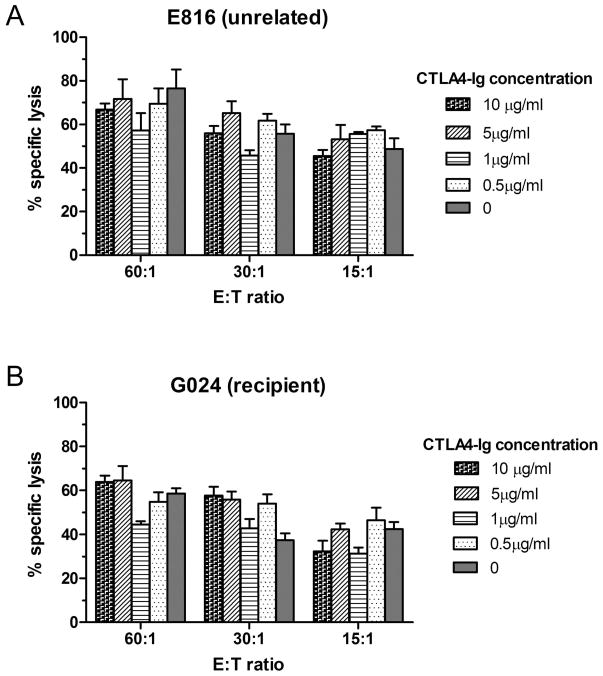
No differences in NK functional activity between the absence and presence of different concentrations of CTLA4-Ig were observed. Figure 5A shows the NK assay of an unrelated dog (E816) which was used as a normal control. The NK cell activity was not affected by adding CTLA4-Ig in vitro. Figure 5B shows the result of recipient dog G024 in Group 2 on day 0 before TBI. Compared to control (E816) the NK cell activity did not change despite 7 days of in vivo CTLA4-Ig treatment, and similarly, G024’s NK cytolytic activity was not affected by adding different concentrations of CTLA4-Ig in vitro.
Figure 5. Effect of CTLA4-Ig on NK cell cytolytic activity.
Specific lysis of canine thyroid adenocarcinoma cells (CTAC) by mononuclear cells from (A) E816 (unrelated control) or (B) G024 (recipient from Group 2) in the absence or presence of different concentrations of CTLA-Ig. Mononuclear cells from G024 were obtained after 7 treatments with CTLA-Ig and prior to total body irradiation on day 0. Data are presented as mean and range of triplicates. E:T ratio, Effector cell to Target cell ratio.
Comparison of effects of CTLA4-Ig and S5 on in vitro T and NK cell function
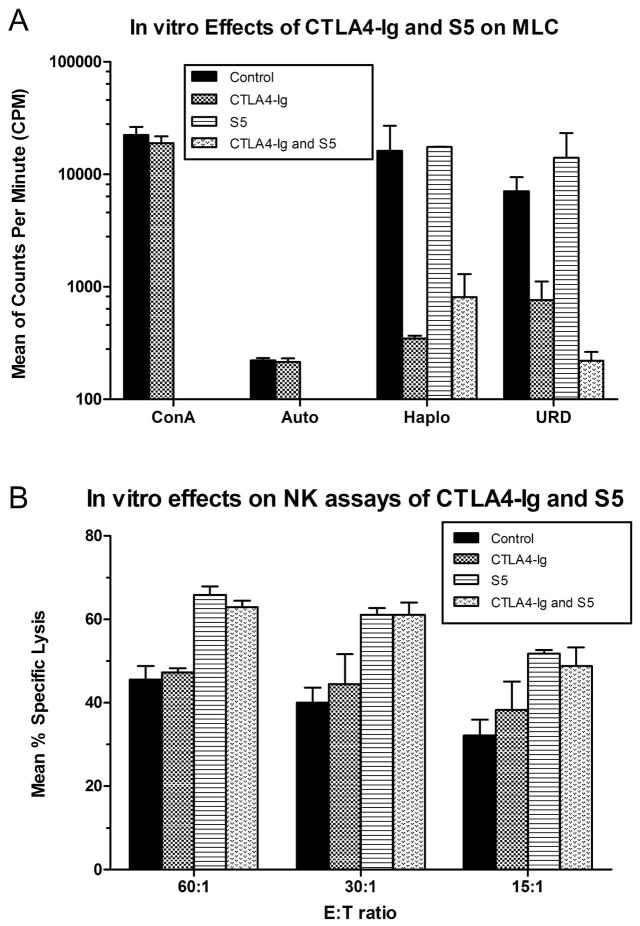
MLC and NK assays comparing in vitro effects of CTLA4-Ig and S5 individually and combined were conducted in three dogs G053, G052 (haploidentical littermate to G053) and E816 (unrelated dog) (Figure 6). Addition of CTLA4-Ig (10 μg/mL) to the medium during 7 days of incubation reduced G053 immunoreactivity in MLC towards its DLA-haploidentical littermate and unrelated donor. No effect on immunoreactivity in MLC was observed with S5 (10 μg/ml) alone, and no additional effect on immunoreactivity was observed with the combination of CTLA4-Ig and S5 (Fig 6A). Specific lysis in the NK assays was enhanced by S5 (10 μg/ml), while no effect was found with CTLA4-Ig (10 μg/ml) alone and no additional effect was observed with the combination of CTLA4-Ig and S5. Both hyporesponsiveness in MLC induced by CTLA4-Ig and enhancement of NK cell activity by S5 were not disrupted by the combination of S5 and CTLA4-Ig, respectively.
Figure 6. In vitro effects of CTLA4-Ig and S5 (canine anti-CD44 mAb) alone and in combination on mixed lymphocyte culture immunoreactvity and NK cell activity.
(A) Mixed lymphocyte culture immunoreactivity of G053, G052 and E816 cells towards autologous cells (auto), DLA haploidentical cells (Haplo) (G052 against G053, and G053 against G052) and unrelated donor cells (URD) (G052 or G053 against E816, or E816 against G052 or G053) in the absence of CTLA4-Ig and S5 (Control), presence of CTLA4-Ig 10 μg/ml only (CTLA4-Ig), presence of S5 10 μg/ml only (S5), or presence of both CTLA4-Ig 10 μg/ml and S5 10 μg/ml (CTLA4-Ig and S5). Data are presented as mean and range of counts per minute (CPM) from triplicates. (B) The specific lysis of canine thyroid carcinoma cells by PMNC is presented as mean and range from 3 dogs (G053, G052 and E816). Abbreviations: ConA, concanavalin-A; E:T ratio, effector: to target cells ratio.
DISCUSSION
In DLA-haploidentical HCT where there is a greater MHC disparity between recipient and donor, there is a higher risk of host-versus-graft (HVG) and graft-versus-host (GVH) reactions. Co-stimulatory inhibition therapy used in transplantation is expected to induce donor-specific immune tolerance to both benefit graft acceptance and prevent GVHD. However, HVG reactions in MHC-mismatched HCT are affected both by NK and T-cells, while GVH reactions are thought to be predominantly mediated by T-cells [43–45]. In this study, we performed a series of studies to evaluate the effects of CTLA4-Ig therapy on host T-cell and NK cell function. T-cell function, as evaluated by MLC demonstrated that pre-transplant infusions of CTLA4-Ig and donor PBMC induced hyporesponsesiveness in recipient dogs towards their DLA-haploidentical donors on day 0 before TBI. Additionally, immunoreactivity against DLA haploidentical donor cells, resembling HVG reactions, could be suppressed by adding CTLA4-Ig to MLC. These findings suggest that pre-transplant treatment with CTLA4-Ig could induce host hyporesponsiveness to DLA-haploidentical HCT by abrogating T-cell immunoreactivity. In contrast, in both in vitro and in vivo studies, no effect of CTLA4-Ig on host NK cell function was observed, indicating that treatment with CTLA4-Ig is unlikely to overcome the critical immune barrier manifested by NK cell activity in MHC-mismatched HCT. Although 7-day courses of CTLA4-Ig 4 mg/kg/day are sufficient to allow sustained engraftment in solid organ [1] and canine DLA-identical marrow transplantation [20], this strategy may not be sufficient to induce immune tolerance in a DLA-haploidentical model as 2 of 3 dogs treated with 4 mg/kg/day in our study failed to engraft. This result is consistent with the finding of trough CTLA4-Ig plasma levels in dogs treated with 4 mg/kg/day being below 1 μg/mL, which in vitro only suppressed T-cell reactivity by approximately 40% (Fig 2). A dose-dependent immunosuppressive effect of CTLA4-Ig has been observed in previous studies performed in other animal models and in humans [46–48]. Therefore, the dose of CTLA4-Ig was increased to 5 mg/kg/day in groups 2 and 3, which resulted in CTLA4-Ig trough levels of greater than 5 μg/mL corresponding to an 80%. decrease in recipient T-cell immunoreactivity. Four of 5 dogs in Groups 2 and Group 3 achieved initial engraftment. Despite the increase in CTLA4-Ig dose, all dogs eventually rejected their grafts. Compared to a similar study where S5 was used instead of CTLA4-Ig in the conditioning regimen prior to DLA-haploidentical transplants, the findings in the current study are inferior, as 43% of the S5 treated dogs had sustained engraftment with a median follow-up of 17 (range 5–55) weeks after HCT [21]. In the present study, we used the same post-grafting immunosuppression regimen which was used in our previous study of S5 based DLA-haploidentical transplantation [21]. In both the current study and the S5 study, most rejections occurred after discontinuation or dose reduction of immunosuppression. As this suggests that discontinuation of immunosuppression at day +100 may not be sufficient to ensure stable engraftment in a DLA-haploidentical transplant model using only 200 cGy TBI-based conditioning, we prolonged immunosuppression in group 4, although without significant effects on the rejection rate.
The mechanism by which S5 facilitates engraftment is still unclear, however in vivo and in vitro experiments have demonstrated that S5 reduces NK function in the post-transplant setting by increasing NK-cell susceptibility to radiation damage [24–26]. In light of this, our findings support the concept of both T- and NK cell barriers being important in MHC-mismatched grafts. Similar results have previously been observed in DLA-mismatched models, where the anti-T-cell reagent, anti-thymocyte serum, failed to enhance engraftment after a myeloablative TBI dose (9.2 Gy) [49]. The critical role of NK cells was also observed in hybrid mice undergoing marrow transplantation from their MHC-haploidentical parents. The mice, that were deficient in all lymphoid cells including NK cells, were unable to reject the parental marrow, while the mice deficient in T, B and NKT cells, rejected the parental marrow similar to the wild type [50].
The current data indicate that persisting NK-cell function could be responsible for graft rejection in our DLA-haploidentical transplantation model. However, alternative mechanisms cannot be ruled out. In a recent study in a primate model of MHC-matched and mismatched nonmyeloablative HCT, the authors observed that a recipient-derived allo-reactive T-cell population emerged at the time of tapering of pharmacological immunosuppression, thus suggesting that despite nonmyeloablative conditioning and post-transplant immunosuppression, a reservoir of alloreactive T-cells remain functional and, in addition to NK-cell activity, could help drive rejection [51].
Although sustained engraftment was not achieved in the current study, our results do suggest a role for CTLA4-Ig, as it facilitated initial engraftment without the occurrence of GVHD, even in recipients of high CD3+ cell number. Although no synergistic effects were observed between CTLA4-Ig and S5 in our in vitro experiments, further studies are needed to establish whether concomitant treatment could abrogate both T-cell and NK cell mediated immune barriers to sustained engraftment. Recently, plerixafor (AMD3100), which reversibly inhibits the binding of stromal derived factor-1 (SDF-1) to its receptor CXCR4, has been shown to provide significant stem cell mobilizing activity with possibly increased engraftment potential [52,53]. In a small descriptive study from our group, it was demonstrated that Plerixafor as a single mobilizing agent provided prompt early engraftment in DLA-haploidentical HCT after a nonmyeloablative preparative regimen consisting of S5 and 200 cGy TBI [54].
In summary, our data demonstrate that donor-specific hyporesponsiveness of T-cells can be induced by administering CTLA4-Ig and donor PBMC infusions before HCT. CTLA4-Ig treatment had no effect on host NK cell function. In our DLA-haploidentical nonmyeloablative HCT model with 200 cGy TBI conditioning, most dogs achieved initial engraftment without GVHD. However, all dogs rejected their allografts after discontinuation or dose reduction of MMF/CSP immunosuppression. These results suggest that future studies are warranted in evaluating the addition of CTLA4-Ig to S5, DLI and possibly other immunomodulatory and stem cell mobilizing agents for maintaining long-term engraftment while preventing GVHD.
Acknowledgments
The authors are indebted to Drs. Howard Shulman and George Sale for pathology studies; to Michele Spector, D.V.M., the investigators who participated in the weekend treatments, and the technicians in the canine facilities. We also would like to thank Stacy Zellmer for DLA typing. The authors are indebted to Richard Boismenu, Ph.D., Repligen Corporation, Needham, MA, who kindly provided RG2077 (recombinant human CTLA4 linked to human IgG1 heavy chain) and generously helped in CTLA4-Ig level measurement. We also appreciate Amgen for the gift of canine G-CSF. We are grateful for the assistance of Helen Crawford, Bonnie Larson, and Sue Carbonneau for manuscript preparation.
Grant support: Research funding was provided by the National Institutes of Health, Bethesda, MD grants P01HL036444, P01CA078902, and P30CA015704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers. YC was supported by a grant from China Scholarship Council and Shandong University. TF was supported by a fellowship from the Kirin Brewery Company, Ltd. BK, was supported by a fellowship from the Danish Cancer Society.
ABBREVIATIONS
- ATS
anti-thymocyte serum
- ConA
concanavalin A
- CPM
counts per minute
- CSP
cyclosporine
- CTAC
canine thyroid adenocarcinoma cells
- CTLA4
cytotoxic T lymphocyte antigen 4
- CXCR4
chemokine receptor 4
- DLA
dog leukocyte antigen
- DLI
donor lymphocyte infusion
- GVH
graft-versus-host
- GVHD
graft-versus-host disease
- HCT
hematopoietic cell transplant
- HVG
host-versus-graft
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MLC
mixed leukocyte culture
- MMF
mycophenolate mofetil
- MNC
mononuclear cell
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PBSC
peripheral blood stem cell
- PTLD
post-transplant lymphoproliferative disease
- SDF-1
stromal derived factor-1
- S5
canine anti-CD44 monoclonal antibody
- TBI
total body irradiation
- TCR
T-cell receptor
Footnotes
AUTHORSHIP:
- Yun Chen drafted and revised the manuscript, analyzed and interpreted data. No conflicts of interest.
- Takahiro Fukuda designed and conducted the study, analyzed and interpreted data, revised manuscript. No conflicts of interest.
- Monica S Thakar designed and conducted the study, revised manuscript. No conflicts of interest.
- Brian Kornblit revised manuscript, analyzed and interpreted data. No conflicts of interest.
- Erlinda B. Santos designed and conducted the study, drafted and revised manuscript, analyzed and interpreted data. No conflicts of interest.
- Rainer Storb designed study, revised manuscript. No conflicts of interest.
- Brenda M. Sandmaier conceived, designed and supervised the study, analyzed and interpreted data, drafted and revised manuscript. No conflicts of interest.
References
- 1.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance (Review) [erratum appears in Immunol Rev. 2004 Feb;197:243] Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 2.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells [Review] Immunol Rev. 1998;165:231–47. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 3.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 4.Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei RQ, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–5. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury SJ, Akalin E, Chandraker A, Turka LA, Linsley PS, Sayegh MH, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4. [PubMed] [Google Scholar]
- 7.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162:1947–51. [PubMed] [Google Scholar]
- 8.Loughran TP, Jr, Deeg HJ, Storb R. Morphologic and phenotypic analysis of canine natural killer cells: Evidence for T-cell lineage. Cell Immunol. 1985;95:207–17. doi: 10.1016/0008-8749(85)90309-0. [DOI] [PubMed] [Google Scholar]
- 9.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–6. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–6. [PubMed] [Google Scholar]
- 12.Wallace PM, Johnson JS, Macmaster JF, Kennedy KA, Gladstone P, Linsley PS. CTLA4Ig treatment amerliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–10. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Yin DP, Sankary HN, Williams J, Krieger N, Fathman CG. Induction of tolerance to small bowel allografts in high-responder rats by combining anti-CD4 with CTLA4Ig. Transplantation. 1996;62:1537–9. doi: 10.1097/00007890-199612150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Levisetti MG, Padrid PA, Szot GL, Mittal N, Meehan SM, Wardrip CL, et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–91. [PubMed] [Google Scholar]
- 15.Shirasugi N, Akiyama Y, Aramaki O, Shibutani S, Matsumoto K, Bashuda H, et al. Role of the CTLA4 pathway in hyporesponsiveness induced by intratracheal delivery of alloantigen. Transplantation. 2003;75:1636–9. doi: 10.1097/01.TP.0000062572.27963.7F. [DOI] [PubMed] [Google Scholar]
- 16.Foster RD, Pham S, Li S, Aitouche A. Long-term acceptance of composite tissue allografts through mixed chimerism and CD28 blockade. Transplantation. 2003;76:988–94. doi: 10.1097/01.TP.0000079827.91675.A3. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim S, Jakobs F, Kittur D, Hess A, Linsley PS, Sanfilippo F, et al. CTLA4Ig inhibits alloantibody responses to repeated blood transfusions. Blood. 1996;88:4594–600. [PubMed] [Google Scholar]
- 18.Speiser DE, Bachmann MF, Shahinian A, Mak TW, Ohashi PS. Acute graft-versus-host disease without costimulation via CD28. Transplantation. 1997;63:1042–4. doi: 10.1097/00007890-199704150-00028. [DOI] [PubMed] [Google Scholar]
- 19.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–54. [PubMed] [Google Scholar]
- 20.Storb R, Yu C, Zaucha JM, Deeg HJ, Georges G, Kiem H-P, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–9. [PubMed] [Google Scholar]
- 21.Fukuda T, Kerbauy FR, Gooley T, Santos EB, Storb R, Sandmaier BM. Dog leukocyte antigen-haploidentical stem cell allografts after anti-CD44 therapy and nonmyeloablative conditioning in a preclinical canine model. Transplantation. 2006;82:332–9. doi: 10.1097/01.tp.0000228908.10775.b0. [DOI] [PubMed] [Google Scholar]
- 22.Sandmaier BM, Storb R, Appelbaum FR, Gallatin WM. An antibody that facilitates hematopoietic engraftment recognizes CD44. Blood. 1990;76:630–5. [PubMed] [Google Scholar]
- 23.Sandmaier BM, Storb R, Bennett KL, Appelbaum FR, Santos EB. Epitope specificity of CD44 for monoclonal antibody dependent facilitation of marrow engraftment in a canine model. Blood. 1998;91:3494–502. [PubMed] [Google Scholar]
- 24.Tan PHS, Santos EB, Rossbach HC, Sandmaier BM. Enhancement of natural killer activity by an antibody to CD44. J Immunol. 1993;150:812–20. [PubMed] [Google Scholar]
- 25.Tan PH, Liu Y, Santos EB, Sandmaier BM. Mechanisms of enhancement of natural killer activity by an antibody to CD44: Increase in conjugate formation and release of tumor necrosis factor α. Cell Immunol. 1995;164:255–64. doi: 10.1006/cimm.1995.1169. [DOI] [PubMed] [Google Scholar]
- 26.Tan PH, Sandmaier BM, Stayton PS. Characterization of an anti-CD44 single-chain FV antibody that stimulates natural killer cell activity and induces TNFα release. Immunol Invest. 1995;24:907–26. doi: 10.3109/08820139509060717. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–7. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 28.Wagner JL, Burnett RC, Works JD, Storb R. Molecular analysis of DLA-DRBB1 polymorphism. Tissue Antigens. 1996;48:554–61. doi: 10.1111/j.1399-0039.1996.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 30.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–73. [PubMed] [Google Scholar]
- 31.Storb R, Raff RF, Graham T, Appelbaum FR, Deeg HJ, Schuening FG, et al. Marrow toxicity of fractionated versus single dose total body irradiation is identical in a canine model. Int J Radiat Oncol Biol Phys. 1993;26:275–83. doi: 10.1016/0360-3016(93)90207-c. [DOI] [PubMed] [Google Scholar]
- 32.Zaucha JM, Zellmer E, Georges G, Little M-T, Storb R, Storer B, et al. G-CSF-mobilized peripheral blood mononuclear cells added to marrow facilitates engraftment in nonmyeloablated canine recipients: CD3 cells are required. Biol Blood Marrow Transplant. 2001;7:613–9. doi: 10.1053/bbmt.2001.v7.pm11760149. [DOI] [PubMed] [Google Scholar]
- 33.Sandmaier BM, Storb R, Santos EB, Krizanac-Bengez L, Lian T, McSweeney PA, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–13. [PubMed] [Google Scholar]
- 34.Lee R, Storb R, Little M-T, Joslyn A, Spector M, Kuhr CS. Percutaneous central dual-lumen catheter for apheresis in the canine. J Invest Surg. 2002;15:337–41. doi: 10.1080/08941930290086155. [DOI] [PubMed] [Google Scholar]
- 35.Deeg HJ, Storb R. Canine marrow transplantation models. Current Topics in Vet Res. 1994;1:103–14. [Google Scholar]
- 36.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–5. [PubMed] [Google Scholar]
- 37.Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high density expression of CD4 by canine neutrophils. Tissue Antigens. 1992;40:75–85. doi: 10.1111/j.1399-0039.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 38.Barsoukov AA, Moore PF, Storb R, Santos EB, Sandmaier BM. The use of an anti-TCRαβ monoclonal antibody to control host-versus-graft reactions in canine marrow allograft recipients conditioned with low dose total body irradiation. Transplantation. 1999;67:1329–35. doi: 10.1097/00007890-199905270-00007. [DOI] [PubMed] [Google Scholar]
- 39.Sandmaier BM, Schuening FG, Bianco JA, Rosenman SJ, Bernstein I, Goehle S, et al. Biochemical characterization of a unique canine myeloid antigen. Leukemia. 1991;5:125–30. [PubMed] [Google Scholar]
- 40.Denkers E, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol. 1985;135:2183–6. [PubMed] [Google Scholar]
- 41.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Veterinary Immunology & Immunopathology. 1993;39:461–6. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- 42.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–6. [PubMed] [Google Scholar]
- 43.Raff RF, Sandmaier BM, Graham T, Loughran TP, Jr, Pettinger M, Storb R. “Resistance” to DLA-nonidentical canine unrelated marrow grafts is unrestricted by the major histocompatibility complex. Exp Hematol. 1994;22:893–7. [PubMed] [Google Scholar]
- 44.Raff RF, Deeg HJ, Loughran TP, Jr, Graham TC, Aprile JA, Sale GE, et al. Characterization of host cells involved in resistance to marrow grafts in dogs transplanted from unrelated DLA-nonidentical donors. Blood. 1986;68:861–8. [PubMed] [Google Scholar]
- 45.Storb R, Weiden PL, Graham TC, Lerner KG, Nelson N, Thomas ED. Hemopoietic grafts between DLA-identical canine littermates following dimethyl myleran. Evidence for resistance to grafts not associated with DLA and abrogated by antithymocyte serum. Transplantation. 1977;24:349–57. doi: 10.1097/00007890-197711000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Srinivas NR, Shyu WC, Weiner RS, Tay LK, Greene DS, Barbhaiya RH. Pharmacokinetics of CTLA4Ig (BMS-188667), a novel immunosuppressive agent, following intravenous and subcutaneous administration to mice. J Pharm Sci. 1995;84:1488–9. doi: 10.1002/jps.2600841217. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas NR, Weiner RS, Warner G, Shyu WC, Davidson T, Fadrowski CG, et al. Pharmacokinetics and pharmacodynamics of CTLA4lg (BMS-188667), a novel immunosuppressive agent, in monkeys following multiple doses. J Pharm Sci. 1996;85:1–4. doi: 10.1021/js950347d. [DOI] [PubMed] [Google Scholar]
- 48.Lundquist L. Abatacept: a novel therapy approved for the treatment of patients with rheumatoid arthritis (Review) Advances in Therapy. 2007;24:333–45. doi: 10.1007/BF02849902. [DOI] [PubMed] [Google Scholar]
- 49.Storb R, Raff RF, Appelbaum FR, Schuening F, Sandmaier B, Shulman H, et al. Failure of antithymocyte serum postgrafting to overcome “resistance” to DLA-nonidentical canine marrow grafts. (Brief Communications) Transplantation. 1988;45:236–9. [PubMed] [Google Scholar]
- 50.Suzue K, Reinherz EL, Koyasu S. Critical role of NK but not NKT cells in acute rejection of parental bone marrow cells in F1 hybrid mice. Eur J Immunol. 2001;31:3147–52. doi: 10.1002/1521-4141(200111)31:11<3147::aid-immu3147>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 51.Larsen CP, Page A, Linzie KH, Russell M, Deane T, Stempora L, et al. An MHC-defined primate model reveals significant rejection of bone marrow after mixed chimerism induction despite full MHC matching. Am J Transplant. 2010;10:2396–409. doi: 10.1111/j.1600-6143.2010.03272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montgomery M, Cottler-Fox M. Mobilization and collection of autologous hematopoietic progenitor/stem cells (Review) Clinical Advances in Hematology and Oncology. 2007;5:127–36. [PubMed] [Google Scholar]
- 53.Cashen AF, Nervi B, DiPersio J. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncology. 2007;3:19–27. doi: 10.2217/14796694.3.1.19. [DOI] [PubMed] [Google Scholar]
- 54.Thakar MS, Santos EB, Fricker S, Bridger G, Storb R, Sandmaier BM. Plerixafor-mobilized stem cells alone are capable of inducing early engraftment across the MHC-haploidentical canine barrier (Letter to the Editor) Blood. 2010;115:916–7. doi: 10.1182/blood-2009-09-245696. [DOI] [PMC free article] [PubMed] [Google Scholar]