Abstract
This study sought to discover if an optimum 1 cm2 area in the non-damaged right hemisphere (RH) was present, which could temporarily improve naming in chronic, nonfluent aphasia patients when suppressed with repetitive transcranial magnetic stimulation (rTMS). Ten minutes of slow, 1 Hz rTMS was applied to suppress different RH ROIs in eight aphasia cases. Picture naming and response time (RT) were examined before, and immediately after rTMS. In aphasia patients, suppression of right pars triangularis (PTr) led to significant increase in pictures named, and significant decrease in RT. Suppression of right pars opercularis (POp), however, led to significant increase in RT, but no change in number of pictures named. Eight normals named all pictures correctly; similar to aphasia patients, RT significantly decreased following rTMS to suppress right PTr, versus right POp. Differential effects following suppression of right PTr versus right POp suggest different functional roles for these regions.
Keywords: TMS, Brain Stimulation, Aphasia, Stroke Rehabilitation
Introduction
In the first few months following stroke, surviving patients commonly show remarkable functional recovery. Neural plasticity (including changes at the molecular, cellular, and systems levels) underlies some of this recovery (Johansson, 2000). Although neural plasticity is an intrinsic property of the nervous system that provides the evolutionary advantage of adapting to changes associated with development and learning, evidence suggests that it is not always successful in coping with injury (Pascual-Leone, Amedi, Fregni, & Merabet, 2005). Indeed, it appears that plasticity following stroke can be the cause of persistent behavioral deficits rather than the vehicle for recovery of function.
Speech and language deficits are common consequences of stroke to the left hemisphere and approximately 20% of aphasia patients are left with hesitant, poorly articulated, agrammatic speech with word-finding problems that persist (Pedersen, Vinter, & Olsen, 2004). The relative importance of the contralesional and ipsilesional hemispheres for recovery from aphasia remains unclear. A hypothesized role of right hemisphere (RH) regions in aphasia recovery dates back to Barlow and Gowers (Barlow, 1877; Gowers, 1886). However, some functional neuroimaging studies suggest that unusually high activation in right perisylvian regions during various language tasks is associated with persistent speech and language deficits, especially in nonfluent aphasia (Belin, et al., 1996; Naeser, et al., 2004; Rosen, et al., 2000; Postman-Caucheteux et al., 2010), while post-stroke emergence of activation of left hemisphere peri-lesional language areas is often correlated with improved behavioral outcome (Heiss, Kessler, Thiel, Ghaemi, & Karbe, 1999; Warburton, Price, Swinburn, & Wise, 1999; Zahn, et al., 2004). These results raise the possibility that cortical activation, at least in certain areas of the RH, might prevent, rather than promote, recovery from aphasia. If so, suppression of specific RH areas might improve language in patients with long-standing, nonfluent aphasia.
Participants and Methods
Informed consent was obtained from aphasia patients and normal controls prior to inclusion in the study. Human subjects approval was given by the Institutional Review Boards at all participating sites. The public trials ClinicalTrials.gov identifier is NCT00608582.
Aphasia Patients
We studied eight right-handed stroke patients (two females) who had single, left hemisphere stroke 1.5 to 30 years prior to the study (Table 1). The cortical and/or subcortical deeper, white matter areas of infarction were compatible with nonfluent aphasia (Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, & Albert, 1989) (Figure 1). The patients had considerable residual word-finding and naming problems. Speech pathology evaluations had documented stable aphasia deficits. Each patient met our minimum entry criterion for picture naming - i.e., a score of at least 3 pictures named correctly on the first 20 items of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2001).
Table 1.
Patient Demographics and Aphasia Characteristics
| Boston Diagnostic Aphasia Exam* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (yrs) |
Gender | Years Post-CVA |
CVA | Right Paresis |
BNT max=20 |
Words per Longest Phrase |
Word Repetition max=10 |
Sentence Repetition max=10 |
Word Comprehension max=37 |
Sentence Comprehension max=12 |
| P1 | 51 | M | 5 | L MCA | Mild | 9 | 7 | 6 | 0 | 28.5 | 5 |
| P2 | 44 | M | 1.5 | L MCA | Mild-Mod | 9 | 5 | 9 | 2 | 33.1 | 6 |
| P3 | 58 | M | 8 | L MCA | Mild-Mod | 16 | 4 | 8 | 2 | 32 | 7 |
| P4 | 52 | M | 10 | L MCA | Moderate | 15 | 1–2 | 8 | 0 | 35.5 | 4 |
| P5 | 76 | M | 2 | L MCA | Severe | 13 | 1 | 6 | 1 | 32.8 | 5 |
| P6 | 52 | M | 9 | L MCA | Severe | 5 | 1 | 3 | 0 | 29 | 9 |
| P7 | 67 | F | 30 | L ICA | Severe | 10 | 1 | 8 | 1 | 26.5 | 3 |
| P8 | 57 | F | 6 | L ICH | Severe | 4 | 1 | 4 | 0 | 26 | 3 |
CVA = cerebro-vascular accident
MCA= Middle Cerebral Artery; ICA= Internal Carotid Artery; ICH= Intracerebral Hemorrhage
BNT = First 20 pictures from the Boston Naming Test, E. Kaplan, H. Goodglass, S. Weintraub, The Boston Naming Test Lippincott, Williams and Wilkins, Philadelphia, 2001.
H. Goodglass, E. Kaplan, B. Baressi, The Assessment of Aphasia and Related Disorders, 3rd Ed., Lippincott, Williams and Wilkins, Philadelphia, PA, , 2001.
Figure 1.
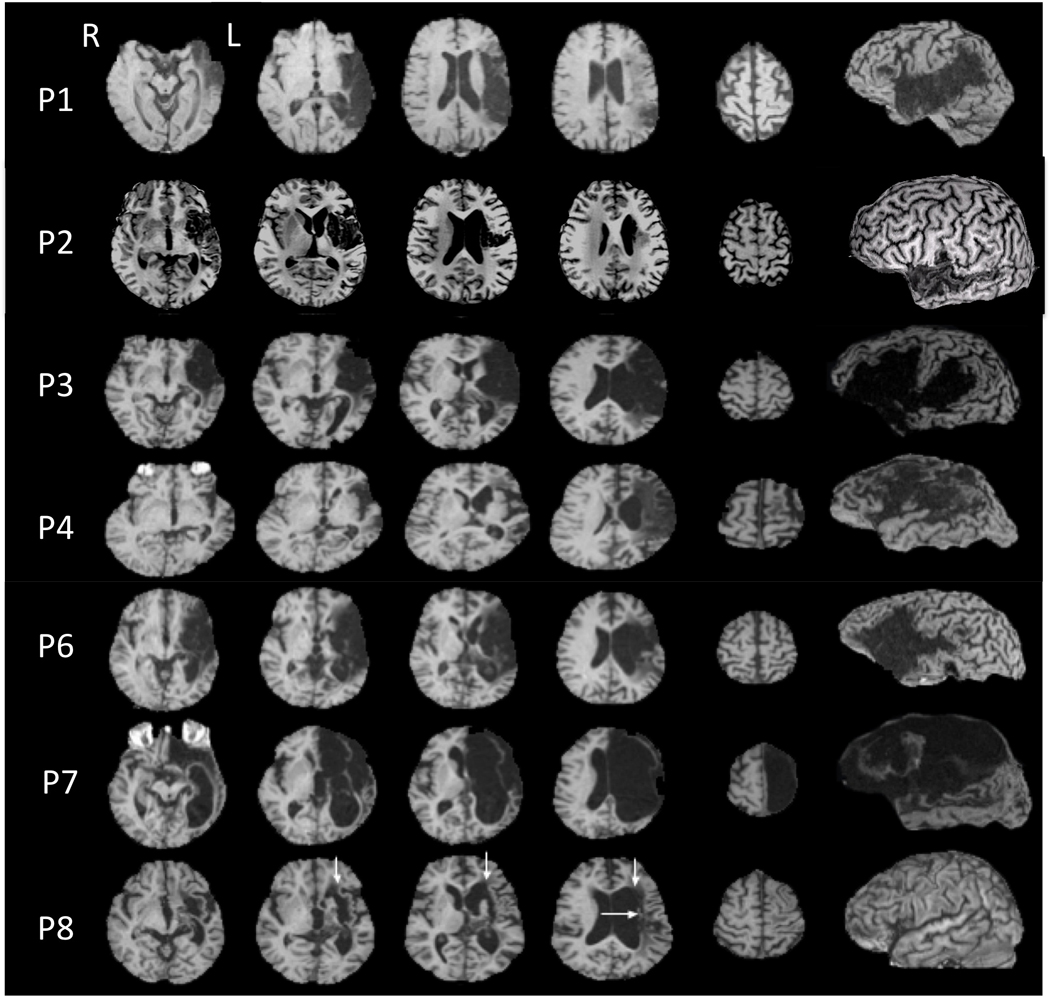
Structural MRI scans for each patient (3-dimensional magnetization prepared rapid gradient echo, 3D MPRAGE), T1-weighted study. The left lateral views are reconstructed from the 3D MPRAGE MRI scans showing left hemisphere cortical lesion sites. Patients are listed in order of severity of aphasia from least to greatest (Table 1). Structural MRI scan for P5 was unavailable for publication. Patients P1–P7 had lesion including left Broca's cortical area and subjacent white matter. P8 had no cortical lesion. Her extensive subcortical white matter lesion included the medial subcallosal fasciculus area located deep to Broca's area, adjacent to the left frontal horn (vertical arrows); and the periventricular white matter area located deep to sensorimotor cortex mouth level, adjacent to the left body of lateral ventricle (horizontal arrow). Extensive lesion in these two white matter areas has been linked to poor prognosis of nonfluent aphasia (Naeser et al., 1989). The mildest patient, P1, had almost no lesion in these two white matter areas; the other patients had some lesion present in one, or both of these deep, white matter areas.
TMS Procedure
We applied 1 Hz rTMS (Robertson, Theoret, & Pascual-Leone, 2003; Rossini, et al., 1994; Wagner, Zahn, Grodzinsky, & Pascual-Leone, 2004; Wassermann, 1998) to transiently suppress activity (Maeda, Keenan, Tormos, Topka, & Pascual-Leone, 2000; Moliadze, Zhao, Eysel, & Funke, 2003; Valero-Cabre, Payne, Rushmore, Lomber, & Pascual-Leone, 2005) in four different RH cortical regions of interest (ROIs) using MRI-guided frameless stereotaxy (Gugino, et al., 2001). We delivered a single train of 600 stimuli at 1 Hz (10 min.), at 90% of each individual patient’s motor threshold intensity for the left first dorsal interosseus muscle as determined by following published guidelines (Rossini, et al., 1994) and in adherence to current safety guidelines (Wassermann, 1998; Rossi, Hallett, Rossini, & Pascual-Leone, 2009).
TMS sessions were conducted at the Harvard-Thorndike General Clinical Research Center. Stimulation was delivered using a Magstim Super Rapid Magnetic Stimulator (Magstim, NY) equipped with an 8-shaped coil (each wing measuring 7 cm in diameter). Mathematical models suggest that when applied at peri-threshold intensity stimulation, this form of stimulation affects a volume of approximately 1 cc of cortex (Wagner, et al., 2004). A frameless stereotactic system (Brainsight, Rogue Industries, Montreal) was used to guide the position of the TMS coil on each patient's scalp so as to target a specific ROI located on the patient's own structural MRI scan. On-line monitoring assured that the defined target ROI was consistently targeted throughout a given rTMS session in each patient individually (Gugino, et al., 2001). In separate rTMS sessions, we evaluated the effect on picture naming (Snodgrass & Vanderwart, 1980) before, and immediately after 10 min. of rTMS to suppress the pars triangularis (PTr), pars opercularis (POp) (Amunts, et al., 2004), motor cortex mouth area (M1), and posterior-superior temporal gyrus (Wernicke's homologue region, STG) in the RH. (Figure 2 and Supplemental Figure 1).
Figure 2.
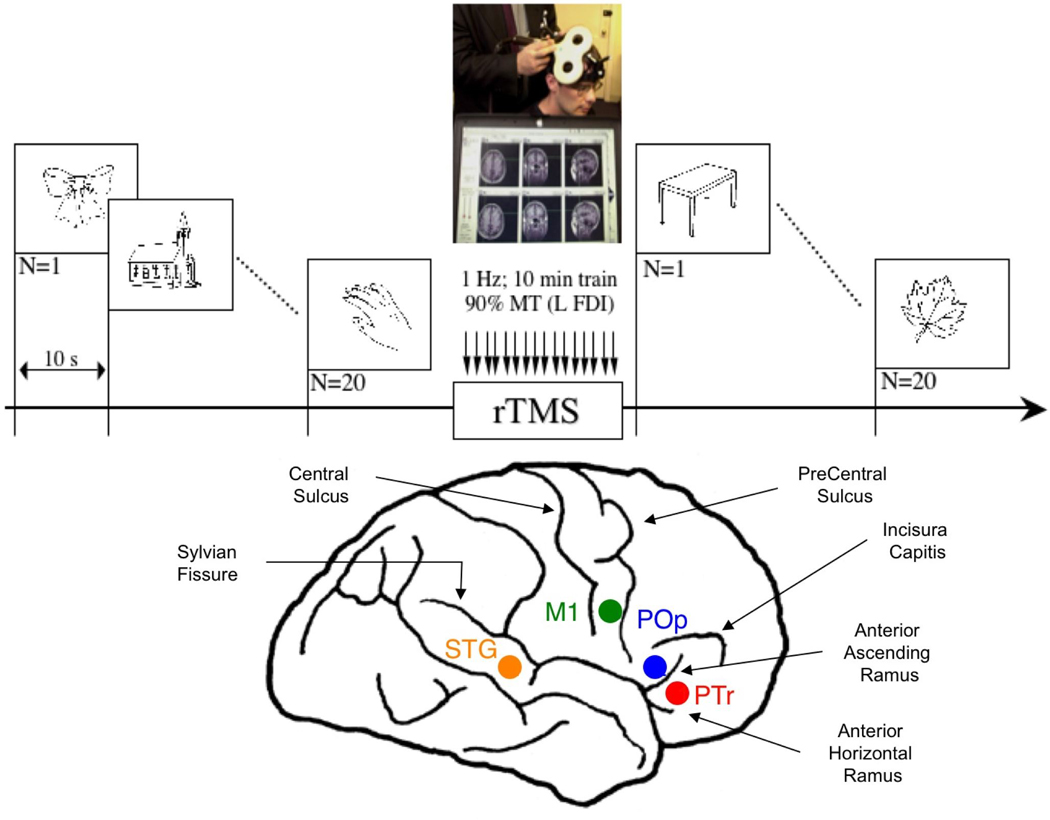
Schematic summary of the experimental design. Top: Picture naming was tested before and after rTMS, using Snodgrass & Vanderwart pictures (Snodgrass & Vanderwart, 1980). Bottom: Location of four right hemisphere cortical ROIs (schematically shown in lateral diagram), which were each suppressed with rTMS in separate sessions.
Sulcal and gyral landmarks were used to define the anatomical location for each cortical ROI in the RH. Broca's area, within the inferior frontal gyrus, includes an anterior portion (PTr) and a posterior portion (POp). These two cortical ROIs are usually, anatomically separated by the anterior ascending ramus of the Sylvian fissure. The PTr ROI was targeted on the gyrus immediately rostral to the anterior ascending ramus. The POp ROI was targeted on the gyrus caudal to the anterior ascending ramus, adjacent to the junction with inferior premotor cortex and rostral to the precentral sulcus. The right motor cortex mouth area (M1) was targeted as defined by the location from which TMS-induced motor evoked potentials of maximal amplitude in the orbicularis oris muscle had been recorded (standard surface EMG techniques). In addition, we targeted the posterior-superior temporal gyrus area (STG, Wernicke's homologue region). In one patient, P3, the right anterior supramarginal gyrus instead of right STG was targeted. In patients P2, P5, and P6, who participated later during the course of this study, no posterior ROI was suppressed with rTMS.
In order to measure the effect of rTMS to suppress each cortical ROI, naming was tested pre- and post- rTMS, using a 20-item Snodgrass & Vanderwart (Snodgrass & Vanderwart, 1980) (S&V) picture list. Prior to the first rTMS session, a baseline naming ability had been established across five different S&V picture lists (A–E). Each list contained 20 different S&V pictures of objects. All lists were carefully matched by Kucera-Francis frequency (familiarity) and number of syllables per word (complexity). Most pictures were monosyllabic words (e.g., bow, church, hand), and no two consecutive picture stimuli began with the same phoneme or belonged to the same semantic category. Within each list, six different internal randomizations were produced (List A1–6, List B1–6, etc.). Each picture was displayed on a laptop computer screen, where the picture was preceded by a 120 ms tone beep (and a fixation dot on the screen), and presented for a maximum of 10 s. Patients were instructed to try to name the picture as quickly as possible. Responses were tape-recorded and response time (RT) for each correctly named picture was later measured using SoundEdit Pro software. The RT was defined as the time between picture presentation and beginning of the production of the correct name of the picture, while ignoring intermediate responses.
Naming performance was assessed before, and immediately following rTMS for a maximum of two rTMS sessions in one day, separated by at least a 30 min break, between the two rTMS sessions. Only four patients received two rTMS sessions on the same day (P4, P5, P7, P8). Naming before the second rTMS session was within 2 SD of baseline S&V naming, regardless of the initial rTMS cortical ROI target, on any given day (Supplemental Table 1), even if the first ROI was the right PTr (Supplemental Table 2a,b). The baseline S&V naming scores for accuracy and RT are shown in Supplemental Table 3a,b.
Normal Controls
We studied the effect of rTMS to suppress the left PTr and POp; and the right PTr and POp in separate rTMS sessions, on the same naming task used in the aphasia patients, in eight right-handed normal controls (five were women). All had normal medical and neurological exams. The mean age was 36.9 years (SD 15.9).
Statistical Analyses
Aphasia Patients
The pre- versus post- naming data for each ROI were analyzed using two separate statistical analysis methods described below.
Analysis Method 1
For each aphasia patient, the pre- rTMS data consisted of the mean and SD across the five S&V lists administered at entry (baseline). The post- rTMS scores (accuracy and RT) for each ROI site were then subtracted from the baseline mean for each case. Data were submitted to a one-way, repeated measures analysis of variance (ANOVA), for site of stimulation (PTr, POp and M1), for both number of pictures named and RT. All post-hoc comparisons of means were carried out using the Tukey correction procedure for multiple comparisons. In addition, change from baseline for each site was tested by a series of one-sample t-tests. Data for STG were not included in the ANOVAs because of missing data. For normal controls, a parallel set of analyses using a two-way ANOVA for site of stimulation (PTr, POp and M1) × hemisphere (L, R) was completed for RT data only.
Analysis Method 2
It is recognized that aphasia patients can vary in language ability from day to day. Thus, to investigate possible intersession variability among our aphasia patients, the entry baseline S&V data were not used as the pre- rTMS naming measure, in this method. In Method 2, the pre- rTMS naming measures for accuracy and RT consisted of data obtained on a single S&V list administered immediately before rTMS suppression of each ROI, for each aphasia patient. The post- rTMS score for each ROI site was then subtracted from the pre- rTMS score for that ROI site for each patient. Data were submitted to two-way repeated measures analyses of variance (ANOVAs) for site of stimulation (PTr, POp, M1) × (pre- and post-) for both number of pictures named and RT. All post-hoc comparisons of means were carried out using the Tukey correction procedure for multiple comparisons. Pre- versus post- score differences for each site were tested by a series of one sample t-tests. Data for STG were not included in the ANOVAs because of missing data in three cases.
Results
Analysis Method 1
Aphasia Patients
Our results (Figure 3) demonstrate a significant site-specific effect of rTMS on both number of pictures named (F= 16.375; df 2,14; p<.001) and RT (F= 8.605; df 2,14; p=.004). Suppression of the right PTr was the only site that led to a significant increase in number of pictures named (p<.001), and a significant decrease in RT (p<.005). On average, this resulted in the correct naming of 3 more items on a list of 20 pictures that was administered after rTMS to suppress the right PTr, than at baseline (Supplemental Table 3a,b). The number of pictures named correctly following suppression of the right PTr was significantly greater than following suppression of the right POp (p<.001) and M1 (p<.001). The speed of response was significantly faster after suppression of right PTr versus right POp (p<.008); but not M1.
Figure 3.
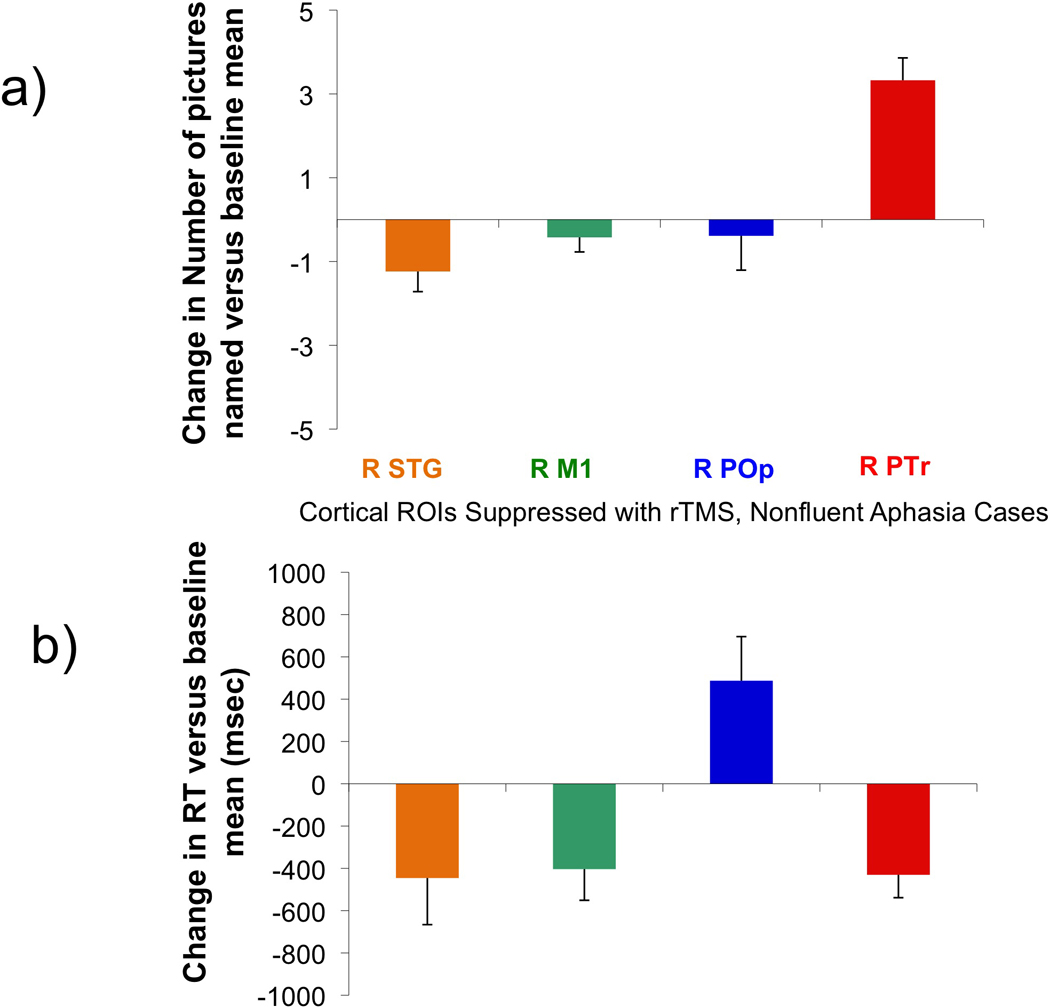
Effect on picture naming for right cortical ROIs that were suppressed with rTMS in the eight aphasia patients. Top (a): Change in number of pictures named versus baseline mean, post-rTMS (± standard error) to suppress each cortical ROI. Picture naming significantly increased only following rTMS to suppress the right PTr (p<.001). Bottom (b): Change in RT versus baseline mean, post-rTMS (± standard error) to suppress each cortical ROI. RT was significantly decreased after rTMS to suppress the right PTr (p<.005) and the right M1 (p<.029), but significantly increased only after rTMS to suppress the right POp (p<.052).
Suppression of the right POp was the only site that led to a significant increase in RT (p<.052), however, there was no change in number of pictures named. Suppression of right POp resulted in significantly longer RT, than suppression of PTr (p<.008) or M1 (p<.031). Suppression of right M1 led to a significant decrease in RT (p<.029); there was no change in number of pictures named. [In a separate analysis for the five patients who had the right STG suppressed, there was a trend towards a significant decrease in number of pictures named (p<.062); there was no significant change in RT (p<.113).] See Figure 3 and Supplemental Table 3a,b. The sequence of when a targeted cortical ROI was suppressed with rTMS (1st, 2nd, 3rd, 4th) was not a factor (Supplemental Table 4a,b,c).
In summary, Analysis Method 1 showed that suppression of right PTr was the only ROI to result in both a significant increase in number of pictures named (p<.001), and decrease in RT (p<.005), while suppression of right POp was the only ROI to result in a significant increase in RT (p<.052). Generally, the more severe patients showed less improvement than the mild-moderate cases. For example, the three more severe patients improved by only 1 or 2 pictures, whereas the mild-moderate patients improved by 4 pictures following suppression of right PTr (Supplemental Table 3a,b). P8, the most severe aphasia case, showed the greatest decrease in number of pictures named (3.45) following suppression of right POp, along with the greatest increase in RT (1443.0).
Normal Controls
The normal controls named all pictures correctly and only the RT data were analyzed statistically. Results indicated a Site Effect (PTr versus POp, p<.003), a Side Effect (L versus R, p<.003), and a trend towards an interaction (p=.10). In these normal controls, RT significantly decreased following rTMS suppression of right versus left PTr (p<.002); and increased following rTMS suppression of left versus right POp (p<.057). RT significantly decreased following rTMS to suppress right PTr, versus right POp (p<.009). See Figure 4.
Figure 4.
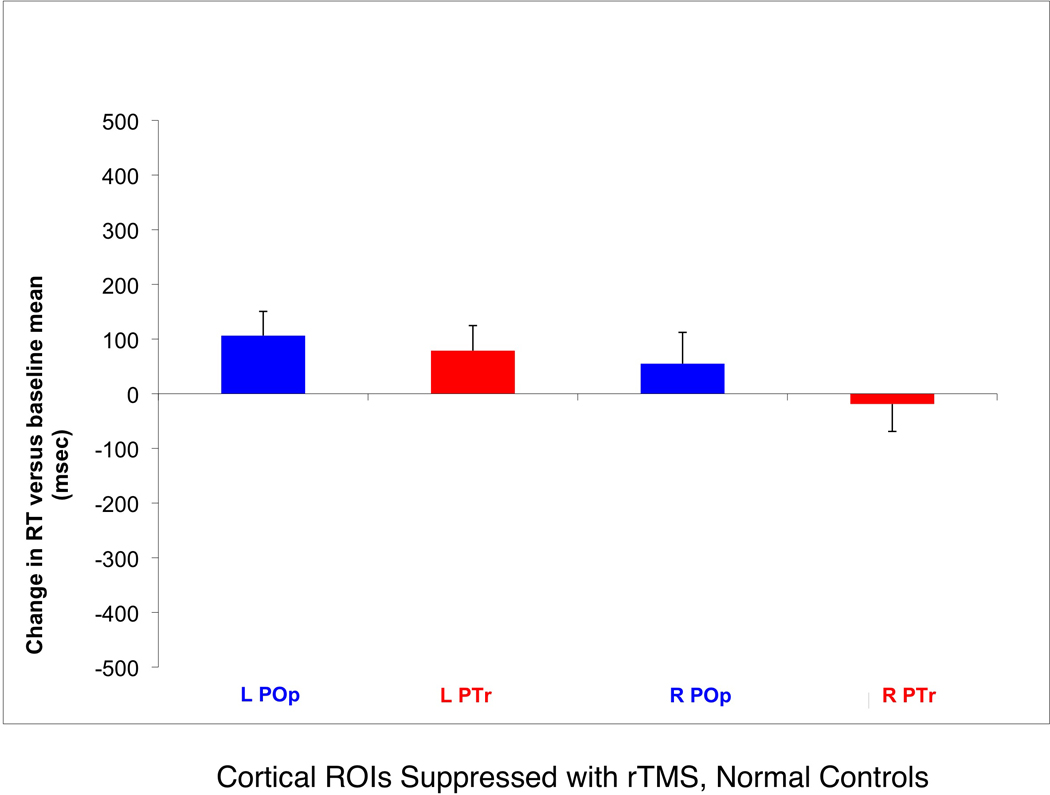
Effect on picture naming for left and right cortical ROIs that were suppressed with rTMS in eight normal controls. Change in RT versus baseline mean, post-rTMS (± standard error) to suppress the left PTr or POp; or right PTr or POp. Note that the RT increased maximally from baseline, after rTMS to suppress left POp, but decreased after rTMS to suppress right PTr. The RT was significantly faster (p<.009) following suppression of right PTr, versus right POp. This is consistent with the findings in the aphasia patients.
Results for Analysis Method 2
Aphasia Patients Only
For accuracy in number of pictures named, pre- rTMS versus post- rTMS comparisons for each ROI site using Analysis Method 2 resulted in similar significant effects to those observed using Analysis Method 1. There was a significant interaction (F=8.437, df 2,14; p=.004). Compared to pre- rTMS scores, only PTr had higher post- rTMS scores (p<.01), whereas the other two regions (POp and M1) had almost no change in post- rTMS scores (n.s.), accounting for the significant interaction. See Table 2a. The pre- and post- rTMS raw naming data are presented in Supplemental Table 5a.
Table 2.
| a. Accuracy for Naming, Mean and SD for each ROI site, pre- and post- rTMS to that site | |||
|---|---|---|---|
| PTr | POp | M1 | |
| Pre | 8.50 (3.9) | 9.29 (2.1) | 8.62 (2.9) |
| Post | 12.00 (3.7) | 8.29 (4.1) | 8.25 (3.1) |
| b. RT, Mean and SD for each ROI site, pre- and post- rTMS to that site | |||
|---|---|---|---|
| PTr | POp | M1 | |
| Pre | 3195.0 (747.64) | 2971.6 (715.31) | 3050.9 (604.8) |
| Post | 2728.7 (461.95) | 3646.5 (634.85) | 2755.6 (486.47) |
Analysis of the aphasia group data showed that none of the three ROI sites differed significantly on naming accuracy pre- rTMS (range, p<.30 to .80). See Table 2a. [For each patient, the single pre- rTMS naming accuracy score for each ROI site fell within 2 SD of that patient’s baseline mean for naming accuracy, shown in Supplemental Table 3a.] The post- rTMS naming accuracy scores for PTr were significantly higher than POp and M1 (p<.01); POp and M1 did not differ on post- rTMS accuracy scores (p<.80). See Table 2a.
For RT, pre- rTMS versus post- rTMS comparisons for each ROI site using Analysis Method 2 resulted in similar significant effects to those observed using Analysis Method 1. There was a significant interaction (F=7.108, df 2,14; p=.007). Compared to pre- rTMS, only POp had increased RT post- rTMS (p<.01), whereas the other two regions (PTr and M1) each had decreased RT post- rTMS (p<.05), accounting for the significant interaction (See Table 2b). The pre- and post- rTMS raw RT data are presented in Supplemental Table 5b.
Analysis of the aphasia group data showed that none of the three ROI sites differed significantly on RT pre- rTMS (all p’s >.10). See Table 2b. [For each patient, the single pre- rTMS RT for each ROI site fell within 2 SD of that patient’s baseline mean for RT, shown in Supplemental Table 3b.] The post- rTMS RT for POp was significantly longer than PTr and M1 (p<.01); PTr and M1 did not differ on post- rTMS RT (p>.9).
In summary, Analysis Method 2 showed that suppression of right PTr was the only ROI to result in both a significant increase in number of pictures named (p<.01), and a significant decrease in RT (p<.05). A significant increase in RT for the right POp (p < .01) was again observed.
Discussion
Our results show that when slow rTMS was used to suppress the right POp in our aphasia patients, RT was significantly increased. Thus, the right POp may contribute to residual language in the incompletely recovered aphasia patients, and play a role in recovery of language in nonfluent aphasia (Barlow, 1877; Blank, Bird, Turkheimer, & Wise, 2003; Gowers, 1886). More striking is the finding that rTMS to suppress a neighboring region, right PTr, significantly improved picture naming and significantly decreased RT. As first demonstrated by Sprague (Sprague, 1966), damage or disruption of a specific brain region might normalize the behavioral dysfunction induced by an initial insult (Kapur, 1996). A second lesion can, for example, release other brain structures from inhibition and hence improve behavior. Temporary "virtual lesions" as induced with 1 Hz rTMS appear ideally suited to systematically explore such principles of brain-behavior relations, and mechanisms of recovery of function (Theoret, Kobayashi, Valero-Cabre, & Pascual-Leone, 2003).
Limitations of available frameless stereotactic systems include the fact that they project the main vector of the induced current from the TMS coil assuming a homogenous spheric field of distribution. This oversimplified model may introduce an error in the current distribution and thus overestimate the focality of stimulation (Wagner, et al., 2004). In addition, differences in conductivity between brain tissue and cerebro-spinal fluid (CSF) result in the potential shunting of induced current by CSF collection (Wagner, et al., 2004). This might affect the focality of rTMS stimulation in the area of the inferior frontal gyrus, adjacent to the Sylvian fissure.
These limitations, however, do not detract from the functional resolution of our findings that demonstrate consistent differential effects of rTMS to suppress the right PTr versus the right POp. Although there is inter-individual variability in the effects, and the mechanisms of action remain unclear, local cortical effects are associated with specific distant effects on cortical and subcortical regions that depend on the strength of the anatomical projections (Valero-Cabre, et al., 2005). The effects of rTMS are not limited to the directly targeted brain region, but spread along functional neural networks (Chouinard, Van Der Werf, Leonard, & Paus, 2003; Siebner, Hartwigsen, Kassuba, & Rothwell, 2009; Valero-Cabre, et al., 2005). Our results suggest that the two portions of right Broca's homologue are integrated in neural networks with fundamentally different effects on residual speech in nonfluent aphasia.
This differential effect was observed across the range of severity of the cases studied. The greatest effect on picture naming accuracy was observed after suppression of the right PTr, where the increase was 4 pictures in mild-moderate cases and 1–2 pictures in the three more severe cases. After suppression of right PTr, a decrease in RT was observed across the mild, moderate and severe cases, but there was no pattern in relationship to degree of severity. Following suppression of right POp a significant increase in RT was observed across the mild, moderate and severe cases; again, there was no clear-cut pattern in relationship to degree of severity. P8, the most severe aphasia case, however, did show the greatest decrease in accuracy and increase in RT following suppression of right POp. Thus, suppression of right POp in more severe aphasia cases could have an especially detrimental effect. These cases may have weaker or fewer remaining neural-network, anatomical projections to access. For the remaining two ROIs, right M1 and right STG, there was no consistent effect on accuracy or RT in relationship to degree of severity.
Lesion size had no effect on the degree of improved accuracy after suppression of the right PTr. P8 (severe patient), who improved by only 1.55 pictures, had the smallest lesion size of all cases (Figure 1). Her lesion was in subcortical white matter areas only, and located in the two lesion site areas adjacent to ventricle, that are associated with severe nonfluent speech (Naeser, Palumbo, Helm-Estabrooks, Stiassny-Eder, & Albert, 1989). Paradoxically, P7 (another severe patient), with the largest lesion size of all cases, also improved by only 1 picture. This latter patient had large cortical-subcortical lesion, that extended from the cortex, deeper into the two lesion site areas adjacent to ventricle, associated with severe nonfluent speech (Figure 1).
The differential effects of rTMS suppression of PTr versus POp were not unique to patients with long-standing nonfluent aphasia because they were also demonstrated in the normal controls. The normal controls named all pictures correctly, but RT was significantly longer following rTMS suppression of left versus right ROIs. The longest RT followed rTMS suppression of left POp. Similar to findings in the aphasia patients, RT was longer compared to baseline, following rTMS suppression of right POp, but RT was shorter compared to baseline, following suppression of right PTr. Therefore, in patients with aphasia, in whom the observed effects of rTMS are much more prominent, existing interhemispheric and cortico-cortical interactions might simply be modified or exaggerated in the attempt to recover function (Pascual-Leone, et al., 2005).
The differential effect was observed in the aphasia patients regardless of the method of statistical analysis used to analyze their pre- versus post- rTMS naming data for each ROI. These two methods included: 1) using the S&V baseline mean and SD, as the pre- rTMS naming measure; or 2) using the pre- rTMS naming data obtained immediately before rTMS suppression of each ROI, as the pre- rTMS naming measure for that ROI. Thus, the absence of any significant difference between S&V baseline measures and pre- rTMS performance measures for any ROI, mitigates the possibility of day-to-day variability or practice effects accounting for improvement.
In the left inferior frontal gyrus, POp is generally occupied by Brodmann Area 44 (BA 44), while PTr tends to correspond to BA 45 (Amunts, et al., 2004). Both of these areas are critical for verbal fluency, but consistent with their cytoarchitectonic differences, their linguistic contributions appear to be distinct. Left BA 45 is preferentially involved in semantic aspects of language processing while left BA 44 contributes primarily to phonological processing (Devlin, Matthews, & Rushworth, 2003; Gold & Buckner, 2002; Nixon, Lazarova, Hodinott-Hill, Gough, & Passingham, 2004; Poldrack, et al., 1999; Gough et al., 2005; Hartwigsen, et al., 2010). In recent DTI studies, different primary, white matter pathways have been observed between different parts of Broca’s area (PTr versus POp), and different parts of posterior language zones. For example, LH pathways between the PTr and the posterior language zone (superior or middle temporal gyrus) have been observed to be primarily via the extreme capsule (Frey et al., 2008; Saur et al., 2008). However, LH pathways between the POp and the posterior language zone (anterior supramarginal gyrus) have been observed to be primarily via the arcuate fasciculus (Frey et al., 2008; Saur et al., 2008; Kaplan et al., 2010). Kaplan et al. (2010) observed similar white matter pathway differences for PTr versus POp, in the RH, as well as in the LH. Thus, the connections between parts of Broca’s area and parts of the posterior language zones have been observed to be similar in the RH and in the LH. However, it is unknown if, or how, these differences in connection pathways for PTr and POp with posterior areas may support aphasia recovery. A possibly greater role for the right POp is discussed below.
A role for the right POp and right ventral premotor cortex (vPMC) was posited in promoting recovery of speech in nonfluent aphasia by Barlow (1877), in a detailed anatomical study. A 10 year-old boy lost speech for only 10 days following a first stroke restricted to L POp and L vPMC. One month later, however, a second stroke occurred, located in the homologous RH areas (R POp and R vPMC). Following the second stroke, he lost all speech again, and there was “loss of voluntary motor power over the muscles concerned in articulation.” The boy died two months later, without any recovery of speech, despite intact left and right PTr. The results from this early post-mortem study suggest that the intact right POp may be more important than intact right PTr in aphasia cases with left frontal lesion.
The potential contribution of the mirror neuron system to recovery in aphasia is unknown. The POp (BA 44) is thought to be the human equivalent of the primates’ F5, a primary locus of visuomotor (mirror) neurons (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Rizzolatti and Craighero, 2004). The mirror neuron system is bilateral, important in child language acquisition and activates during both production and perception of similar actions (Rizzolatti and Craighero, 2004; Wilson et al., 2004; Iacoboni, 2008 for review). The POp has been observed to mediate “observation-execution matching for the goals of arm/hand actions” (Kemmerer and Gonzalez-Castille, 2010, for review). The POp also participates in non-language related motor functions (Binkofski, et al., 2003), and is part of a parieto-premotor network that integrates sensory (visual and auditory) inputs with related motor representations for hand- and face-related actions, possibly including articulation and language. Such a sensory input to an action matching system bears similarity to the implementation system that includes Wernicke's and Broca’s areas in an audio-phonological-articulatory loop (A. R. Damasio, 1992). In our other rTMS treatment studies where the right PTr was suppressed with rTMS for longer rTMS treatments (20 minutes, 1200 pulses) and over more days (10), long-term follow-up showed significant improvement in naming of “Tools/Implements” at 8 months post-rTMS (Naeser et al. 2005a,b), and with naming Actions at 6 months post-rTMS (Hamilton et al., 2010). While unknown, these improvements could be associated with the mirror neuron system.
A recent rTMS study (Meister, Wilson, Deblieck, Wu, & Iacoboni, 2007) has supported the role of ventral premotor cortex as necessary for phonemic categorization in speech perception, within the “motor theory of speech perception” (Liberman & Mattingly, 1985). Suppression of right POp in our aphasia patients could have interrupted these functional phonological circuits, slowing picture naming.
Another possible mechanism could involve u-fiber connections between PTr and POp. DTI tractography has been used to show the presence of u-fibers between PTr and POp (Naeser et al., 2010). Neurons in BA 45 with rich prefrontal connections, might serve to modulate activity of neurons in BA 44 through inhibitory interaction. If so, possible hyperactivity of neurons in right BA 45, could excessively suppress right BA 44 and hinder recovery in nonfluent aphasia patients. Suppression of this hyperactivity in right BA 45 with 1 Hz rTMS might permit better modulation of right BA 44 (in part, via u-fibers), and consequently promote better modulation of other right and left temporo-parietal regions important for naming (H. Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004; Price, Warburton, Moore, Frackowiak, & Friston, 2001). Longer rTMS sessions (20 minutes) to suppress right PTr, repeated over 10 days can lead to sustained improvements in naming and phrase length for several months in chronic nonfluent aphasia patients, and warrant careful clinical trial evaluation (Martin, et al., 2009; Naeser, Martin, Nicholas, Baker, Seekins, Helm-Estabrooks, et al., 2005; Naeser, Martin, Nicholas, Baker, Seekins, Kobayashi, et al., 2005; Barwood et al., 2011; Weiduschat et al., 2011).
The present study demonstrates the critical importance of the focality of a TMS target area to suppress in the RH with chronic, nonfluent aphasia patients in order to improve naming. Future studies are important to investigate the focality of TMS target areas, in order to promote optimal language recovery in additional types of aphasia with different lesion site patterns.
Highlights.
>We studied chronic aphasia patients with one stroke in the left hemisphere. >We sought optimal brain area to treat with slow, Transcranial Magnetic Stimulation. >The optimal cortical area in the undamaged (right) hemisphere was pars triangularis. >This improved accuracy and speed in naming pictures. >Results have application for enhanced rehabilitation in stroke patients with aphasia.
Supplementary Material
Supplemental Figure 1: 3-D reconstructed right, lateral view of the structural MRI scan for each of the 8 aphasia patients, displaying locations for the cortical ROIs that were suppressed with rTMS: pars opercularis (yellow square), pars triangularis (red triangle), posterior-superior temporal gyrus (orange diamond), and the motor cortex mouth area (green circle).
Acknowledgements
The authors would like to thank Mark Thivierge for the invaluable administrative support and Heidi Seekins for help with data acquisition.
Statement of Funding: Research supported by NIH grant RO1 DC05672 from the National Institute on Deafness and Other Communication Disorders, Bethesda, MD and a grant from the Medical Research Service, Department of Veterans Affairs, Washington, D.C. (to M.A.N.); a K24 NIH award (RRO18875), BBVA Chair in Translational Medicine, Harvard Clinical and Translational Science Center (UL1 RR025758), RO1-NS 47754, and RO1-NS 20068 (to A.P.-L.); the Harvard-Thorndike General Clinical Research Center (NCRR MO1 RR01032); and NIH grant P30 DC05207, National Institute on Deafness and Other Communication Disorders (to the Harold Goodglass Boston University Aphasia Research Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space--the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Barlow T. Brit Med J. 1877:103–104. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, JD OS, et al. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol. 2011 doi: 10.1111/j.1468-1331.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology. 1996;47(6):1504–1511. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Butler A, Buccino G, Heide W, Fink G, Freund HJ, et al. Mirror apraxia affects the peripersonal mirror space. A combined lesion and cerebral activation study. Exp Brain Res. 2003;153(2):210–219. doi: 10.1007/s00221-003-1594-2. [DOI] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJ. Speech production after stroke: the role of the right pars opercularis. Ann Neurol. 2003;54(3):310–320. doi: 10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90(2):1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. N Engl J Med. 1992;326(8):531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers WR. A Manual of Diseases of the Nervous System. London: Churchill; 1886. [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112(10):1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RH, Sanders L, Benson J, Faseyitan O, Norise C, Naeser M, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang. 2010;113(1):45–50. doi: 10.1016/j.bandl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Price CJ, Baumgaertner A, Geiss G, Koehnke M, Ulmer S, et al. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia. 2010;48(10):3155–3163. doi: 10.1016/j.neuropsychologia.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45(4):430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. The role of premotor cortex in speech perception: evidence from fMRI and rTMS. J Physiol Paris. 2008;102(1–3):31–34. doi: 10.1016/j.jphysparis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Johansson BB. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke. 2000;31(1):223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- Kaplan E, Naeser MA, Martin PI, Ho M, Wang Y, Baker E, et al. Horizontal portion of arcuate fasciculus fibers track to pars opercularis, not pars triangularis, in right and left hemispheres: a DTI study. Neuroimage. 2010;52(2):436–444. doi: 10.1016/j.neuroimage.2010.04.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297(5582):846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, et al. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang. 2009;111(1):20–35. doi: 10.1016/j.bandl.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister IG, Wilson SM, Deblieck C, Wu AD, Iacoboni M. The essential role of premotor cortex in speech perception. Curr Biol. 2007;17(19):1692–1696. doi: 10.1016/j.cub.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol. 2003;553(Pt 2):665–679. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22(1):29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005a;93(1):95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, et al. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005b;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112(Pt 1):1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16(2):289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis. 2004;17(1):35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci. 2010;22(6):1299–1318. doi: 10.1162/jocn.2009.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13(4):419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cogn Neurosci. 2003;15(7):948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55(12):1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009;45(9):1035–1042. doi: 10.1016/j.cortex.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153(743):1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Theoret H, Kobayashi M, Valero-Cabre A, Pascual-Leone A. Exploring paradoxical functional facilitation with TMS. Suppl Clin Neurophysiol. 2003;56:211–219. doi: 10.1016/s1567-424x(09)70224-7. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2005;163(1):1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Wagner TA, Zahn M, Grodzinsky AJ, Pascual-Leone A. Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans Biomed Eng. 2004;51(9):1586–1598. doi: 10.1109/TBME.2004.827925. [DOI] [PubMed] [Google Scholar]
- Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66(2):155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Thiel A, Rubi-Fessen I, Hartmann A, Kessler J, Merl P, et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke. 2011;42(2):409–415. doi: 10.1161/STROKEAHA.110.597864. [DOI] [PubMed] [Google Scholar]
- Zahn R, Drews E, Specht K, Kemeny S, Reith W, Willmes K, et al. Recovery of semantic word processing in global aphasia: a functional MRI study. Brain Res Cogn Brain Res. 2004;18(3):322–336. doi: 10.1016/j.cogbrainres.2003.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: 3-D reconstructed right, lateral view of the structural MRI scan for each of the 8 aphasia patients, displaying locations for the cortical ROIs that were suppressed with rTMS: pars opercularis (yellow square), pars triangularis (red triangle), posterior-superior temporal gyrus (orange diamond), and the motor cortex mouth area (green circle).


