Abstract
Cell membrane consists of various lipids such as phosphatidylserine (PS), phosphatidylcholine (PC), and phosphatidlethanolamine (PE). Among them, PS is a molecular marker of apoptosis, because it is located to the inner leaflet of plasma membrane generally but it is moved to the outer leaflet during programmed cell death. The process of apoptosis has been implicated in the fusion of muscle progenitor cells, myoblasts, into myotubes. However, it remained unclear whether PS regulates muscle cell differentiation directly. In this paper, localization of PS to the outer leaflet of plasma membrane in proliferating primary myoblasts and during fusion of these myoblasts into myotubes is validated using Annexin V. Moreover, we show the presence of PS clusters at the cell-cell contact points, suggesting the importance of membrane ruffling and PS exposure for the myogenic cell fusion. Confirming this conclusion, experimentally constructed PS, but not PC liposomes dramatically enhance the formation of myotubes from myoblasts, thus demonstrating a direct positive effect of PS on the muscle cell fusion. In contrast, myoblasts exposed to PC liposomes produce long myotubes with low numbers of myonuclei. Moreover, pharmacological masking of PS on the myoblast surface inhibits fusion of these cells into myotubes in a dose-dependent manner
Keywords: phosphatidylserine, myoblast, myotube, fusion, liposome, annexin V
1. Introduction
The cell plasma membrane largely consists of a phospholipid bilayer containing phopsphatidylcholine (PC), phosphatidylserine (PS) and phosphatidylethanolamine (PE) [1,2]. PS is a hallmark of apoptosis because in normal healthy cells it is localized to the inner lipid layer, or leaflet, of the plasma membrane, but during early apoptosis PS localizes to the outer leaflet by phospholipid(s) scramblase [3,4]. The exposed PS recruits various immune cells and in particular is one of the ‘eat me’ signals present on the surface of apoptotic cells signaling macrophage engulfment [5].
During muscle maintenance and repair, muscle stem or satellite, cells activate, proliferate and give rise to myoblasts, which can both proliferate and differentiate into myotubes. Myotubes are the final product of the muscle lineage, where each cell is post-mitotic and multinucleated and is produced by the fusion of many myoblasts [6]. Some growth factors [7–9], macrophages [10,11], and leukocytes in general [12] can control muscle regeneration and specifically, cell fusion. However, the molecular nature of fusogen(s) remains unknown and it is yet to be determined what specific molecules in the plasma membrane of myoblasts play a crucial role during fusion of these cells into myotubes or myofibers. To date, several molecules that are thought to control myoblast fusion, such as caveolin-3 [13,14], myoferin [15] and nephrin [16], were reported to cluster at the cell membrane, specifically localizing at the cell-cell contact regions during myotube formation. However, none of these molecules were demonstrated to directly regulate the membrane fusion process. Additionally, damaging stimuli that are known to promote apoptosis as well as direct experimental induction of apoptosis have been shown to enhance myotube formation [17,18]. However, no molecular mechanism connecting apoptosis with myoblast fusion was revealed, until now.
In this paper, we demonstrate that one of the key membrane-bound regulators of myoblast fusion into myotubes is PS, which provides the first molecular identification of a fusogen and gives an explanation as to why induction of apoptosis promotes terminal muscle differentiation.
2. Materials and Methods
2.1. Cell culture of mouse primary myoblasts
Primary myoblasts were isolated from young (2–4 month) C 57/Bl6 mice (Jackson Lab., ME) as described [20]. Briefly, tibialis and gastrocnemius muscles were dissociated into myofibers by a digest for 1 hr at 37°C in DMEM with 250 units/ml Collagenase Type II (Sigma-Aldrich, MO), containing 1% Penicillin-Streptomycin. Digested muscles were washed with PBS two times and triturated into myofibers in Ham’s F10 (Mediatech, VA), 20% Bovine growth serum (BGS; Hyclone, IL) and 1% Penicillin-streptomycin. In order to get primary myoblast from myofibers, myofibers were incubated in Ham’s F10 including 1% Pnicillin-Streptomycin and 2 U/ml Dispase for 1 hr at 37°C incubator with shaking. Isolated satellite cells were cultured in growth media (Ham’s F10, 20% BGS, 5 ng/ml bFGF (Invitrogen, CA) and 1% Penicillin-Streptomycin). Satellite cells gave rise to primary myoblasts in growth medium. Primary myoblasts were used for no more than 10 passages.
2.2. Preparation of SUV (small, unilamellar vesicles) liposomes
Phosphatidylserine and phosphatidylcholine were purchased from Avanti polar lipids (Alabaster, AL). PS and 50:50% PS:PC SUV liposomes were made as described [21]. Briefly, 2.6 µmole each phospholipid (PL) was prepared in a glass test tube (13 × 100 mm), in chloroform and then dried under a nitrogen stream in the hood, followed by vacuum drying for 1 hr. Dried PL was resuspended with 2.6 ml HBS solution for 1 hr at room temperature and then vortexed to mix. Resuspended PL was sonicated until clear using a bath sonicator (75T, VWR, PA) for 5–10 min. PC, PS and PC:PS liposomes were added at 40, 80, and 80 µM concentrations to myoblasts cultured in DM for 48 hours.
2.3. Immunocytochemistry
Immunostaining was performed as described [19]. Briefly, differentiated myotubes were fixed in 70% EtOH over night at 4°C. Cells were permeabilized with PBS + 1% FBS + 0.25% Triton X-100, incubated with 1 µg/ml of primary antibodies for 1 hr at room temperature, washed in staining buffer (PBS + 1% FBS), incubated with fluorochrome conjugated secondary antibodies and Hoechst, washed again and mounted.
2.4. Differentiation of mouse primary myoblasts
Primary myoblasts were seeded onto 6 well plates at a density of 5 × 105 cells/well in growth medium. Culture medium was switched to differentiation medium (DMEM containing 2% Horse serum and 1% Penicillin-Streptomycin), for 48 hrs at 37°C under 5% CO2. Exposed PS was detected using Alexa Fluor® 488 conjugated Annexin V (Invitrogen, CA), as recommended by the manufacturer [23].
2.5. Statistical analysis
Quantified data are expressed as mean ± sd. Significance testing was performed using T-test of variance to compare data from different experimental groups. Three independent experiments were conducted to obtain the means. P-values of < 0.05 were considered statistically significant.
3. Results
3.1. Phosphadidylserine is enriched at cell-cell contact regions during myoblast differentiation into myotubes
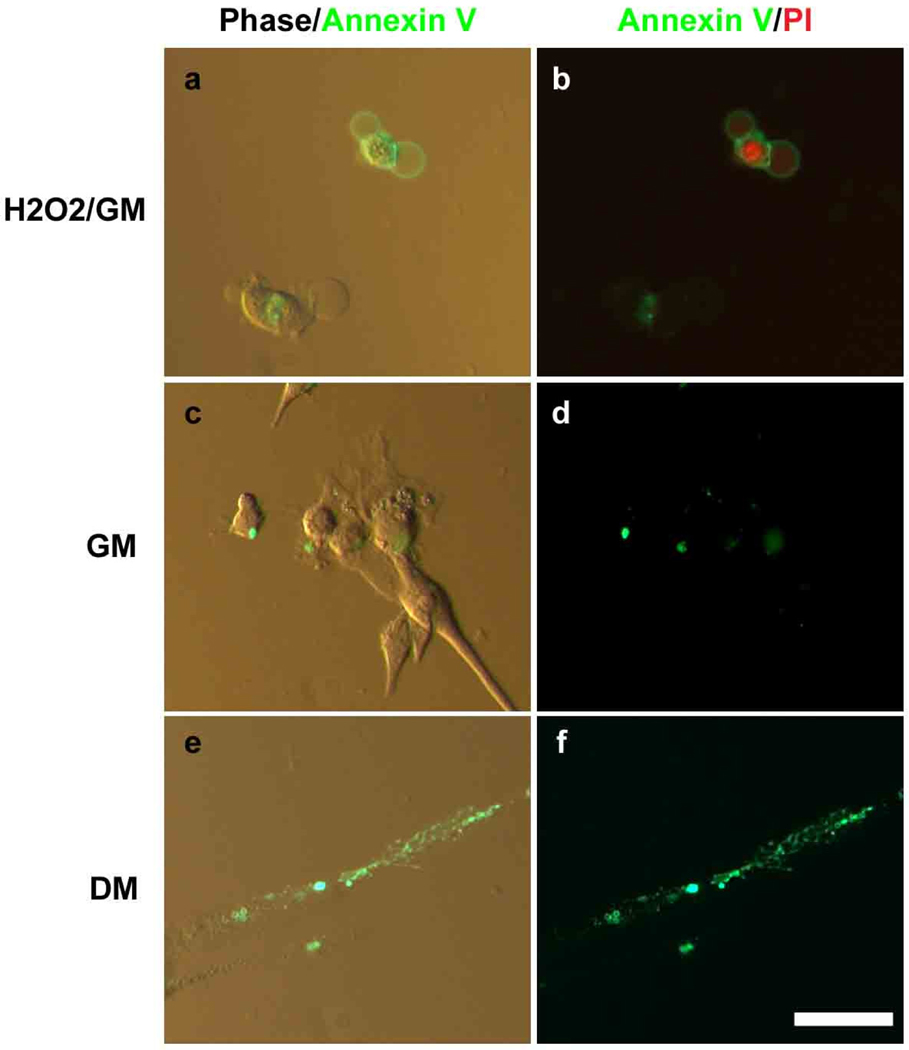
To determine whether phophatidylserine (PS) is exposed on the outer leaflet of the plasma membrane during fusion of primary myoblast into de-novo myotubes, mitogenic growth medium (GM) was replaced by the mitogen-low differentiation medium (DM) where myoblasts normally form myotubes by 48 hrs, and these cell cultures were stained by Alexa Fluor 488 conjugated-Annexin V (Fig. 1). H2O2 treated myoblasts were used as a positive control for PS translocation and Annexin V staining, as well as for propidium iodide (PI) staining. All cells were co-stained by PI in order to clearly distinguish membrane permeable (apoptotic) cells from live, fusing cells. H2O2 treated cells were easily detected by PI and Annexin V, indicating they had either localized PS to the outer surface of their membranes (Fig. 1a, b) or their membranes were antibody permeable. Some Annexin V staining was seen in live, PI-excluding myoblasts in GM (Fig. 1c, d), indicating these cells had localized PS to the outer leaflet of the plasma membrane. In DM, however, fused myotubes which excluded PI and thus were not apoptotic, had significantly more Annexin V staining than myoblasts cultured in GM, and furthermore, the highest presence of PS clusters was identified at the cell-cell contact regions of apparently fusing myoblasts (Fig. 1e, f). These results reveal that re-location of PS from the inner to the outer leaflet of the plasma membrane in myoblasts and myotubes is not caused by the process of apoptosis and might be specific to cell-cell fusion.
Figure 1.
PS was exposed on the outer leaflet of the plasma membrane and was enriched at the cell-cell contact regions during myoblast differentiation into myotubes. H2O2 treated myoblasts were used as a positive control for apoptosis and for PS translocation (a, b). Apoptotic cell nuclei were detected with PI (red). The myoblasts were cultured in GM (c, d) and DM (e, f) for 48 hours and then stained using Annexin V (green). Scale bar, 20 µm.
3.2. PS treated myoblasts form robust myotubes
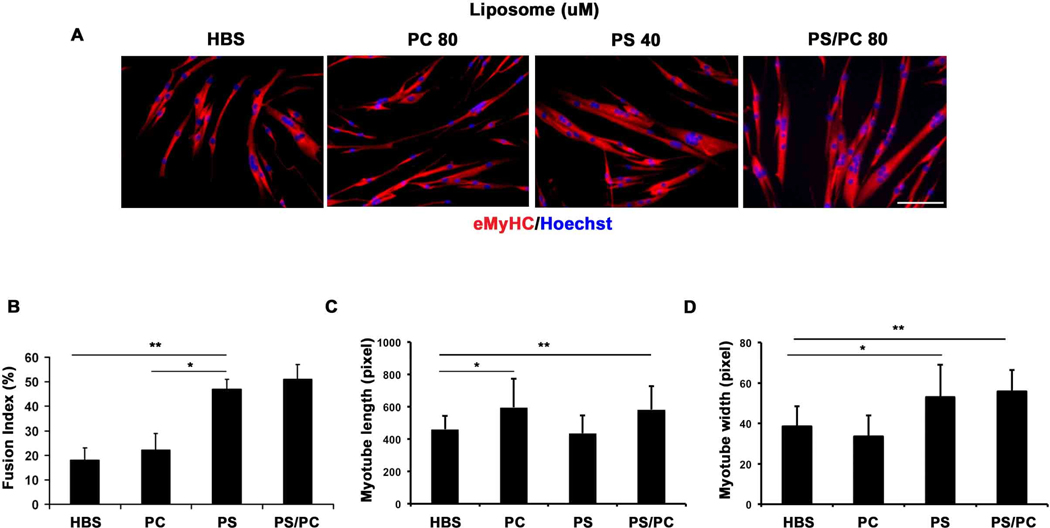
In order to address whether PS just correlates with or actually causes fusion of primary myoblasts into multinucleated myotubes, we generated PS liposomes (as well as a negative control PC liposomes), and added them to myoblasts that were cultured for 48 hrs in DM. Myoblast treated with PS liposomes and a 50:50% mixture of PS:PC liposomes, but not with PC liposomes alone, displayed greatly enhanced fusogenic properties, based on the quantification of the width of the de-novo formed, eMyHC expressing myotubes which have more than 2 nuclei, and on the counts of the myonuclei in these myotubes (Fig. 2A, quantified in B). Myoblasts treated with PC liposomes alone formed long narrow myotubes with a low number of myonuclei, suggesting a defect in myogenic cell fusion (Fig. 2 C). In contrast, the width and the number of myonuclei (fusion index) of de-novo myotubes were enhanced by adding PS or PS:PC liposomes. PS:PC liposomes increased the width, length and fusion index of the myotubes, suggesting that while PS directly and specifically enhanced the myoblast fusion, the high concentration of liposomes (80 uM) in PC and PS:PC cultures could have an indirect effect on myotube length. These results strongly suggest that PS liposomes on the myoblast cell surface directly enhance cell-cell contacts and promote myogenic fusion into multinucleated myotubes of larger width and with more myonuclei.
Figure 2.
PS treated myoblasts form robust myotubes. (A) PS 100%, PS:PC (50%:50%) or PC 100% liposomes were added to myoblasts that were cultured in DM for 48 hours and then fixed with 70% EtOH for 24 hours and stained with anti-eMyHC specific antibody (red). Nuclei were stained with Hoechst. Scale bar, 100 µm. (B) The fusion index was calculated by quantifying the percent of myonuclei in myotubes that have more than 2 nuclei, out of the total cell nuclei number. (C, D) The length and the width of the de-novo formed myotubes that have more than 2 nuclei were quantified. *p= 0.0006; **p= 0.0004 (B). *p= 0.0388; **p=0.0398 (C). *p=0.0248; **p=0.0024 (D).
3.3. Myoblast fusion index is decreased by masking PS with Annexin V or PS-specific antibody
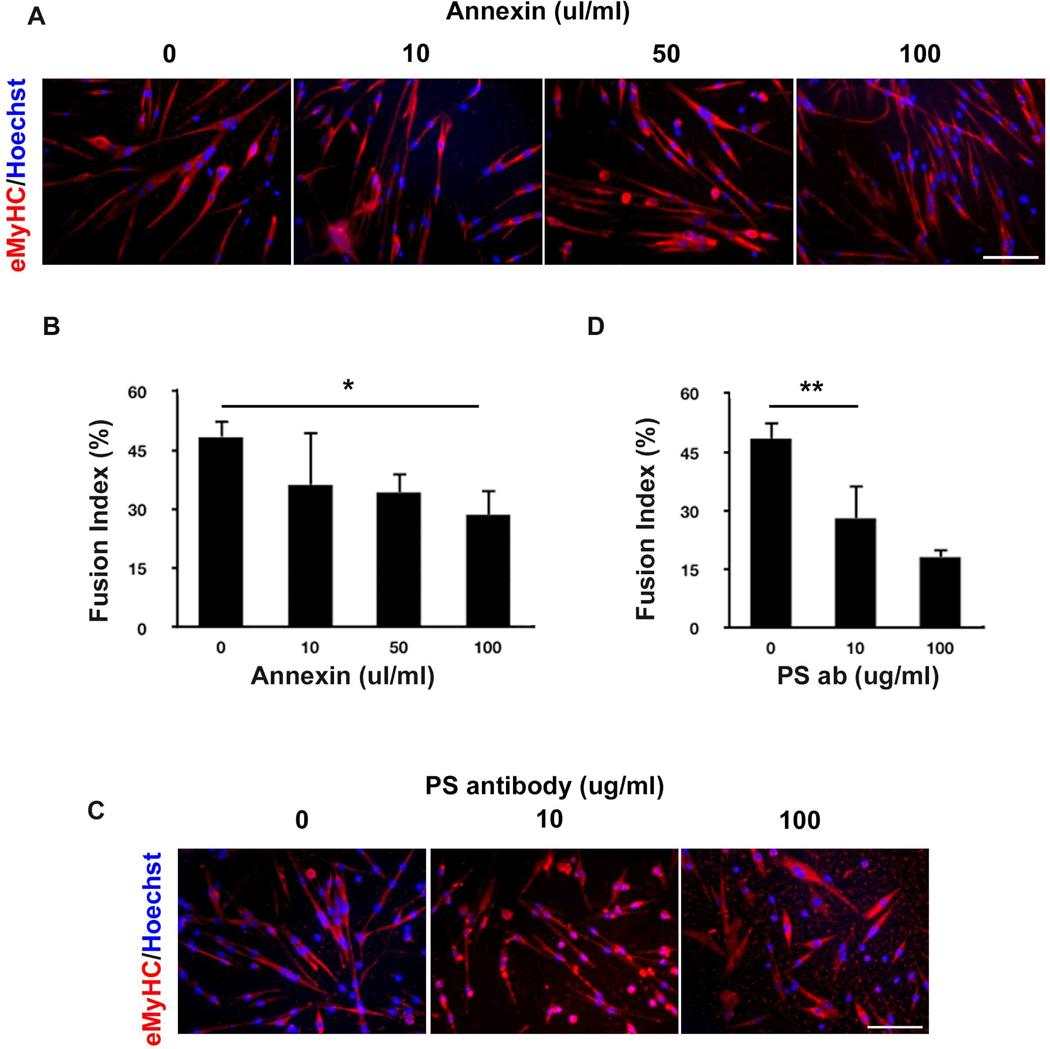
To confirm and extrapolate these data, we blocked PS on the cell surface of myoblasts cultured in DM by treating these cells with Annexin V or with PS-specific antibody, and assayed the ability of these myoblasts to fuse into myotubes. Indeed, the myoblast fusion index was significantly decreased in a dose-dependent manner when PS was experimentally masked (Fig. 3A, C, quantified in B, D). These data establish that translocation of the PS to the outer leaflet of the plasma membrane directly and positively regulates the fusion of primary myoblasts into multinucleated myotubes.
Figure 3.
Myoblast fusion index is decreased by masking PS with Annexin V or PS-specific antibody. (A) Annexin V or (B) PS-specific antibody was added to myoblasts in DM for 48 hours. De-novo myotubes were detected by immunostaining with eMyHC-specific antibody. Scale bar, 100 µm. The percent of myonuclei in myotubes out of total cell nuclei number was quantified for Annexin V (C) and for PS-specific antibody (D). *p=0.0002; **p=0.0005.
4. Discussion
Phosphatidylserine (PS) is a hallmark of eukaryotic cell apoptosis and its translocation from the inner to outer leaflet of the membrane is well known [3,4]. While previous work suggested that some membrane bound proteins may regulate myoblast fusion and that apoptosis generally promotes myotube formation, the molecular regulator of this event remained unknown and the mechanism by which apoptosis promotes myotube generation was undefined. This work is the first to provide a molecular explanation to both of these questions, as it occurs in primary mouse myoblasts that were freshly generated from muscle stem cells. Specifically, we show that PS is moved to the outer leaflet of the plasma membrane in primary myoblasts and that this process plays a crucial causal role during myoblast fusion. Even though PS was localized to the outer leaflet in myoblasts fusing into myotubes, these cells were not undergoing apoptotic pathway. Hence, these data uncover that membrane ruffling occurs independently of apoptosis, which changes the dominant paradigm. As shown in figure 1, some of PS is present on the outer membrane leaflet of proliferating myoblasts [24]. The function of exposed PS during myoblast cell proliferation is unknown, but we propose that it predisposes these cells for the fusion events. Interestingly, according to a previous report, the presence of PS at the cell-cell contact regions in C2C12 and H9C2 cell lines was only transient [25]. However, our data in primary myoblasts suggest that PS is present in the outer membrane leaflet broadly and persistently, particularly when these myogenic cells are cultured for 48 hours in DM that is known to promote myoblast differentiation into myotubes. Thus, our work not only validates but also greatly clarifies the localization of PS in proliferating and differentiating muscle progenitor cells.
Importantly, when PS liposomes were added exogenously, fusion of myoblasts into multinucleated myotubes was significantly and specifically enhanced; conversely, masking of PS with Annexin V or an antibody, inhibited the fusogenic properties of myoblasts (Fig. 2). Therefore, PS localization to the outer membrane that is unrelated to cell-death, directly promotes the physiological fusogenic properties of primary myoblasts. Future identification of the PS receptor in myoblasts will further enhance our understanding of the cell-cell fusion process and of the molecular regulation of muscle regeneration.
Highlights.
PS broadly and persistently trans-locates to the outer leaflet of plasma membrane duirng myoblast fusion into myotubes. Robust myotubes are formed when PS liposomes are added exogenously. PS increases the width of de-novo myotubes and the numbers of myonuclei, but not the myotube length. Annexin V or PS antibody inhibits myotube formation by masking exposed PS.
Acknowledgements
We would like to thank Dr. Michael Conboy for helpful discussion and critical reading of the manuscript. This work was supported by NIH/NIA AG 027252 and CIRM RN1-00532 grants to IMC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236:11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 2.Verkleij AJ, Zwaal RF, Roelofsen B, et al. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 3.Fadok VA, Voelker DR, Campbell PA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 4.Smeets EF, Comfurius P, Bevers EM, et al. Calcium-induced transbilayer scrambling of fluorescent phospholipid analogs in platelets and erythrocytes. Biochim Biophys Acta. 1994;1195:281–286. doi: 10.1016/0005-2736(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 5.Li W. Eat-me signals: Keys to molecular phagocyte biology and "appetite" control. J Cell Physiol. 2011 doi: 10.1002/jcp.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidekamm E, Schudt C, Brdiczka D. Physical properties of muscle cell membranes during fusion. A fluorescence polarization study with the ionophore A23187. Biochim Biophys Acta. 1976;443:169–180. doi: 10.1016/0005-2736(76)90500-9. [DOI] [PubMed] [Google Scholar]
- 7.Schabort EJ, van der Merwe M, Niesler CU. TGF-beta isoforms inhibit IGF-1-induced migration and regulate terminal differentiation in a cell-specific manner. J Muscle Res Cell Motil. 2011;31:359–367. doi: 10.1007/s10974-011-9241-1. [DOI] [PubMed] [Google Scholar]
- 8.Griffin CA, Apponi LH, Long KK, et al. Chemokine expression and control of muscle cell migration during myogenesis. J Cell Sci. 2010;123:3052–3060. doi: 10.1242/jcs.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uaesoontrachoon K, Yoo HJ, Tudor EM, et al. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Pajcini KV, Pomerantz JH, Alkan O, et al. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol. 1995;54:121–128. doi: 10.1097/00005072-199501000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Velica P, Khanim FL, Bunce CM. Prostaglandin D2 inhibits C2C12 myogenesis. Mol Cell Endocrinol. 2010;319:71–78. doi: 10.1016/j.mce.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Stoppani E, Rossi S, Meacci E, et al. Point mutated caveolin-3 form (P104L) impairs myoblast differentiation via Akt and p38 signalling reduction, leading to an immature cell signature. Biochim Biophys Acta. 2011;1812:468–479. doi: 10.1016/j.bbadis.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Volonte D, Peoples AJ, Galbiati F. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol Biol Cell. 2003;14:4075–4088. doi: 10.1091/mbc.E03-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posey AD, Jr, Demonbreun A, McNally EM. Ferlin proteins in myoblast fusion and muscle growth. Curr Top Dev Biol. 2011;96:203–230. doi: 10.1016/B978-0-12-385940-2.00008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn RL, Huang P, Kawahara G, et al. A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc Natl Acad Sci U S A. 2009;106:9274–9279. doi: 10.1073/pnas.0904398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray TV, McMahon JM, Howley BA, et al. A non-apoptotic role for caspase-9 in muscle differentiation. J Cell Sci. 2008;121:3786–3793. doi: 10.1242/jcs.024547. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi K, Dohmae N, Morishima N. Endoplasmic reticulum stress increases myofiber formation in vitro. FASEB J. 2007;21:2994–3003. doi: 10.1096/fj.06-6408com. [DOI] [PubMed] [Google Scholar]
- 19.Conboy MJ, Cerletti M, Wagers AJ, et al. Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:165–173. doi: 10.1007/978-1-60761-063-2_11. [DOI] [PubMed] [Google Scholar]
- 20.Conboy MJ, Conboy IM. Preparation of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:149–163. doi: 10.1007/978-1-60761-063-2_10. [DOI] [PubMed] [Google Scholar]
- 21.Smith SA, Morrissey JH. Rapid and efficient incorporation of tissue factor into liposomes. J Thromb Haemost. 2004;2:1155–1162. doi: 10.1111/j.1538-7836.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 22.Conboy IM, Conboy MJ, Smythe GM, et al. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 23.Koopman G, Reutelingsperger CP, Kuijten GA, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 24.Krijnen PA, Sipkens JA, Molling JW, et al. Inhibition of Rho-ROCK signaling induces apoptotic and non-apoptotic PS exposure in cardiomyocytes via inhibition of flippase. J Mol Cell Cardiol. 2010;49:781–790. doi: 10.1016/j.yjmcc.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 25.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]