Abstract
Drug dependence is characterized bydysregulation of brain reward systems and increased sensitivity to stress. Chronic exposure to drugs of abuse is associated with increased expression of the neuropeptide dynorphin, the endogenous ligand for kappa opioid receptors (KORs). Activation of KORs causes depressive- and aversive-like responses in rodents, raising the possibility that drug-induced upregulation of dynorphinplays a role independence-associated negative states. Here we used “binge” exposure to cocaine (3 daily intraperitoneal injections of 15 mg/kg for 14 days) to examine the development of dependence-like behavior in the intracranial self-stimulation (ICSS) test and the forced swim test (FST). When rats were tested immediately before their first scheduled injection of each day—a period of drug withdrawal corresponding to 20 hr after their last injection on the previous day—there were exposure-dependent increases in ICSS thresholds (a putative indicator of anhedonia) and decreases in latencies to immobility in the FST (a putative indicator of behavioral despair). Administration of the long-lasting KOR antagonist norBNI (20 μg, intracerebroventricular) before the beginning of the binge regimen attenuated the development of cocaine withdrawal-induced anhedonia in the ICSS test. In contrast, administration of norBNI in the midst of the binge regimen had no effect on expression of cocaine withdrawal-induced anhedonia in the ICSS test, although it did attenuate despair-like behavior in the FST. These data suggest that blockade of KORs before exposure to a stressor (in this case, cocaine withdrawal or forced swimming) can attenuate the development of stress-induced behavioral adaptations.
Keywords: intracranial self-stimulation, addiction, dynorphin, binge cocaine, norBNI
1. Introduction
Drug abuse can lead to dependence, which is characterized by negative affective states, including anhedonia (defined as an inability to experience pleasure from rewarding stimuli), anxiety, and altered stress responses during drug withdrawal (Koob and Le Moal, 2001). Combined with a tolerance to the hedonic effects of the drug, these negative affective states are thought to be a driving force of craving and relapse (Koob and Le Moal, 2001). In human studies of relapse, most people report negative emotional states just prior to the relapse, with anxiety and depression being the most common moods described (Brownell et al., 1986). Stress can potentiate negative affective states in drug abstinent people (Fox et al., 2008) and has been shown to trigger craving and relapse (Shaham et al., 2000; Sinha et al., 2006). Taken together, these findings suggest that preventing or reversing the neurobiological changes responsible for drug-induced negative affective states would decrease addictive behavior.
One well-documented neurobiological change in response to chronic exposure to psychostimulants, opiates, nicotine, and ethanol is an increase in activity of the kappa opioid receptor (KOR) system within brain circuits related to motivated behavior (for review see Shippenberg et al., 2007).This includes elevations in levels of the KOR ligand dynorphin, KOR receptors, and coupling of KORs to G-proteins (Hurd and Herkenham, 1993; Mathieu-Kia and Besson, 1998; Piras et al., 2010; Spangler et al., 1993; Zhou et al., 2008). Activation of KORs produces depression or depressive-like behaviors in humans and rodents (Bals-Kubik et al., 1989; Pfeiffer et al., 1986; Todtenkopf et al., 2004; Wadenberg, 2003), whereas KOR blockade has antidepressant-like effects (Mague et al., 2003; McLaughlin et al., 2003; Newton et al., 2002; Pliakas et al., 2001).Dynorphin has also been shown to play a role in the dysphoric component of stress (Land et al., 2008), and KOR antagonists have anxioytic effects in rodents (Knoll et al., 2007).Taken together, these findings raise the possibility that KOR activation contributes to drug withdrawal-induced negative affective states. Indeed, several lines of evidence support a connection between KOR function and the motivational aspects of drugs of abuse: KOR agonists can under some circumstances potentiate the reinforcing effects of cocaine (McLaughlin et al., 2006; Negus, 2004), whereas KOR antagonists can prevent stress-induced reinstatement of drug seeking behavior(Aldrich et al., 2009; Beardsley et al., 2005) and attenuate drug self-administration under conditions of dependence (Walker and Koob, 2007; Wee et al., 2009).In addition, KOR antagonists block aspects of nicotine-associated withdrawal (Jackson et al., 2010).
The purpose of the present study was to examine the role of KORs in the development and expression of behavioral signs of cocaine withdrawal. We hypothesized that if KORs play a crucial role in cocaine withdrawal-induced negative affective states, then the KOR antagonist norBNI would attenuate behaviors that reflect these states. We treated rats for 14 days with a ‘binge’ pattern of cocaine administration, a treatmentregimen that has been characterized using behavioral (Unterwald et al., 1994), neurochemical (Maisonneuve et al., 1995), and molecular(Spangler et al., 1993; Yuferov et al., 2003)endpoints, in order tostandardize drug exposure among rats. This pattern of “binge” cocaine mimics common patterns of cocaine abuse in humans (Kreek and Koob, 1998) and has been shown to produce withdrawal-associated anhedonia (Goussakov et al., 2006).We measured the effects of withdrawal from binge pattern cocaine administration on brain stimulation reward using intracranial self-stimulation (ICSS), a test that is highly sensitive to the function of brain reward systems and thus optimally suited to quantifythe onset and offset of motivational symptoms of cocaine withdrawal.We administered the KOR antagonist norBNI either before the start ofthe cocaine binge regimen(to assess the role of KORs in the development of negative affective states)or in the middle of the regimen, after signs of anhedonia had already developed(to assess the role of KORs in the expression of negative affective states). When we found that norBNI did not block cocaine-induced anhedonia once ithad been established, we used a second behavioral test (the forced swim test [FST]; Mague et al., 2003) to determine if KOR antagonismduring a binge could block other facets of withdrawal-induced depressive-like states.
2. Experimental Procedures
2.1. Subjects and surgery
A total of 194adultmale Sprague-Dawley rats (Charles River Laboratories) were used for these studies. Upon arrival, rats were group-housed (3/cage) with a 12h/12h light/dark cycle and all experiments were conducted during the light phase. Rats were maintained on ad libitum food and water. Prior to surgery, each rat (∼350 g, 3 mos of age) was anesthetized with sodium pentobarbital (65 mg/kg, IP; Abbott Laboratories, North Chicago, IL) supplemented with subcutaneous atropine (0.25 mg/kg, Sigma, St. Louis, MO) to minimize bronchial secretions, and immobilized in a stereotaxic instrument. For studies involving only ICSS electrodes, 24 rats were implanted with stainless steel monopolar electrodes (0.25 mm diameter; Plastics One, Roanoke, VA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to Bregma, 1.7 mm lateral to midline, and 7.8 mm below dura; Paxinos & Watson 1986). The electrodes were coated with polyamide insulation except at the tip. A non-insulated stainless steel wire was used as the anode and wrapped around a stainless steel screw embedded in the skull just posterior to the electrode, and the entire assembly was coated with acrylic cement. For studies involving ICSS electrodes and intracerebroventricular (ICV) administration of norBNI, 92 rats were implanted with a stainless steel guide cannula (26-gauge; Plastics One) with an internal dummy stylet extending 1.0 mm beyond the guide cannula tip aimed at the lateral ventricle (0.8mm posterior to Bregma, 1.4mm lateral to midline, and 3.4mm below dura (final depth); Paxinos & Watson 1986). A stimulating electrode was then implanted into the right medial forebrain bundle at the level of the lateral hypothalamus at an angle 10 degrees posterior to the normal (4.2mm posterior to Bregma, 1.7mm lateral to midline, and 7.9mm below dura; Paxinos & Watson 1986). The electrode and cannulae were fixed in place with four skull screws and dental cement. For forced swim test studies involving ICV administration of norBNI, thirty rats were implanted with ICV guide cannula as above. Following surgery, the rats were housed singly. All animal procedures were approved by the Institutional Animal Care and Use Committee of McLean Hospital in strict accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (1996).
2.2. Intracranial self-stimulation
After one week of recovery from surgery, rats were trained to respond for brain stimulation using a continuous reinforcement schedule (FR1) at 158 Hz, where each lever press earned a 500 ms train of square wave cathodal pulses (100 ms per pulse), as described (Carlezon and Chartoff, 2007). The stimulation current was adjusted (final range: 180 – 250 μA) for each rat to the lowest value that would sustain a reliable rate of responding (average of 40 responses per 50 s). After the minimal effective current was found for each rat, it was kept constant throughout the remainder of training and testing. These procedures have been described in detail (Carlezon and Chartoff, 2007; Ebner et al., 2010).
To characterize the functions relating response strength to reward magnitude (rate-frequency function), a least-squares line of best fit was plotted across the frequencies that sustained responding at 20, 30, 40, 50, and 60% of the maximum rate using customized analysis software. The stimulation frequency at which the line intersected the X-axis (theta 0) was defined as the ICSS threshold (see Carlezon and Chartoff, 2007). Rats were trained until mean ICSS thresholds remained stable (± 10% for 4 consecutive days). For each rat, the average threshold over these 4 days was used as the baseline threshold for the remainder of the experiment.
Upon stable responding, rats were subsequently divided into treatment groups that were balanced with respect to baseline ICSS thresholds. A 14-d regimen of “binge” cocaine treatments was initiated (Spangler et al., 1993), in which rats were given 3 daily intraperitoneal (IP) injections, at 1-hr intervals, of either saline or cocaine (15 mg/kg/injection; Sigma). Rats weighed ∼400 g at the start of the binge cocaine regimen. In sixteen rats, ICSS thresholds were determined for each rat in 60 mintests (four rate-frequency determinations) twenty-one hours after the last cocaine injection of each day (i.e. immediately prior to first cocaine injection of the next day). The mean threshold was compared to the baseline (pretreatment) threshold for each rat to determine a daily percent change from pre-binge baseline. At the end of the 14-day binge, ICSS thresholds were determined on abstinence days 3, 5, and 9 and compared back to pre-binge baseline thresholds. A separate group of eight rats did not receive any ICSS during the 14-d binge regimen; these rats were used to determine if ICSS during withdrawal might be affecting the severity or duration of cocaine withdrawal. Each day these rats were brought to the room in which ICSS was conducted, treated with binge cocaine in their home cages, and immediately returned to the animal colony. ICSS thresholds were determined for these rats on days 3, 5, and 9 after the end of the binge cocaine regimen. For rats with ICSS electrodes and ICVcannulae, vehicle (water) or the KORantagonistnorBNI (nor-Binaltorphimine,20 μg in 2 μl; National Institute of Drug Abuse Drug Supply Program [Bethesda, MD]) was microinjected into the lateral ventricle 24 hr prior to the start of the binge cocaine regimen (d0; see Fig. 3A) or immediately after ICSS thresholds were measured (and just prior to binge cocaine injections) on cocaine injection d7 and again on d10 (see Fig. 4A). The timing of norBNI administration was based on prior reports showing that this KOR antagonist has immediate, but transient (<1hr), antagonist effects at mu and delta opioid receptors, whereas KOR-specific antagonism becomes maximal at >4hr and lasts for up to 85 days (Horan et al., 1992; Potter et al., 2011).ICV infusions were administered using a syringe pump and Hamilton syringes connected to polyethylene (PE) tubing, which was fitted to an injector stylette (30-gauge; Plastics One) extending 1.0mm beyond the end of the cannula. All rats received infusions at a rate of 0.5 μl/min for 4 min, and infusions were followed by a 3 min wait period to allow for diffusion of drug before removing the injection stylettes. Data were analyzed with 2-way ANOVAs (Treatment × Time) with repeated measures on Time. Significant effects were analyzed further using Bonferroni/Dunn's post hoc tests.
Figure 3.
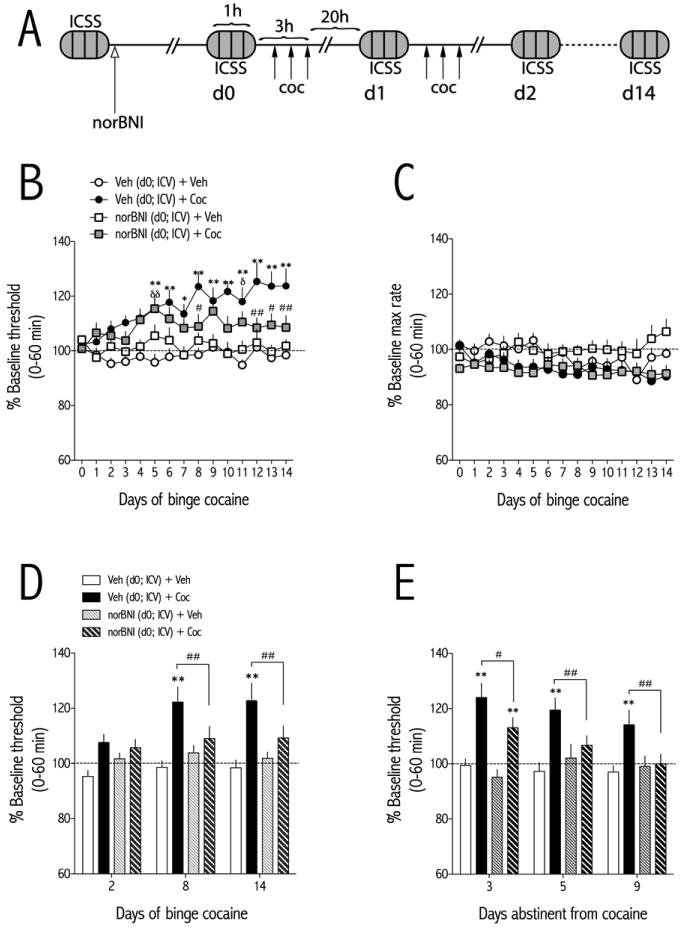
NorBNI(20 μg, ICV) administered 24 hr prior to the start of binge pattern cocaine (3×15 mg/kg/d, IP)attenuates the development of withdrawal-induced increases inICSS thresholds. (A) Schematic of experimental design. NorBNIor vehicleis administered 24 hr prior to d0. ICSS behavior is recorded 1 hr prior to the first cocaine injection of each day. (B) NorBNI attenuates the development of cocaine withdrawal-induced increases inICSS thresholds. Rats were treated with vehicle (ICV) and binge vehicle (n=14), vehicle (ICV) and binge cocaine (n=18), norBNI (ICV) and binge vehicle (n=8), or norBNI (ICV) and binge cocaine (n=16) and ICSS thresholds were measured 21-hr after the end of each daily binge. (C) NorBNI does not significantly affect maximum rates of responding at any time point during the 14-day binge. (D) ICSS thresholds for each treatment are shown at early (day 2), mid (day 8) and late (day 14) stages of the binge pattern cocaine regimen. NorBNI pretreatment significantly blocks binge cocaine-induced elevations in ICSS thresholds at days 8 and 14. (E) After cessation of binge cocaine, ICSS thresholds return to vehicle control levels over time. Data are expressed as a percent of pre-binge baseline thresholds (±SEM) during 60-min tests. *P<0.05, **P<0.01 comparing vehicle (ICV) and binge cocaine to vehicle (ICV) and binge vehicle-treated rats at the same time points;δδP<0.01 comparing norBNI (ICV) and binge cocaine to vehicle (ICV) and binge vehicle-treated rats at the same time points;#P<0.05, ##P<0.01 comparing vehicle (ICV) and binge cocaine to norBNI (ICV) and binge cocaine at the indicated time points. Bonferroni (Dunn's) posthoc tests.
Figure 4.
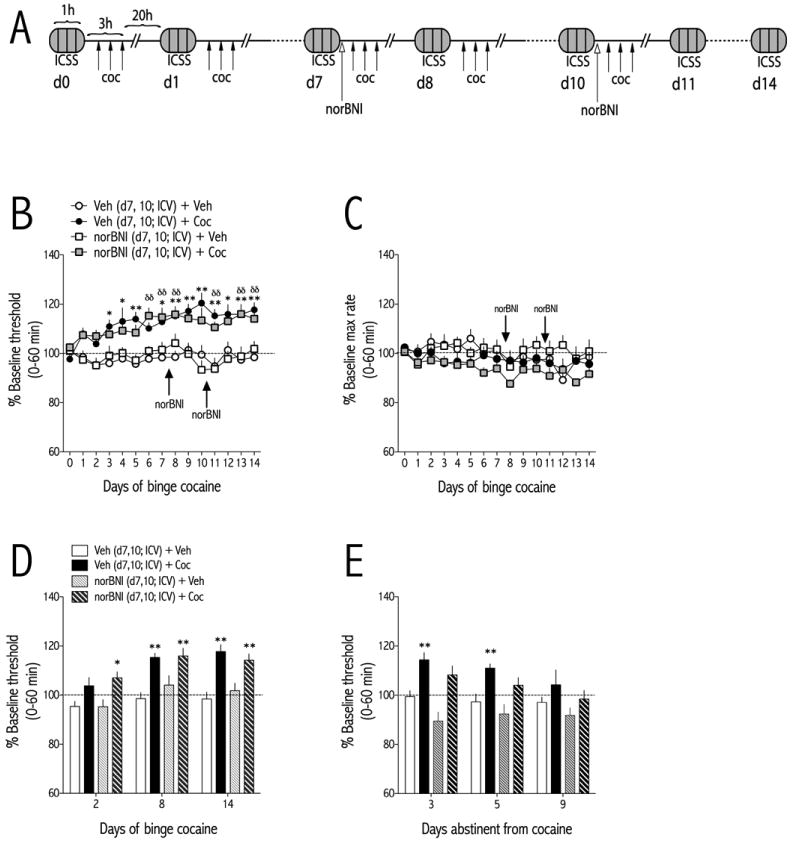
NorBNI (20 μg/injection, d7 and d10; ICV) administered in the middle of a 14-day binge pattern cocaine regimen (3×15 mg/kg/d, IP) has no effect onICSS thresholds. (A) Schematic of experimental design. NorBNIor vehicle is administered immediately after the ICSS test and 1 hr prior to initiation of the cocaine binge regimen on binge days 7 and 10. (B) NorBNI has no effect on the expression of cocaine withdrawal-induced increases in ICSS thresholds. Rats were treated with vehicle (ICV) and binge vehicle (n=14), vehicle (ICV) and binge cocaine (n=14), norBNI (ICV) and binge vehicle (n=7), or norBNI (ICV) and binge cocaine (n=15) and ICSS thresholds were measured 21-hr after the end of each daily binge. (C) NorBNI does not significantly affect maximum rates of responding at any time point during the 14-day binge. (D) ICSS thresholds for each treatment are shown at early (day 2), mid (day 8) and late (day 14) stages of the binge pattern cocaine regimen. (E) After cessation of binge cocaine, ICSS thresholds return to vehicle control levels over time. Data are expressed as a percent of pre-binge baseline thresholds (±SEM) during 60-min tests. *P<0.05, **P<0.01 compared to vehicle-treated rats at the same time points; δδP<0.01 comparing norBNI (ICV) and binge cocaine to vehicle (ICV) and binge vehicle-treated rats at the same time points;#P<0.05, ##P<0.01 comparing vehicle (ICV) and binge cocaine to norBNI (ICV) and binge cocaine at the indicated time points. Bonferroni (Dunn's) posthoc tests.
2.3. Forced swim test (FST)
Seventy-eight rats were used in experiments involving forced swimming; all rats weighed ∼350 gm at the time of the FST. Typically the FST is a 2-d procedure in which rats swim under conditions in which escape is not possible. On the first day, rats are forced to swim for 15 min. The rats initially struggle to escape from the water, but eventually adopt a posture of immobility in which they make only the movements necessary to keep their heads above water. When the rats are retested on the second day, 24 hr later, the latency to become immobile is decreased. This facilitated immobility is reduced when treatments with antidepressant efficacy in humansare administered in the time period between the first and second test (Carlezon et al., 2002; Detke et al., 1995). Since our rats had different treatment histories prior to the first test— as opposed to after the first test—we examined behavior during the first test only.
In one experiment, 48 rats were used to determine whether binge cocaine facilitated immobility in the FST. Separate groups of rats were treated with the binge cocaine regimen for 2, 7, or 14 days, andthe FST was conducted 20-hr after the last cocaine injection. Data were analyzed using a two-way ANOVA (Treatment × Binge Day). In a second experiment, 30 rats were used to determine whether administration of the KOR antagonist norBNI during the cocaine binge (d7 and d10) would block thefacilitatory effects of binge cocaine on immobility in the FST. Rats with ICV cannula were treated with the binge cocaine regimen [3 daily injections, at 1-hr intervals, of either saline or cocaine (15 mg/kg/injection, IP)] for 14 days. Just prior to the first cocaine injection on days 7 and 10, rats were microinjected with vehicle (deionized water) or norBNI (20 μg in 2 μl; ICV), exactly as described above for the ICSS tests. Twenty-four hours after the last cocaine injection, the FST was conducted. Data were analyzed using a one-way ANOVA (Treatment × Binge Day), followed by post hoc simple main effects tests.
For all FST studies, forced swimming was conducted in clear Plexiglas cylinders (65 cm tall × 25 cm diameter) filled to 48 cm with 25°C water. After 15 min of forced swimming, the rats were removed form the water, dried with towels, and placed in a warmed enclosure for 30 min. The cylinders were emptied and cleaned between rats. Forced swimming was videotaped from the side of the cylinders, and trained raters unaware of the treatment conditions scored the videotapes. Latency to become immobile was defined as the time at which the rat first initiated a stationary posture that did not reflect attempts to escape from the water. To qualify as immobility, this posture had to be clearly visible and maintained for≥2.0 sec.
2.4. Histology
After testing, rats with ICSS electrodes and/or ICVcannulaewere overdosed with pentobarbital (130 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brains were removed from the skull and immersed in the fixative for 20 h at 4°C. After fixation, the brains were stored for 4 days in 20% glycerol, 0.1 M PBS (pH 7.4), and then 40- μm sections were cut through the lateral hypothalamus and lateral ventricle at the level of the NAcand cresyl violet stained for histological analysis of electrode and cannula placements, respectively. Only rats in which the ICVmicroinjections and ICSS electrodes were targeted appropriately were included in analyses.
3. Results
3.1 Withdrawal from binge cocaine is associated withincreasedICSS thresholds (anhedonia)
Rats were treated three times daily with cocaine (15 mg/kg/injection, IP), at 1-hr intervals. Twenty hours after the last cocaine injection of each day, a 1-hrICSS test session was conducted, comprising 4, 15-min rate-frequency determinations. For each rat, the mean ICSS threshold (Hz) was compared to the baseline threshold (Hz) obtained during training to obtain a percent change from baseline threshold. The effect of binge cocaine on ICSS thresholds depended on an interaction between treatment and day [F(13,182) = 5.10, p<0.0001; Fig. 1A]. Post-hoc analyses with Bonferroni (Dunn's) corrections showed that ICSS thresholds of cocaine-treated rats were significantly higher than those from vehicle-treated rats from d6 to d14 of the binge cocaine treatment regimen (p<0.01). There was no effect of binge cocaine on the maximum rates of responding for ICSS(Fig. 1B). After cocaine treatments had stopped, ICSS thresholds were measured on d3, d5, and d9 (days abstinent from cocaine). The effect of prior cocaine treatment on ICSS thresholds depended on an interaction between treatment and abstinence day [F(2,28) = 3.51, p<0.05; Fig. 1C]. Post-hoc analyses showed that ICSS thresholds of cocaine-treated rats were significantly higher than those from vehicle-treated rats on d3 (p<0.01) and d9 (p<0.05) of abstinence. ICSS thresholds of cocaine-treated rats had significantly decreased from d3 when measured on d5 and d9 (p<0.05).
Figure 1.
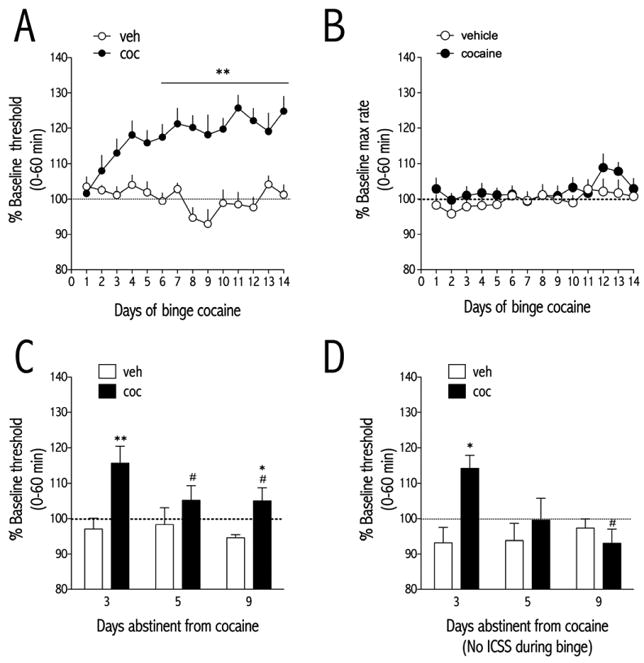
Cocaine administered in a binge pattern (Spangler et al., 1993) elevates ICSS thresholds. (A) Effect of vehicle (Veh; saline) or cocaine (Coc; 3×15 mg/kg/d, IP) on ICSS thresholds (Veh, n=7; Coc, n=9) measured 21-hr after the end of each daily binge. Data are expressed as a percent of pre-binge baseline thresholds (±SEM) during 60-min tests. Treatment with cocaine for 6 - 14 days causes significant elevations in ICSS thresholds during daily withdrawal. (B) Binge pattern cocaine does not affect maximum rates of responding for ICSS. Data are expressed as a percent of pre-binge baseline maximum rates of responding during 60-min tests. (C) ICSS thresholds were significantly elevated in rats treated with binge cocaine compared to rats treated with vehicle on days 3 and 9 of abstinence from binge cocaine. (D) Rats treated with an identical 14-day regimen of binge pattern cocaine, but without daily ICSS exposure, showed a similar increase in ICSS thresholds after cessation of cocaine administration. ICSS thresholds were significantly elevated in rats treated with binge cocaine compared to rats treated with vehicle on day 3 of abstinence from binge cocaine. For A and B, data were collected 21-hr after the last cocaine treatment of each day (and 1 hr before the time of the first injection of the day). For C and D, data were collected at the same time each day. *P<0.05, **P<0.01 compared to vehicle-treated rats at the same time point; #P<0.05, ##P<0.01 compared to abstinence day 3 of corresponding treatment. Bonferroni (Dunn's) posthoc tests.
To determine whether the effect of binge cocaine on ICSS thresholds depended on rats receiving cocaine and ICSS behavior concurrently, a separate group of rats was trained in ICSS but administered the 14-d binge cocaine regimen in the absence of any ICSS behavior. ICSS thresholds were then measured on d3, d5, and d9 after the last cocaine injection. The effect of prior cocaine treatment on ICSS thresholds depended on an interaction between treatment and abstinence day [F(2,12) = 5.51, p<0.05; Fig. 1D]. Post-hoc analysis showed that ICSS thresholds of cocaine-treated rats were significantly higher than those from vehicle-treated rats on d3 (p<0.05) of abstinence.
3.2Withdrawal from binge cocaine is associated with decreased latency to immobility in the FST
Rats were treated three times daily with cocaine (15 mg/kg/injection, IP), at 1-hr intervals. Behavior in the FST was measured 20-hr after the last cocaine injection in rats treated with binge cocaine for either 2, 7, or 14 days. The time to immobility depended on treatment [F(1,42) = 6.29, p<0.05; Fig. 2]. Given the between-subjects design of this experiment, simple main effects tests were performed for each time point. Rats treated with binge cocaine for 14—but not 2 or 7—days had significantly lower latencies to immobility than rats treated with vehicle [F(7,7) = 8.36, p<0.05], reflecting a depressive-like effect.
Figure 2.
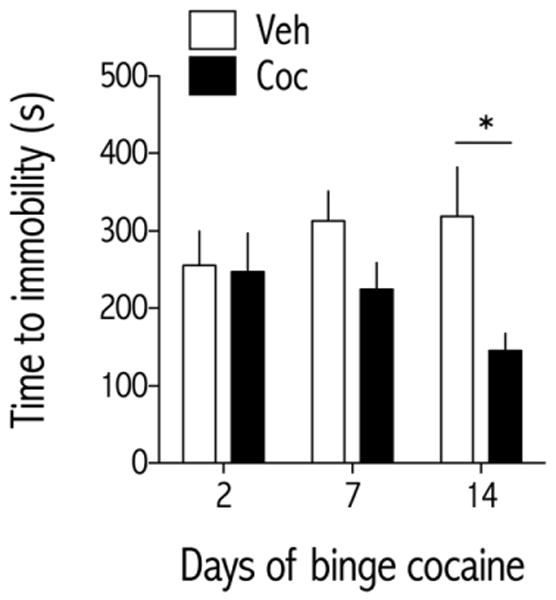
Effect of binge pattern cocaine on FST behavior. Rats were treated with vehicle (Veh, saline) or cocaine (Coc; 3×15 mg/kg/d, IP) for 2, 7, or 14 daysin a between-subject design (N=8 rats/group), and time to immobility was measured during a 15-min forced swim session 24 hr after the last cocaine treatment of the indicated day. Data are expressed as time (s) to become immobile (±SEM). **P<0.01 compared to vehicle-treated rats at the same time point;Simple main effects tests on treatment for each treatment day.
3.3 Blocking KORs before initiation ofa cocaine binge decreases cocaine withdrawal-inducedanhedonia
Rats with ICVcannulae and ICSS electrodes were injected ICV with vehicle (deionized water) or norBNI (20 μg) 24-hr prior to the first cocaine injection, which was administered on d0 (Fig. 3A). Before analyzing the effect of norBNI pretreatment on ICSS thresholds, we compared how ICV vehicle affected ICSS thresholds during binge vehicle administration. Using a 2-way ANOVA (ICV vehicle treatment time × day) with repeated measures on day, we found no significant effect of treatment, day, or interaction on ICSS thresholds. Therefore for this, and subsequent analyses, we combined the ICV vehicle + binge vehicle treatment groups. The effect of KOR blockade on binge cocaine-induced anhedonia depended on an interaction between treatment and day [F(42,728) = 2.24, p<0.0001; Fig. 3B]. Post-hoc analyses with Bonferroni (Dunn's) corrections showed that ICSS thresholds of cocaine-treated rats that received ICV vehicle were significantly higher than those from vehicle-treated control rats starting at d5 of the binge cocaine treatment regimen (p<0.01). In contrast, ICSS thresholds of cocaine-treated rats that received ICVnorBNI were only significantly higher than those from vehicle-treated control rats on d5 of the binge cocaine treatment regimen. Furthermore, thresholds of cocaine-treated rats that received ICVnorBNI were significantly less than those from cocaine-treated rats that received ICV vehicle on days 8,12-14. Maximum rates of responding depended on an interaction between treatment × day [F(42,728) = 1.85, p<0.01; Fig. 3B]. However, post hoc tests revealed no significant differences among groups. To examine the effects of KOR blockade in more detail, we analyzed early (d2), mid (d8), and late (d14) “snapshots” of the effects of norBNI pretreatment on cocaine withdrawal-induced anhedonia. ICSS thresholds depended on main effects of treatment [F(3,52) = 5.63, p<0.01] and time [F(2,104) = 5.34, p<0.01] (Fig. 3D).
After cocaine treatments had stopped, ICSS thresholds were measured on d3, d5, and d9 (days abstinent from cocaine). During abstinence, ICSS thresholds depended on an interaction between treatment and abstinence day [F(6,104) = 3.65, p<0.01; Fig. 3E]. Post-hoc analyses showed that ICSS thresholds of cocaine-treated rats administered ICV vehicle were significantly higher than those from vehicle-treated control rats on d3, d5, and d9 (p<0.01) of abstinence, whereas thresholds of cocaine-treated rats administered ICVnorBNI were only significantly higher than those from controls on d3 (p<0.01). Furthermore,ICSS thresholds of cocaine-treated rats administered ICVnorBNIwere significantly less than those from cocaine-treated rats administered ICV vehicle on abstinence days 3, 5, and 9 (p<0.01).
3.4Blocking KORsduring a cocaine binge has no effect on cocaine withdrawal-induced anhedonia
Rats with ICVcannulae and ICSS electrodes were injected ICV with vehicle (water) or norBNI (20 μg) immediately after ICSS testing on binge cocaine d7 and d10during a 14-d binge cocaine regimen (Fig. 4A). One hour after norBNI infusion, the first cocaine injection of the corresponding days' binge treatment was administered. ICSS thresholds depended on an interaction between treatment and day [F(42,644) = 1.74, p<0.01; Fig. 4B]. Post-hoc analysis showed that ICSS thresholds of cocaine-treated rats that received ICVvehicle were significantly higher than those from vehicle-treated control rats starting on d3 of the binge cocaine treatment regimen (p<0.01). However, norBNI treatment on d7 and d10 had no effect on cocaine-induced increases in ICSS thresholds. ICSS thresholds of cocaine-treated rats that received ICVnorBNI were significantly higher than those from vehicle-treated control rats starting on d6 of the binge cocaine treatment regimen (p<0.01). There was no effect of treatment or day on maximum rates of responding (Fig. 4C).To examine the effects of KOR blockade in more detail, we analyzed early (d2), mid (d8), and late (d14) “snapshots” of the effects of norBNI pretreatment on cocaine withdrawal-induced anhedonia. ICSS thresholds depended on main effects of treatment [F(3,46) = 15.34, p<0.0001] and day [F(2,92) = 12.04, p<0.0001] (Fig. 4D).After cocaine treatments had stopped, ICSS thresholds were measured on d3, d5, and d9 (days abstinent from cocaine). During abstinence, ICSS thresholds depended on main effects of treatment [F(3,46) = 7.00, p<0.001] and day [F(2,92) = 4.23, p<0.05] (Fig. 4E). Post-hoc analyses showed that ICSS thresholds of cocaine-treated rats administered ICV vehicle were significantly higher than those from vehicle-treated control rats on d3 and d5 (p<0.01) of abstinence, whereas thresholds of cocaine-treated rats administered ICVnorBNI were not significantly different from controls on an abstinence day.
3.4Blocking KORsduring a cocaine binge prevents cocaine withdrawal-induced depressive-like effects in the FST
Rats with ICVcannulae were injected with vehicle (deionized water) or norBNI (20 μg) into the lateral ventricle 1-hr prior to the first daily cocaine injection on d7 and d10 of a 14-d binge cocaine regimen. Behavior in the FST was measured 20-hr after the last cocaine injection on d14. The time to immobility depended on treatment [F(3,28) = 4.43, p<0.05; Fig. 5]. Bonferroni's post-hoc analysis showed that rats treated binge cocaineand ICV vehicle had a significantly shorter time to immobility than rats treated with binge cocaine and ICVnorBNI and control rats treated with vehicle (p<0.05).
Figure 5.
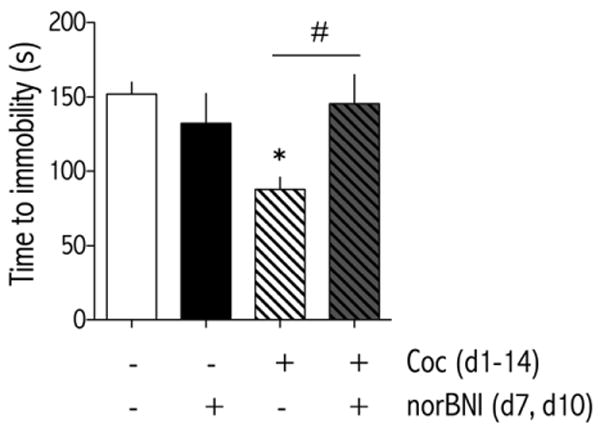
NorBNI (20 μg/injection, d7 and d10; ICV) administered in the middle of a 14-day binge pattern cocaine regimen (3×15 mg/kg/d, IP) blocks withdrawal-associated behavioral despair in theFST. Rats were treated with vehicle (d7, 10; ICV) and binge vehicle (n=8), vehicle (d7, 10; ICV) and binge cocaine (n=8), norBNI (d7, 10; ICV) and binge vehicle (n=8), or norBNI (d7, 10; ICV) and binge cocaine (n=8) and time to immobility was measured during a 15-min forced swim session 24 hr after the last binge cocaine treatment of d14. Data are expressed as time (s) to become immobile (±SEM). *P<0.05 compared to vehicle-treated rats at the same time point. #P<0.05 comparing groups under bar. Bonferroni (Dunn's) posthoc tests.
3.5 Histology
A representative image of an ICV guide cannula is presented in Fig. 6. ICSS electrode placements were indistinguishable from those described previously (Ebner et al., 2010; Muschamp et al., 2011; Tomasiewicz et al., 2008).
Figure 6.
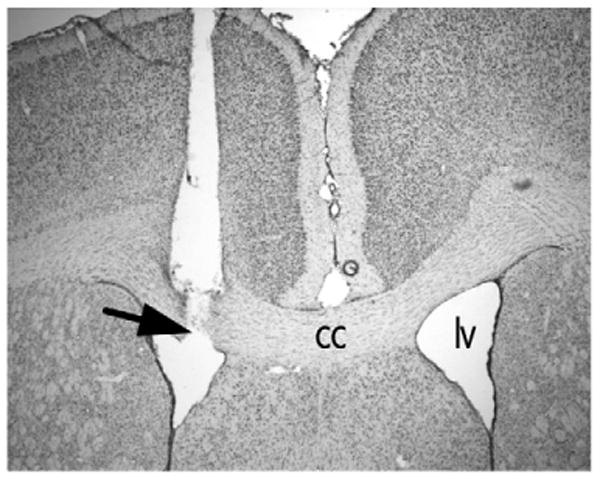
Placement of ICV cannula as determined by cresyl violet stain in a representative coronal brain section at the level of the nucleus accumbens. Arrow shows injector needle track terminating in lateral ventricle. Abbreviations, cc, corpus callosum; lv, lateral ventricle.
4. Discussion
Weshow that blockade of KORs attenuates the developmentbut not the expressionof cocaine withdrawal-induced anhedonia. Specifically, we found that administration of the long-lasting KOR antagonist norBNIinto the lateral ventricle prior to the start of binge pattern cocaine administration decreased cocaine withdrawal-induced increases in ICSS thresholds. In contrast, administration of norBNI in the middle of the regimen, after development of anhedonia, failed to attenuate the expression of elevated ICSS thresholds in cocaine-treated rats. Further studies showed that despite a lack of effect on the expression of already-developed cocaine-induced anhedonia, norBNI completely prevented cocaine withdrawal-induced depressive-like effects upon exposure to a new stressor—forced swimming—suggesting a dissociation in the neurobiological mechanisms subservinganhedonia and increased stress sensitivity after binge cocaine. These findings are consistent with previous studies linking KORs and addictive behaviors (Beardsley et al., 2005; Jackson et al., 2010; Negus, 2004; Walker and Koob, 2007; Wee et al., 2009). In addition, they are broadly consistent with previous work showing that treatment with KOR antagonists before the first exposure to a stress—in this case, cocaine withdrawal or forced swim stress—is highly effective at blocking stress-induced behavioral adaptations (see Knoll and Carlezon Jr, 2010; Knoll et al., 2007). The fact thata regimen of norBNIthat failed to block the depressive effects of cocaine withdrawal that had already developed in the ICSS test was simultaneously effective at blocking depressive like effects in response to a new stressor (FST) raises the possibility that KOR antagonists prevent the development of stress-induced neuroadaptations more readily than they reverse those that have already occurred.
Anhedonia is a hallmark symptom of psychostimulant withdrawaland major depression in humans(APA, 2000). ICSS thresholdsserve as an operational measure of reward function (Ahmed et al., 2002; Barr et al., 2002; Carlezon and Chartoff, 2007), and previous work has shown that withdrawal from chronic cocaine exposure increases stimulation thresholds(Baldo et al., 1999; Goussakov et al., 2006; Markou and Koob, 1991). Here we show that over a period of several days,ICSS thresholds become elevated compared to pre-drug baseline thresholds when measured just prior to each daily binge (in the absence of acute drug effects). Theexpression of decreased reward sensitivity during the 14-day binge cocaine regimen may be analogous to the daily “crash” experienced by human cocaine users(Gawin, 1991), which is thought to reflect motivational dependence on cocaine and contribute tocompulsive drug use(Ahmed et al., 2002; Kenny, 2007; Koob and Le Moal, 2001). Although acute cocaine has profound effects on locomotor activity, cocaine withdrawal-induced anhedonia was not associated with changes in the maximum rates of responding for stimulation, suggesting that motor retardation is not the cause of increased ICSS thresholds. During the first 9 days of abstinence frombinge cocaine administrationICSS thresholds decline linearly until they approach threshold values maintained by binge saline-treated rats. This is generally consistent with clinical observations of the time course of cocaine withdrawal-induced anhedonia and craving(Gawin, 1991; Gawin and Kleber, 1986), and with findings that KOR antagonists decrease reinstatement of drug seeking in animal models of craving (Aldrich et al., 2009; Beardsley et al., 2005; Walker and Koob, 2007; Wee et al., 2009).It is possible that cocaine-induced increases in ICSS thresholds depend on an interaction between binge pattern cocaine administration and ICSS behavior itself. However, we found that rats treated for 14 days with binge pattern cocaine in the absence of daily ICSS showqualitatively similar increases in ICSS thresholds at the end of the binge regimen.
Cocaine addicts report an increased sensitivity to stress that can trigger craving and relapse (Fox et al., 2008; Sinha et al., 2006). The forced swim test is a stress-sensitized behavior in which stress-induced neuroadaptations increase measures of immobility(Knoll and Carlezon, 2010). Although the FST does not model depression per se, it is sensitive to manipulations that have antidepressant and pro-depressive effects in humans (Detke et al., 1995; Mague et al., 2003; Pfeiffer et al., 1986; Pliakas et al., 2001). Inasmuch as long-term exposure to cocaine can produce behavioral adaptations that qualitatively resemble exposure to forced swim stress itself, our data raise the possibility that cocaine and stress cause common neuroadaptations that render rats more prone to depressive-like phenotypes. One such neuroadaptation could be elevated activity of the transcription factor CREB (cAMP response element binding protein) in the nucleus accumbens: drugs of abuse and stress have common effects on CREB function in this region, and mimicking elevated CREB function with viral vectors produces depressive-like phenotypes (Carlezon et al., 1998; Muschamp et al., 2011; Pliakas et al., 2001).
Multiple studies have demonstrated that KOR agonists have depressive-like, and KOR blockade has antidepressant-like, effects in ICSS and FST behaviors (for review see Knoll and Carlezon Jr, 2010). Combined with reports of cocaine-induced elevations in dynorphin systems, it has been suggested that dynorphin is one of the primary mediators of negative affective states (an anti-reward system) following drug withdrawal (Bruchas et al., 2010; Kreek and Koob, 1998; Wee and Koob, 2010). We found that administration of the long-lasting KOR antagonist norBNI prior to the start of a 14-day cocaine binge attenuated—but did not completely prevent—the development of cocaine withdrawal-induced anhedonia:ICSS thresholds initially increased after each day of binge cocaine administration such that they were significantly elevated compared to vehicle-treated control rats by day 5, similar to effects observed in binge cocaine treated rats administered ICV vehicle. However, for the remainder of the binge pattern cocaine regimen, ICSS thresholds in rats treated with ICVnorBNI and cocaine were lower than thresholds in rats treated with ICV vehicleandcocaine and not significantly greater than thresholds in vehicle-treated control rats.During abstinence, reward thresholds of rats treated with norBNI prior to the start of the binge pattern cocaine treatments recovered more quickly to control values, most likely because they never reached maximal levels.This type of outcome raises the possibility that dynorphin is only one of several factors that contribute to the motivational aspects of cocaine withdrawal.
We found that when norBNI was administered to rats after 7 days of binge cocaine—a time point at which cocaine withdrawal-induced anhedonia had already developed—there was no effect on cocaine withdrawal-induced anhedonia.We were surprised that norBNI administered on day 7 had no effect on cocaine-induced elevations of ICSS thresholds, and thought it possible that higher doses might be needed to reverse this effect once it had already developed. Therefore we treated rats with norBNI a second time (day 10), which was also without effect.In contrast, this exact same KOR antagonist treatment regimen completely blocked cocaine withdrawal-induced decreases in latency to immobility in the FST. This is consistent with prior studies showing antidepressant-like effects of KOR antagonists and dynorphin gene disruption in the FST(Mague et al., 2003; McLaughlin et al., 2003; Pliakas et al., 2001). At first one might predict that norBNI itself should increase time to immobility, as seen in previous studies with KOR antagonists (Mague et al., 2003; Pliakas et al., 2001). One potential explanation for this outcome is that in the 1-day version of the FST used in the current study, rats treated with binge vehicle have not experienced a stressor before exposure to forced swimming that is sufficient to triggerneuroadaptations sensitive to blockade by norBNI. In addition, it is important to note that our data suggest that cumulative treatment history can have profound effects on baseline latencies to immobility in the FST. Group-housed rats treated repeatedly with cocaine or vehicle (Fig. 2) show baseline latencies to immobility that are considerably higher than those of singly-housed rats surgically implanted with an ICV guide cannula (Fig. 5), consistent with previous work showing that singly housed rats show greater immobility in the FST than group housed rats in an enriched environment (Konkle et al., 2010). These results emphasize the importance of the use of appropriately matched control groups in these studies.
A parsimonious explanation for the delayed effects of norBNI administered prior to the start of binge cocaine on withdrawal-induced increases in ICSS thresholds is that several days are required for dynorphinto increase to a level where it contributes to withdrawal-associated negative affective states. This is consistent with the finding that it takes three days of binge cocaine treatments to significantly increase preprodynorphin mRNA levels in the caudate putamen (Spangler et al., 1993).Following this logic, release of elevated dynorphin would directly increase ICSS thresholds. However, administration ofnorBNI after 7 days of binge cocaine failed to reduce cocaine withdrawal-induced increases in ICSS thresholds. Alternatively, it is possible that norBNIrequires 7-8 days to become active under these experimental conditions.We do not think this is a likely explanation for several reasons. First, it has been shown that a 24-hr pretreatment with norBNI is sufficient to completely block KOR agonist-induced increases in ICSS thresholds (Potter et al., 2011).Second, norBNIadministered on days 7 and 10 of the 14-day cocaine binge does not have significant effects on ICSS thresholds when measured for the remainder of the binge as well as for nine days after, during cocaine abstinence. Third,norBNI administered on days 7 and 10 blocks cocaine withdrawal-induced decreases in immobility in the FSTmeasured on day 1 of abstinence from binge cocaine. Taken together, these findings suggest that dynorphin is most likely having complex effects on affective state through multiple neural circuits. Neuroadaptations downstream from—but dependent on—KOR activation may ultimately be responsible for cocaine withdrawal-induced increases in ICSS thresholds. In contrast, the ability of norBNI administered during binge pattern cocaine administration to block cocaine-induced increases in immobility in the FST suggests a direct role for swim stress-induced dynorphin release(see also Pliakas et al., 2001).
The neural substrates underlying the effects of KOR activation on cocaine withdrawal-induced negative affective states are not known.KORs are found throughout brain regions that regulate motivated behavior, including the mesolimbic dopamine system, amygdala, and hypothalamic pituitary adrenal (HPA) axis(Mansour et al., 1987). There is substantial evidence implicating the nucleus accumbens in the depressive-like effects of dynorphin: manipulations that elevate dynorphin expression in the nucleus accumbens increase ICSS thresholds, immobility in the FST, and learned helplessness (Muschamp et al., 2011; Newton et al., 2002; Pliakas et al., 2001), and activation of KORs causes profound decreases in dopamine release in the nucleus accumbens(Carlezon et al., 2006; Ebner et al., 2010). Likewise, basal dopamine release is increased in the nucleus accumbens of KOR knockout mice and in response to norBNI(Chefer et al., 2005; Chefer et al., 2006), suggesting that in our study, norBNI might have rewarding properties on its own that counteract cocaine withdrawal-induced anhedonia. However, this is not likely since norBNI had no effect on ICSS thresholds or rates of responding when administered on its own.There is also a growing body of literature demonstrating a relationship between dynorphin and corticotrophin releasing factor (CRF). Dynorphin and CRF are colocalized in the hypothalamus(Roth et al., 1983), and dynorphinstimulates CRF release in rats and humans (Buckingham and Cooper, 1986). ACRF antagonist has been shown to block KOR agonist-induced reinstatement of cocaine seeking in non-human primates with a prolonged history of cocaine exposure (Valdez et al., 2007), which is consistent with the idea that KOR-dependent, downstream effectors are ultimately responsible for cocaine withdrawal-associated negative affective states.Finally, it has been shown that KOR antagonists have anxiolytic effects when microinjected directly into the amygdala(Knoll et al., in press), and LTP-like neuroplasticity occurs in the basolateral amygdala in rats treated with binge cocaine for 7 days (Goussakov et al., 2006). These data raise the possibility that the ability of norBNI to block cocaine withdrawal-induced despair-like behavior is mediated within amygdalar circuitry.
Previous work has suggested the potential utility of KOR antagonists in the treatment of addictive disorders(Aldrich et al., 2009; Beardsley et al., 2005; Jackson et al., 2010; Walker and Koob, 2007; Wee et al., 2009). Our data are broadly consistent with this work, but suggest that KOR antagonists would be most effective if given as a prophylactic treatment—before a stress occurs—as opposed to an acute treatment given after stress-induced behavioral adaptations have already been triggered. From this perspective, KOR antagonists may be particularly usefulin rehabilitating addicts who are free of acute withdrawal symptoms but are at risk for stress-induced relapse to drug use. KOR antagonists may also be useful for treating the anhedonia that can promote self-medication with psychostimulants(Markou et al., 1998).
Highlights.
Withdrawal from binge pattern cocaine produces depressive-like effects in rats.
The kappa opioid receptor antagonist norBNI attenuates the development of withdrawal-induced anhedonia.
NorBNI has no effect once anhedonia is established, but blocks withdrawal-induced behavioral despair.
Acknowledgments
We thank Steve Mague, Brian Gillis, and Katie Famous for technical assistance with these studies. Work supported by National Institute of Health grants DA014789 and MH063266(to WAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci U S A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, D.C.: 2000. [Google Scholar]
- Baldo BA, Koob GF, Markou A. Role of adenosine A2 receptors in brain stimulation reward under baseline conditions and during cocaine withdrawal in rats. J Neurosci. 1999;19:11017–11026. doi: 10.1523/JNEUROSCI.19-24-11017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-Like Effects of the kappa-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nature protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, Renshaw PF. Antidepressant-like effects of cytidine in the forced swim test in rats. Biol Psychiatry. 2002;51:882–889. doi: 10.1016/s0006-3223(01)01344-0. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goussakov I, Chartoff EH, Tsvetkov E, Gerety LP, Meloni EG, Carlezon WA, Jr, Bolshakov VY. LTP in the lateral amygdala during cocaine withdrawal. Eur J Neurosci. 2006;23:239–250. doi: 10.1111/j.1460-9568.2005.04538.x. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular Alterations in the Neostriatum of Human Cocaine Addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Jackson K, Carroll F, Negus S, Damaj M. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-Like Effects of kappa-Opioid Receptor Antagonists in Models of Unlearned and Learned Fear in Rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Kentner AC, Baker SL, Stewart A, Bielajew C. Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J Amer Assoc Lab Animal Sci. 2010;49:427–436. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The Dysphoric Component of Stress Is Encoded by Activation of the Dynorphin kappa-Opioid System. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, Meloni EG, Carlezon WA., Jr Activation of CREB in the Nucleus Accumbens Shell Produces Anhedonia and Resistance to Extinction of Fear in Rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Piras AP, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5′-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–757. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EC. Repeated exposure to the kappa-opioid receptor agonist salvinorin A modulates extracellular signal regulated kinase and reward sensitivity. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, Weber E, Barchas JD, Chang D, Chang JK. Immunoreactive dynorphin-(1-8) and corticotropin- releasing factor in subpopulation of hypothalamic neurons. Science. 1983;219:189–191. doi: 10.1126/science.6129700. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. The Journal of pharmacology and experimental therapeutics. 1994;270:1387–1396. [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. TJ Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG. A Review of the Properties of Spiradoline: A Potent and Selective k-Opioid Receptor Agonist. CNS Drug Reviews. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2007;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob G. The role of the dynorphin–κ opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman J, Koob G. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


