Abstract
Both the neuropeptide, corticotropin-releasing factor (CRF) and the serotonin 1A (5-HT1A) receptor systems have been implicated in anxiety disorders and there is evidence that the two systems interact with each other to affect behavior. Both systems have individually been shown to affect prepulse inhibition (PPI) of the acoustic startle response. PPI is a form of sensorimotor gating that is reduced in patients with anxiety disorders including post-traumatic stress and panic disorder. Here, we examined whether the two systems interact or counteract each other to affect acoustic startle amplitude, PPI and habituation of the startle response. In experiment 1, Brown Norway (BN) and Wistar-Kyoto (WKY) rats were administered ether an intraperitoneal (IP) injection of saline or the 5-HT1A receptor agonist, 8-OH-DPAT 10 min prior to receiving an intracerebroventricular (ICV) infusion of either saline or CRF (0.3 µg). In a second experiment, rats were administered either an IP injection of saline or the 5-HT1A receptor antagonist, WAY 100,635 10 min prior to receiving an ICV infusion of saline or CRF. Thirty min after the ICV infusion, the startle response and PPI were assessed. As we have previously shown, the dose of CRF used in these experiments reduced PPI in BN rats and had no effect on PPI in WKY rats. Administration of 8-OH-DPAT alone had no effect on PPI in either rat strain when the data from the two strains were examined separately. Administration of 8-OHDPAT added to the effect of CRF in BN rats, and the combination of 8-OH-DPAT and CRF significantly reduced PPI in WKY rats. CRF alone had no effect on baseline startle amplitude in either rat strain, but CRF enhanced the 8-OH-DPAT-induced increase in startle in both strains. Administration of WAY 100,635 did not affect the CRF-induced change in PPI and there were no interactions between CRF and WAY 100,635 on baseline startle. The results suggest that activation of the 5-HT1A receptor can potentiate the effect of CRF on endophenotypes of anxiety disorders in animal models.
Keywords: Acoustic Startle Response; Anxiety Models; Corticotropin-Releasing Factor; Prepulse Inhibition; 8-OH-DPAT; WAY 100,635
1. Introduction
1.1Corticotropin-Releasing Factor and Serotonin in Anxiety Models
Corticotropin-releasing factor (CRF), a 41-amino acid peptide, is synthesized in hypothalamic (Vale et al., 1981) and extra-hypothalamic brain regions including the central nucleus of the amygdala, hippocampus, and frontal cortex (Swanson et al., 1983). The neuropeptide acts as both a hypothalamic releasing hormone and a neurotransmitter to affect endocrine, autonomic, and behavioral responses to stress (Bale and Vale, 2004; Gray, 1993). There are two CRF G protein-coupled receptors, CRF1 and CRF2 (Chang et al., 1993; Lovenberg et al., 1995), which are expressed in brain regions known to modulate startle amplitude and prepulse inhibition (PPI) of the startle response, including the basolateral amygdala, hippocampus, and frontal cortex (Swerdlow et al., 2001; Van Pett et al., 2000). Additionally, there is a projection from CRF-containing neurons in the central nucleus of the amygdala to the major dopaminergic, serotonergic and noradrenergic nuclei (see Gray, 1993 for review). Levels of CRF in cerebrospinal fluid are higher in patients with post-traumatic stress disorder (PTSD) than in controls (Baker et al., 1999; Bremner et al., 1997; Sautter et al., 2003).
Within 10 years of the identification of CRF, the anxiogenic effect of the peptide was convincingly demonstrated (Britton et al., 1986; Dunn and Berridge, 1990; Dunn and File, 1987). Over the past 30 years, a considerable body of evidence has demonstrated that CRF has behavioral effects that are independent of HPA axis activation. Both intracerebroventricular (ICV) (Jones et al., 1998; Spina et al., 2002) and intra-bed nucleus of the stria terminalis (BNST) infusion of CRF (Sahugue et al., 2006) increase anxiety-like behavior. Further, CRF overexpressing mice show more anxiety-like behavior (van Gaalen et al., 2002; Stenzel-Poore et al., 1994), and CRF1 receptor knockout (KO) mice show less anxiety-like behavior than wild type (WT) mice (Contarino et al., 1999; Smith et al., 1998; Timpl et al., 1998). Conditional KO of the CRF1 receptor in the limbic system only is anxiolytic even though the pituitary CRF receptor system is intact (Muller et al., 2003). In some studies, the CRF2 receptor also appears to mediate an anxiogenic effect of CRF (Takahashi et al., 2001). However, CRF2 receptor KO mice show more anxiety-like behavior than WT mice (Bale et al., 2000). It appears that the two receptors mediate opposing effects of CRF on some behaviors (Kishimoto et al., 2000; Risbrough et al., 2004).
The serotonin 5-HT1A receptor system has also been shown to have a role in animal models of anxiety and to be altered in clinical anxiety. 5-HT1A receptors are located on both soma and dendrites of serotonergic neurons in the raphe nuclei, as well as on post-synaptic membranes in cortico-limbic brain regions (see Barnes and Sharpe, 1999 for review). The results of a number of studies reveal that mice lacking 5-HT1A receptors show more anxiety-like behavior that WT controls (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Additionally, Breese et al., (2004) showed that a 5-HT1A receptor agonist reduces a stress-induced increase in anxiety-like behavior. 5-HT1A receptor binding is reduced in patients with panic disorder (Nash et al., 2008) and social anxiety disorder (Lanzenberger et al., 2007). Together, these data suggest that activation of the 5-HT1A receptor is anxiolytic.
CRF interacts with the serotonergic dorsal raphe nucleus in complex and dynamic ways to affect behavior (see Valentino et al., 2010 for review). Low-dose CRF decreases extracellular concentrations of 5-HT in the lateral striatum (Price et al., 1998) and nucleus accumbens (Lukkes et al., 2008) while relatively high doses increase 5-HT concentrations in these brain regions. However, both low and high doses of CRF decrease the discharge rate of dorsal raphe neurons (Price et al., 1998). Waselus et al., (2011) suggest that CRF has both direct and indirect effects on the dorsal raphe, which might explain why low and high doses of CRF do not have opposing effects on the discharge rate of dorsal raphe neurons. Restraint stress increases extracellular concentrations 5-HT in the central nucleus of the amygdala, and this effect is blocked by a non-selective CRF receptor antagonist (Mo et al., 2008). Further, CRF and a 5-HT2 receptor agonist act synergistically to increase anxiety-like behavior (Magalhaes et al., 2010). Activation of 5-HT1A receptors can also affect CRF-induced changes in behavior. Selective 5-HT1A receptor agonists attenuate CRF-induced grooming, but do not affect CRF-induced changes in locomotor activity (Lazosky and Britton, 1991). Both CRF and the 5-HT1A receptor system have been individually implicated in anxiety disorders such as PTSD in which the startle response and prepulse inhibition of the response may be altered. However, the combined effects of CRF and drugs that affect the 5-HT1A receptor on these behaviors have not yet been examined.
1.2 The Acoustic Startle Response and Prepulse Inhibition
The acoustic startle response is a reflexive response to a high-intensity acoustic stimulus with a fast rise-time, and is expressed in whole body muscle contractions (see Koch and Schnitzler, 1997 for review). Startle amplitude is diminished if the startling stimulus is preceded by a low-intensity, non-startling prepulse stimulus, a phenomenon referred to as prepulse inhibition (PPI) (Hammond et al., 1972). PPI, a measure of sensorimotor gating, is thought to occur so that processing of the prepulse stimulus is not interrupted by the high-intensity startling stimulus (see Koch and Schnitzler, 1997). PPI can be achieved in humans and animals with nearly identical parameters, making it a useful endophenotype for disorders which are characterized by deficits in sensorimotor gating (see Braff et al., 2001). PPI is reduced in patients with anxiety disorders including panic disorder (Ludewig et al., 2002) and posttraumatic stress disorder (PTSD) (Grillon et al., 1998). In PTSD patients, good habituation of the startle response predicts positive treatment outcome (Jaycox et al., 1998; van Minnen et al., 2002).
Risbrough and Stein (2006) have discussed evidence suggesting that the startle response and PPI are useful tools for assessing the role of CRF in anxiety disorders. CRF increases acoustic startle amplitude in rats (Liang et al., 1992a; Swerdlow, 1989), although this effect is strain-dependent (Conti, 2005; Conti et al., 2002). The effect of CRF on startle amplitude is not altered by lesions of the PVN (Liang et al., 1992b), suggesting that it is not the result of glucocorticoid release. In rats, nonselective CRF antagonists, as well as selective CRF1 receptor antagonists, attenuate the effect of CRF on startle (Schulz et al., 1996; Swerdlow et al., 1989). In mice, blockade of the CRF2 receptor, as well as the CRF1 receptor attenuates the effect of CRF on startle (Risbrough et al., 2003). However, CRF overexpressing mice show a smaller startle response than WT mice (Dirks et al., 2002), perhaps due to other compensatory changes than can occur in transgenic animals. ICV infusion of CRF reduces PPI in both rats (Conti, 2005; Conti et al., 2002; Sutherland et al, 2008; Tejeda et al., 2010) and mice (Risbrough et al., 2004). Bakshi et al., (2011) find that CRF has long-lasting effects on PPI in rats. Additionally, transgenic mice over-expressing CRF show reduced PPI compared to WT controls (Dirks et al., 2002). Repeated infusion of CRF into the basolateral amygdala, but not the prefrontal cortex, reduces PPI (Bijlsma et al., 2011). In mice, the effect of CRF on PPI in is mediated by the CRF1 receptor, while the CRF2 receptor mediates an opposing response (Risbrough et al., 2004). Additionally, the CRF1 receptor blockade, but not the glucocorticoid receptor blockade attenuates the reduction in PPI seen in CRF over-expressing mice (Groenink et al., 2008). However, we have found that the CRF1 receptor does not mediate the effect of CRF on PPI in Brown Norway rats (Sutherland and Conti, 2011).
The 5-HT1A receptor agonist, 8-OH-DPAT, has been shown to increase baseline startle amplitude (Davis et al., 1986). However, 5-HT1A receptor knockout mice and wild-type mice show equivalent baseline startle (Dirks et al., 2001). The role of the 5-HT1A receptor in PPI has also been examined. An indolamine hallucinogen which is an agonist at the 5-HT1A receptor decreases PPI and this effect is blocked by the selective 5-HT1A receptor antagonist, WAY 100,635 (Krebs-Thomson et al., 2006). The 5-HT1A receptor agonist, 8-OH-DPAT has also been shown to decrease PPI in rats (Rigdon and Weatherspoon, 1992), although some find that this effect is only seen in rats with high baseline levels of PPI (Gogos and van den Busse, 2007). The effect of 8-OH-DPAT on PPI is attenuated by a selective 5-HT1A receptor antagonist (Sipes and Geyer, 1995). While low-dose 8-OH-DPAT alone decreases PPI in rats, it attenuates the decrease in PPI caused by the NMDA receptor antagonist, MK-801(Bubenikova-Valesova et al., 2007). The role of the 5-HT1A receptor in PPI in mice has also been studied. PPI is equivalent in 5-HT1A receptor knockout and wild-type mice (Dirks et al., 2001). Additionally, maternal separation-induced increases in startle amplitude and decreases in PPI are not attenuated by deletion of the 5-HT1A receptor (Groenink et al., 2011). Never-the-less, 8-OH-DPAT does have effects on PPI in mice. While the 5-HT1A receptor agonist either decreases PPI or has no effect on PPI in rats, the agonist increases PPI in WT mice, although the effect is strain-dependent (Dulawa et al., 2000; Dulawa and Geyer, 2000). However, 8-OH-DPAT does not affect PPI in 5-HT1A receptor KO mice (Dulawa et al., 2000) suggesting that although the effect of 8-OH-DPAT on mice and rats may not be the same, the 5-HT1A receptor does mediate the effect of 8-OH-DPAT in mice.
The potential interactions between CRF and the 5-HT1A receptor on baseline startle, PPI and startle habituation have not been examined. In the present studies, we assessed such potential interactions by administering the selective 5-HT1A receptor agonist, 8-OH-DPAT or the selective antagonist, WAY 100,635, prior to infusing CRF (ICV). Given that CRF is anxiogenic, that 5-HT1A knockout mice show enhanced anxiety-like behavior and that 5-HT1A agonists are anxiolytic, we sought to assess whether a 5-HT1A agonist would attenuate and a 5-HT1A antagonist would potentiate the effect of CRF on startle amplitude and PPI.
2. Methods
2.1 Animals
Brown Norway (BN) rats (Harlan Sprague-Dawley) and Wistar-Kyoto (WKY) rats (Charles River) were 10 weeks old upon arrival and were maintained on a 12-hour light/dark cycle with food and water available ad libitum. Rats were group-housed for 1–2 weeks prior to undergoing surgery to have guide cannula aimed at the lateral ventricle for infusion of CRF. Following surgery, the rats were single-housed. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental group sizes ranged from 7–10 rats/group. All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2 Stereotaxic Surgery and ICV Infusion Procedure
Rats were anesthetized with isoflurane-in-oxygen (2.0%) and placed in a Kopf stereotaxic instrument equipped with blunt ear bars. The incisor bar was set to −3.0. A stainless steel guide cannula (22 gauge; Plastics One, Roanoke, VA, USA) was aimed at the lateral ventricle (AP −1.0 mm, ML 2.0 mm from Bregma; 4.4 mm ventral from the skull) (Paxinos and Watson, 1986). Two jewelers’ screws were placed into the skull and the assembly was held in place with dental cement. A dummy cannula was placed into the guide. Rats were allowed to recover for 5–7 days prior to testing.
For ICV infusion, a 28-gauge cannula attached to PE 20 tubing was inserted into the guide cannula and extended 0.5 mm beyond. A 10.0 µl Hamilton syringe was used to manually deliver saline or CRF (0.3 µg in 6.0 µl). The flow of infusate was monitored via introduction of an air bubble into the infusion line. The infusion cannula was kept in place for an additional minute following infusion.
2.3 Startle Response and PPI Testing
Startle amplitude and PPI were measured in two identical startle chambers (SR-LAB, San Diego Instruments, San Diego, CA, USA) consisting of a nonrestrictive Plexiglas cylinder (9 cm diameter, 18.5 cm length) mounted on a platform located inside a sound- and vibration-attenuating cabinet equipped with a 5-watt incandescent bulb and a fan for ventilation. A piezoelectric accelerometer, mounted under each cylinder, detected whole-body startle responses. From the onset of each startle stimulus, output signals from the accelerometer were recorded once/msec for 100 msec by the computer. Signals were rectified, digitized, and stored by the SR-LAB program. Startle response sensitivities were standardized across chambers using a standard calibration tube each day. White noise stimuli were delivered through a horn tweeter controlled by the SR-LAB program.
Rats were placed into a testing chamber for a 5 min acclimation period prior to the delivery of any stimulus. The session was conducted using a 70 dB white noise background. On the first and last 6 trials of the session, a startling stimulus (50 dB above background (or 120 dB0), 40 msec) was presented alone. The remaining trials were presented in a pseudorandom order and included 12 additional trials (middle trials) with the startling stimulus alone (used to calculate % PPI and average startle amplitude), and 12 trials/prepulse stimulus intensity on which a prepulse stimulus (20 msec) preceded the startling stimulus by 100 msec. The prepulse stimuli were 3, 6, 12, 15 or 18 dB above background. Additionally, there were 8 trials on which no stimulus was presented, but activity within the chamber was monitored. The inter-trial interval averaged 20 seconds. Testing was performed between 10 a.m. and 4 p.m.
2.4 Drug and CRF Administration
In Experiment 1, the selective 5-HT1A receptor agonist, 8-OH- DPAT (DPAT; 1.0 mg/kg; IP) or saline was injected 10 min prior to ICV infusion of CRF (0.3 µg in 6.0 µl) or saline. This dose of CRF was used because we have previously shown that it reduces PPI in BN, but not in WKY rats (Conti, 2005). Thus, we could examine the effect of DPAT and WAY 100,635 in animals which show an effect of CRF on PPI as well as in those that do not. PPI was tested 30 min after the ICV infusion. In Experiment 2, the selective 5-HT1A receptor antagonist, WAY 100,635 (WAY; 1.0 mg/kg; IP) or saline was injected 10 min before CRF (ICV), and testing began 30 min after the ICV infusion. CRF was a generous gift from Dr. Jean Rivier, The Salk Institute.
2.5 Data Analysis
Startle amplitude on the 12 middle trials during which the startling stimulus alone was presented was averaged. These data were used in the analyses of baseline startle amplitude. Percent prepulse inhibition was calculated as 100-100*(average startle amplitude on the prepulse trials/average startle amplitude on the middle startle stimulus alone trials). Percent habituation was calculated as 100-100*(average startle amplitude on the last 6 startle alone trials/average startle amplitude on the first 6 startle alone trials). Percent prepulse inhibition data were analyzed with analysis of variance (ANOVA) with rat strain, IP injection (saline or 5-HT1A compound) and ICV infusion (saline or CRF) as between-subjects factors, and prepulse stimulus intensity as a within-subjects factor. Baseline startle amplitude data and percent habituation data were analyzed using the three between-subjects factors. Data for the 8-OH-DPAT experiment and the WAY 100,635 experiment were subjected to separate analyses.
3. Results
3.1 Experiment 1: Effect of IP Injection of the 5-HT1A receptor agonist, 8-OH-DPAT, on CRF-Induced changes in Startle and PPI
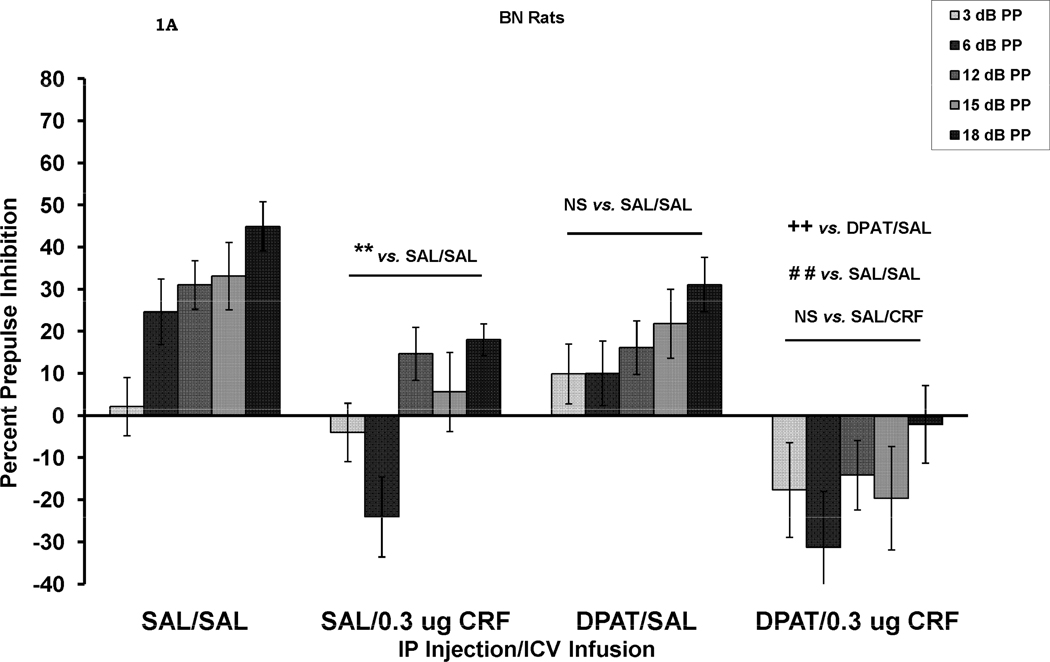
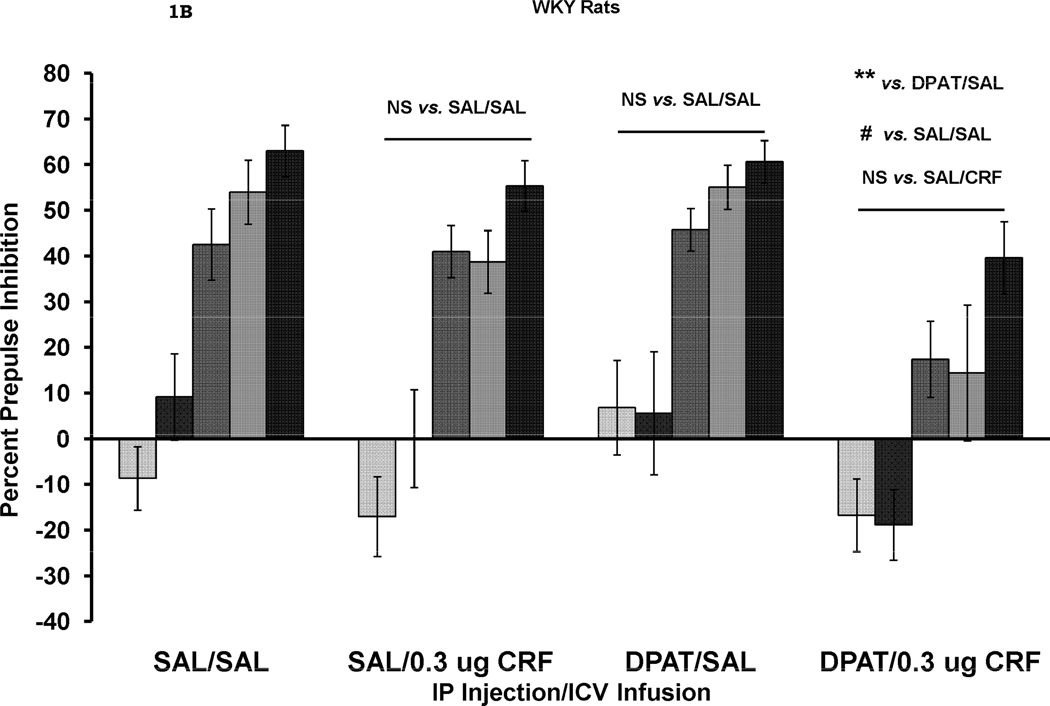
The effects of CRF and DPAT treatment alone and in combination on PPI are shown in Figure 1. A four-way ANOVA, with rat strain, IP injection (SAL vs. DPAT), ICV infusion (SAL vs. CRF) as between-subjects factors, and prepulse stimulus intensity as a within-subjects factor was initially used to test for overall main effects and interactions. The results of this analysis revealed a main effect for rat strain, F (1, 64) = 12.65, p = .001, with BN rats showing less PPI than WKY rats, a significant main effect for IP injection, F (1, 64) = 4.92, p < .05, with DPAT-treated rats showing less PPI than SAL-treated rats, and a significant main effect for ICV infusion, F (1, 64) = 25.5, p <.001, with CRF-treated rats showing less PPI than SAL-treated rats. There were no 2-way interactions between any of these between-subjects factors. There was also a significant effect of prepulse stimulus intensity, F (4, 256) = 93.3, p < .001. A significant prepulse stimulus intensity X rat strain interaction, F (4, 256) = 18.0 p < .001, was due to the fact that prepulse intensity had a greater effect on WKY rats than on BN rats. A significant prepulse intensity X IP injection interaction, F (4, 256) = 3.47, p < .01, was due to the fact that prepulse intensity had a greater effect on SAL-treated than DPAT-treated rats. Finally, a significant prepulse intensity X ICV infusion interaction, F (4, 256) = 2.85, p < .05, was due to the fact that prepulse intensity had a greater effect on SAL-treated than on CRF-treated rats. There were no 3- way or 4-way interactions involving prepulse intensity.
Figure 1.
Percent prepulse inhibition (mean ± SEM) at each of five prepulse stimulus intensities (3–18 dB above background) in BN rats (1A) and WKY rats (1B). Rats received an IP injection of either saline (SAL) or 8-OH-DAT (DPAT) followed 10 min later by an ICV infusion of either SAL or CRF. The results of all analyses are reported in the text. PPI was reduced by CRF alone (SAL/CRF) in the BN rats, ** p = .005. DPAT alone (DPAT/SAL) had a small, but not significant effect on PPI. The combination of DPAT and CRF (DPAT/CRF) also caused a significant reduction in PPI which was larger than the reduction seen in the SAL/CRF treated group. In WKY rats (Figure 1B), neither CRF nor DPAT alone reduced PPI. However, the combination of the two caused a significant reduction in PPI ** p < .01; ++ p = .005; # p < .02; ## p = .001.
Inspection of Figure 1A suggests that both CRF and DPAT alone decreased PPI in BN rats and that the two together had an additive effect resulting in prepulse facilitation. In this strain, there was a significant effect of IP injection, F (1, 31) = 4.34, p < .05 and a significant effect of ICV infusion, F (1, 31) = 19.3, p < .001. There was a significant difference between the group treated with SAL (ICV) alone and the group treated with CRF (ICV) alone (SAL/SAL vs. SAL/CRF), F (1, 14) = 11.19, p = .005, while effect of DPAT alone (SAL/SAL vs. DPAT/SAL) was not significant, p > .05 in BN rats. Never-the-less, the combined treatment of DPAT and CRF reduced PPI over the effect of DPAT alone, F (1, 17) = 10.5, p = .005, suggesting that the small effect of DPAT added to the effect of CRF. Additionally, the combined treatment with DPAT and CRF reduced PPI compared to the SAL/SAL control group, F (1, 16) = 18.4, p = .001, while there was no difference between the combined effect of DPAT/CRF and the effect of CRF alone (SAL/CRF). In WKY rats (Figure 1B), there was an overall significant effect of CRF on PPI, F (1, 33) = 7.44, p < .01, but no effect of DPAT. However, inspection of Figure 1B suggests that neither treatment alone had an effect on PPI in WKY rats, but that together, the two treatments did reduce PPI. Indeed, in WKY rats, there was no effect of CRF on PPI in the absence of DPAT (SAL/SAL vs. SAL/CRF), p > .05, and no effect of DPAT in the absence of CRF (SAL/SAL vs. DPAT/SAL), p > .05. However, when the effects of DPAT alone were compared to the combined effects of CRF and DPAT (DPAT/SAL vs. DPAT/CRF) a significant difference was seen, F (1, 16) = 8.76, p < .01. Additionally, PPI was significantly lower in the DPAT/CRF group than in the SAL/SAL group, F (1, 16) = 6.6, p < .02. However, the combined treatment did not reduce PPI compared to the CRF alone (SAL/CRF) group. Thus, CRF appeared to add to the effect of DPAT.
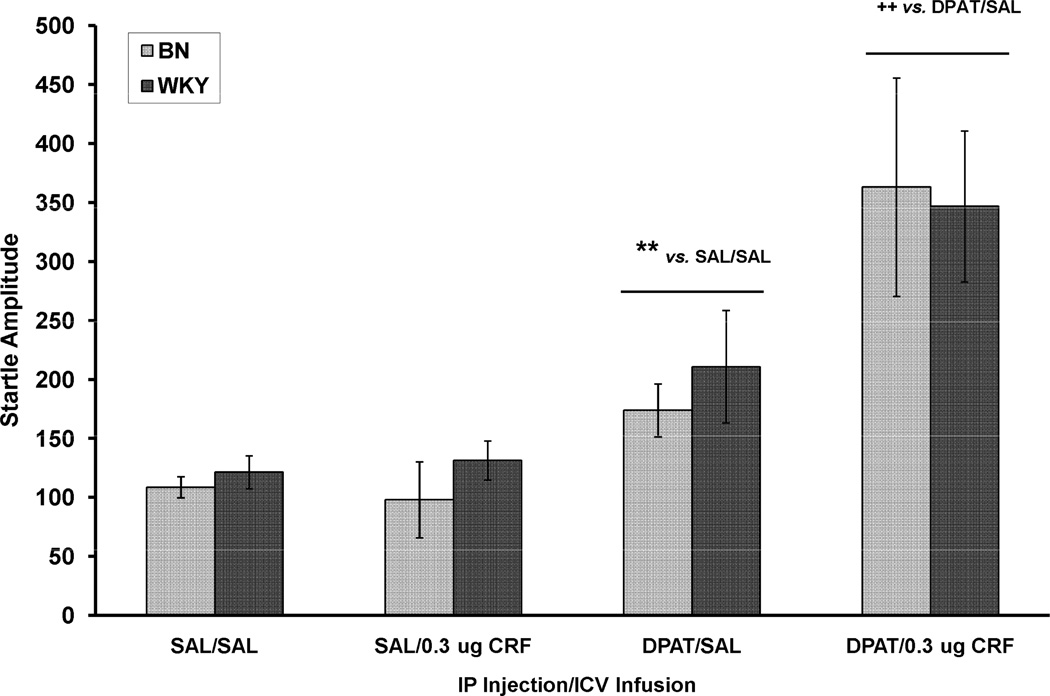
The effects of CRF and DPAT treatment alone and in combination on baseline startle amplitude are shown in Figure 2. An overall 3-way ANOVA with rat strain, IP injection and ICV infusion as between-subjects factors revealed a significant effect of IP injection, F (1, 64) = 25.0, p < .001, with DPAT increasing the startle response. There was also a significant effect of ICV infusion, F (1, 64) = 6.52, p < .02, with CRF increasing startle amplitude. However, this effect of CRF appears only in rats that were also treated with DPAT, as revealed by a significant IP X ICV interaction, F (1, 64) = 6.57, p < .02. Thus, a dose of CRF which alone, has no effect on startle amplitude enhanced the effect of DPAT on startle.
Figure 2.
Baseline startle amplitude (mean ± SEM) in BN and WKY rats on the trials from which the data used to calculate percent PPI were collected. CRF alone (SAL/CRF) did not affect startle in either rat strain. DPAT alone (DPAT/SAL) significantly increased startle amplitude ** p = .01. The combination of DPAT and CRF (DPAT/CRF) had a greater effect on startle amplitude than DPAT alone ++ p < .02 vs. DPAT/SAL.
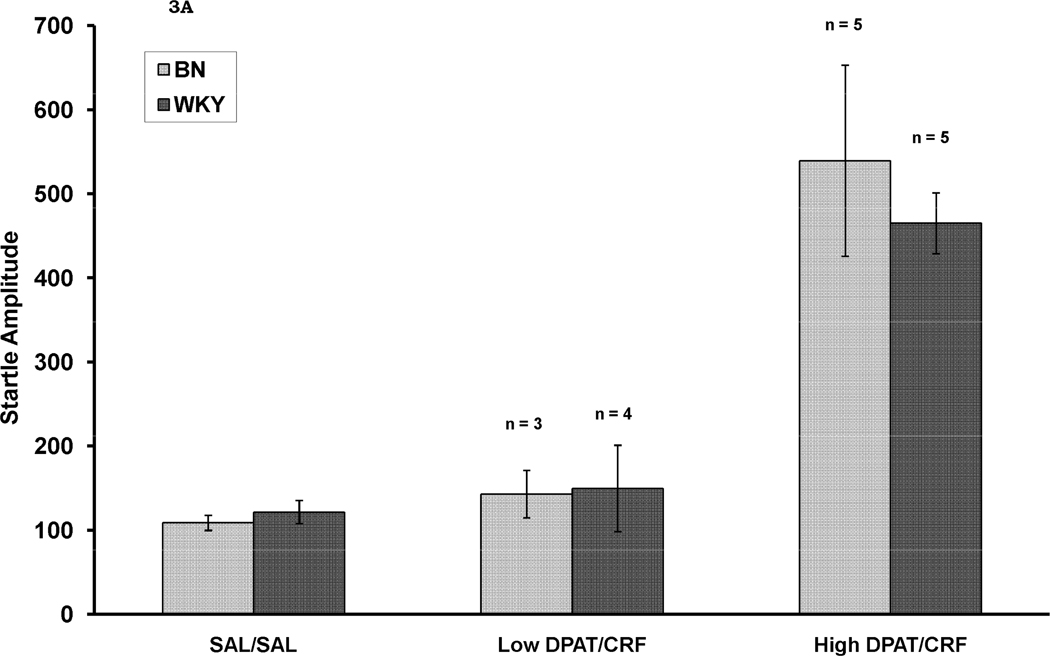
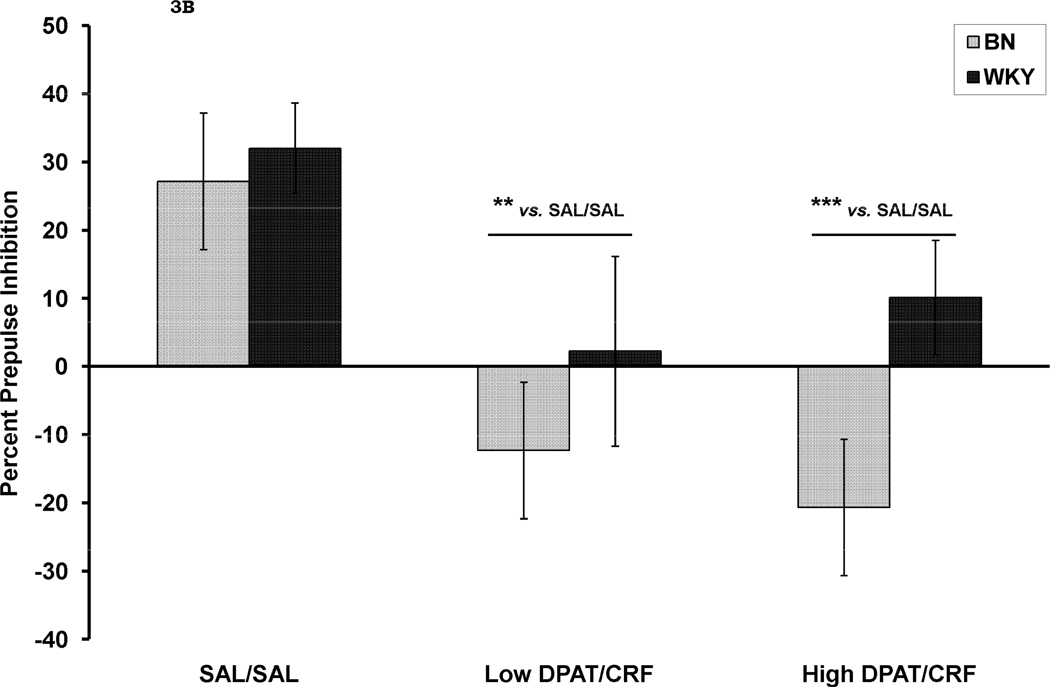
Given that the combination of DPAT and CRF both decreased percent PPI and increased baseline startle we sought to assess whether the decrease in PPI was dependent on an increase in startle amplitude. To do this, we performed a median split on the startle amplitude data so that we could examine whether PPI was reduced in both the rats of the DPAT/CRF group that did not show an increase in baseline startle as well as in those that did show an increase in baseline startle. Figure 3A shows baseline startle amplitude in controls (SAL/SAL) as well as in rats that were treated with DPAT/CRF which did not show an increase in startle amplitude (Low DPAT/CRF) and in those that did show an increase in baseline startle (High DPAT/CRF). As seen in Figure 3B, both of the DPAT/CRF-treated sub-groups showed a decrease in percent PPI. Here, there was a significant effect of rat strain, F (1, 30) = 4.5, p < .05. There was also a significant effect of treatment group, F (2, 30) = 11.5, p < .001, and Tukey post-hoc tests revealed that both the Low DPAT/CRF group (p= .002) and the High DPAT/CRF group (p = .001) showing less PPI than the SAL/SAL group. There was no strain X group interaction. Thus, the decrease in percent PPI was not caused by the increase in baseline startle.
Figure 3.
Startle amplitude (Figure 3A) and percent PPI averaged across all prepulse stimulus intensities (Figure 3 B) in rats in which the combination of DPAT and CRF not increase startle amplitude (Low DPAT/CRF) and in those in which startle amplitude was increased by the combined treatment. In Figure 3B, it can be seen that percent PPI was reduced by the combined treatment whether baseline startle was increased or not. ** p = .002 vs. SAL/SAL; *** p = .001 vs. SAL/SAL.
The effects of rat strain and of treatments on percent habituation of the startle response is shown in Table 1. There was no main effect of rat strain (p > .05), IP injection (SAL vs. DPAT), p > .05, or of ICV infusion (SAL vs. CRF), p > .05. There was a significant rat strain X ICV infusion interaction, F (1, 64) = 4.87, p < .05. There was also a significant IP X ICV interaction, F (1, 64) = 4.61, p < .05, which appears to be due to BN rats although there was no 3-way interaction involving rat strain: DPAT decreased percent habituation in BN rats that received SAL (ICV) but not in those that received CRF (ICV).
Table 1.
The effects of CRF and DPAT on percent habituation (mean ± SEM) of the startle response in BN and WKY rats. Neither treatment alone, nor the combination of the treatments affected within-session habituation. Percent habituation was calculated as 100-100*(average startle amplitude on the last 6 startle alone trials/average startle amplitude on the first 6 startle alone trials).
| BN Rats | WKY Rats | |
|---|---|---|
| SAL/SAL | 44.9 ± 9.0 | 58.7 ± 4.2 |
| SAL/0.3 µg CRF | 53.0 ± 6.9 | 40.5 ± 9.9 |
| DPAT/SAL | 34.1 ± 8.2 | 54.9 ± 4.7 |
| DPAT/0.3 µg CRF | 61.4 ± 8.7 | 62.8 ± 5.2 |
3.2 Experiment 2: Effect of IP Injection of the 5-HT1A receptor antagonist, WAY, on CRF-Induced changes in Startle and PPI
The effects of rat strain, IP injection of WAY and ICV infusion of CRF on percent PPI are shown in Table 2A. An overall ANOVA using these three between-subjects factors was conducted, as well as prepulse stimulus intensity as a within-subjects factor was performed. The results revealed that there was a significant effect of rat strain, F (1, 69) = 41.4, p < .001. There was no effect of IP injection of WAY (p > .05). There was both a trend towards an effect of ICV infusion of CRF, F (1, 69) = 3.21, p = .077 and towards a rat strain X ICV infusion interaction, F (1, 69) = 3.24, p = .076. There was a significant effect of prepulse stimulus intensity, F (4, 276) = 92.0, p < .001. A significant prepulse intensity X rat strain interaction, F (4, 276) = 16.3, p < .001 with WKY rats being more sensitive to the effect of prepulse stimulus intensity than BN rats. There was also a significant prepulse intensity X rat strain X IP injection interaction, F (4, 276) = 3.1, p < .02. This complex interaction appears to be due to the fact that prepulse intensity had less of an effect in BN rats treated with WAY than in those treated with SAL and that this effect on the response to prepulse intensity was not as great in WKY rats.
Table 2.
The effects of the 5-HT1A antagonist, WAY (IP) and CRF (ICV) alone or in combination on percent prepulse inhibition (2A), baseline startle amplitude (2B) and percent habituation of the startle response (2C).
| A. Percent prepulse inhibition (mean ± SEM) at each of five prepulse stimulus intensities (3–18 dB above background) in BN rats and WKY rats. Rats received an IP injection of either saline (SAL) or the 5-HT1A receptor antagonist, WAY, followed 10 min later by an ICV infusion of either SAL or CRF. The results of all analyses are reported in the text. In BN rats, CRF alone significantly reduced PPI. This effect was not altered by administration of WAY. WAY alone had no effect on PPI. In WKY rats neither treatment alone, nor the combination of treatments affected PPI. | |||||
|---|---|---|---|---|---|
| 3 dB PP | 6 dB PP | 12 dB PP | 15dB PP | 18 dB PP | |
| BN Rats | |||||
| SAL/SAL | 3.8 ± 2.7 | 5.3 ± 3.3 | 18.6 ± 2.6 | 24.9 ± 4.0 | 36.9 ± 3.4 |
| SAL/0.3 µg CRF | −2.6 ± 5.6 | −10.2 ± 7.3 | 10.2 ± 5.9 | 13.5 ± 5.8 | 20.5 ± 4.5 |
| WAY/SAL | 1.1 ± 3.2 | −0.8 ± 5.6 | 9.1 ± 4.6 | 18.2 ± 5.2 | 29.8 ± 4.0 |
| WAY/0.3 µg CRF | −1.0 ± 3.7 | −15.8 ± 7.3 | −2.4 ± 9.9 | 2.4 ± 11.0 | 11.7 ± 6.0 |
| WKY Rats | |||||
| SAL/SAL | 6.8 ± 5.4 | 18.2 ± 13.5 | 38.0 ± 5.2 | 47.6 ± 5.3 | 56.0 ± 4.9 |
| SAL/0.3 µg CRF | 3.2 ± 4.3 | 8.0 ± 7.6 | 38.8 ± 10.3 | 44.7 ± 6.5 | 56.1 ± 5.4 |
| WAY/SAL | −5.3 ± 7.1 | −11.0 ± 10.3 | 44.5 ± 5.6 | 46.6 ± 5.4 | 60.4 ± 6.5 |
| WAY/0.3 µg CRF | −5.2 ± 13.7 | 2.5 ± 11.8 | 49.2 ± 4.4 | 43.5 ± 9.2 | 61.4 ± 4.6 |
| B. Baseline startle amplitude (mean ± SEM) in BN and WKY rats on the trials from which the data used to calculate percent PPI were collected. | |||
|---|---|---|---|
| BN Rats | WKY Rats | ||
| SAL/SAL | 144.3 ± 20.6 | 120.9 ± 9.9 | |
| SAL/0.3 µg CRF | 101.4 ± 9.6 | 124.8 ± 15.9 | |
| WAY/SAL | 97.1 ± 9.6 | 75.6 ± 15.5 |
|
| WAY/0.3 µg CRF | 81.7 ± 10.0 | 58.4 ± 11.2 | |
| C. The effect of CRF and WAY on percent habituation (mean ± SEM) in BN and WKY rats. Neither treatment alone, nor the combination of treatments affected within-session habituation. Percent habituation was calculated as 100-100*(average startle amplitude on the last 6 startle alone trials/average startle amplitude on the first 6 startle alone trials). | ||
|---|---|---|
| BN Rats | WKY Rats | |
| SAL/SAL | 14.1 ± 8.1 | 48.1 ± 9.4 |
| SAL/0.3 µg CRF | 29.7 ± 10.9 | 53.0 ± 4.0 |
| WAY/SAL | 30.6 ± 11.0 | 60.0 ± 6.5 |
| WAY/0.3 µg CRF | 36.9 ± 8.1 | 54.6 ± 6.8 |
Overall ANOVA (see text) revealed that WAY caused a significant reduction in baseline startle amplitude (** p < .001). This effect was not rat strain-dependent, and it did not interact with CRF.
Table 2B shows the effect of CRF and WAY, alone and in combination of the amplitude of the baseline startle response in both rat strains. IP injection, ICV infusion and rat strain were between-subjects factors. There was a significant effect of IP injection, F (1, 69) = 22.4, p < .001, with WAY causing a reduction in startle amplitude. There was also a trend for CRF to decrease startle amplitude, F (1, 69) = 3.6, p = .062. There were no interactions. Using the same factors, the percent habituation data were also subjected to a 3-way ANOVA (Table 2C). The results revealed a significant main effect of rat strain, F (1, 69) = 18.7, p < .001 with BN rats showing less habituation than WKY rats. There were no other main effects or interactions.
4. Discussion
CRF (0.3 µg) reduced PPI in BN, but not in WKY rats as we have previously shown (Conti, 2005). The 5-HT1A receptor agonist, DPAT, cause a small, but not significant reduction in PPI when administered in the absence of CRF, and added to the effect of CRF on PPI in BN rats. In fact, when the CRF and DPAT were used in combination in this rat strain, prepulse facilitation was found. While neither CRF nor DPAT alone affected PPI in WKY rats, the combined treatment significantly reduced PPI. Thus, sub-threshold doses of CRF and DPAT appear to act synergistically to reduce PPI in WKY rats. However, since only a single dose of DPAT was used and since the effect of the combined DPAT/CRF treatment was not significantly different than the effect CRF alone, it is difficult to be sure that the two treatments resulted in a synergistic effect. Although there was an overall significant main effect of DPAT on PPI (reduction in PPI), it is unclear why DPAT alone failed to significantly reduce PPI in this experiment when data from each rat strain were examined separately. Others have shown that DPAT does result in such a reduction (Gogos and van den Busse, 2007; Rigdon and Weatherspoon, 1992), although some find that this effect is only seen in rats with high baseline levels of PPI (Gogos and van den Busse, 2007). Never-the-less, an effect of DPAT on PPI was revealed when the agonist was administered to CRF-treated rats of either rat strain. Thus, CRF effects may interact with 5-HT1A agonist effects to reduce PPI. The results of this study also show that the effect of the combined treatment with CRF and DPAT on PPI were not due to alterations in baseline startle amplitude.
CRF alone did not alter baseline startle amplitude in either rat strain while DPAT alone caused a small, but significant increase in baseline startle amplitude. However, when administered with the non-effective dose of CRF, DPAT resulted in a larger increase in startle amplitude than it did when administered alone. Others have shown that nicotine withdrawal-induced enhancement of baseline startle is potentiated DPAT (Rasmussen et al., 1997). Given the results of the present study, it would be interesting to know whether this effect of withdrawal is mediated by CRF. Both CRF and the 5-HT1A receptor system have been individually implicated in stress-related anxiety disorders. However, both animal studies (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998) and clinical studies suggest that reductions in the 5-HT1A receptor contribute to the disorders (Lanzenberger et al., 2007; Nash et al., 2008). Thus, this synergistic effect to increase startle amplitude was unexpected. This study is the first in which the combined treatment with CRF and a 5-HT1A receptor agonist on either startle or PPI was examined. The results suggest that further studies of the interactions between these two agonists on anxiety-like behavior are warranted.
Given the results of studies showing that 5-HT1A receptor null mice show more anxiety-like behavior that WT controls (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998), one might have expected that blockade of the receptor with WAY, would potentiate the effect of CRF on PPI. This did not occur. It should be noted that the dose of WAY used in the present experiments does attenuate behavioral effects caused by DPAT (De Vry et al., 2004). Additionally, WAY alone had no effect on PPI. Yohimbine-induced decreases in PPI are actually blocked by WAY (Powell et al., 2005). Yet, others have shown that WAY does not attenuate the apomorphine-induced decrease in PPI, while it does block the apomorphine-induced increase in baseline startle amplitude (Gogos et al., 2010). Additionally, the nicotine withdrawal-induced increase in startle amplitude is not blocked by WAY (Rasmussen et al., 1997). Thus, blockade of the 5-HT1A receptor appears to diminish the reduction in PPI caused by some treatments, but not by all treatments. Given the effect of 5-HT1A receptor gene knockout on anxiety-like behaviors, it might also have been expected that WAY would reduce baseline startle amplitude. This, in fact, was the case in the present studies.
Here, we show that a 5-HT1A receptor agonist can potentiate the effect of CRF on PPI and startle, while administration of a 5-HT1A receptor antagonist is not sufficient to block the effects of CRF on these behaviors. This may be due to the fact that it is more likely that CRF affects serotonergic neurotransmission rather than that 5-HT affects CRF transmission. Thus, what our results might suggest is that CRF actually potentiates an effect of 5-HT at the 5-HT1A receptor, so that a sub-threshold dose of a 5-HT1A receptor agonist, such as that used here, becomes effective and then adds to the effect of CRF on PPI and startle amplitude. When the 5-HT1A is blocked, CRF remains free to affect PPI independently of 5-HT. Other studies have been conducted in order to explore the potential interactions between the effects of CRF and other neurotransmitters systems on PPI and startle. We have shown that neither serotonin depletion nor administration of the serotonin (5-HT) 5-HT2A/2C receptor antagonist, ketanserin attenuate the effect of CRF on PPI (Sutherland et al., 2008). However, ketanserin does block the CRF-induced increase in baseline startle amplitude and serotonin depletion enhanced the CRF-induced increase in startle (Sutherland et al., 2008). Others have shown that neither deletion of the dopamine D1 or D2 receptors, nor administration of antagonists for these receptors affect CRF-induced changes in startle or PPI (Vinkers et al., 2007). The roles of norepinephrine receptors in CRF-induces changes in startle and PPI have also been examined. In those studies, Gresack and Risbrough (2010) found that both the α2-adrenergic receptor agonist, clonidine, and the α1-adrenergic receptor antagonist, prazosin, blocked the CRF-induced increase in baseline startle amplitude, but neither drug affected the CRF-induced decrease in PPI. The β-adrenergic receptor antagonist, propranolol, had no did not affect either the CRF-induced change in startle or PPI. Thus, it appears that while the effect of CRF on PPI and startle can be potentiated by activation of 5-HT1A receptors, CRF can also act independently to reduce PPI.
The 5-HT1A receptor agonist, 8-OH-DPAT, and CRF additivley or synergistically decreased prepulse inhibition of the startle response depending on rat strain.
8-OH-DPAT and CRF acted synergistically to increase baseline startle amplitude.
The 5-HT1A receptor antagonist, WAY 100,635, neither attenuated nor potentiated the effect of CRF on baseline startle or prepulse inhibition.
We studied the interaction between CRF and the 5-HT1A receptor system > We examined baseline acoustic startle as well as prepulse inhibition > The 5-HT1A receptor agonist, 8-OH-DPAT, enhanced the effect of CRF on PPI > The 5-HT1A receptor agonist, 8-OH-DPAT, enhanced the effect of CRF on startle > The 5-HT1A receptor antagonist, WAY 100635, did not interact with CRF
Acknowledgements
The expert technical assistance of Ms. Jennifer Costill is greatly appreciated. This work was supported by MH065467.
Abbreviations
- CRF
Corticotropin-Releasing Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Alsene KM, Roseboom PH, Connors EE. Enduring sensorimotor gating abnormalities following predator exposure or corticotropin-releasing factor in rats: A model for PTSD-like information-processing deficits? Neuropharmacol. 2011 doi: 10.1016/j.neuropharm.2011.01.040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behavior and are hypersensitive to stress. Nat. Genetics. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Ann. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central serotonin receptors and their function. Neuropharmacol. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bijlsma EY, Van Leeuwen MLF, Westphal KGC, Olivier B, Groenink L. Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: Differential involvement of the basolateral amygdala and medial prefrontal cortex. Neurosci. 2011;173:82–92. doi: 10.1016/j.neuroscience.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacol. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am. J. Psychiat. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and a benzodiazepine receptor antagonists and a –HT1A-receptor agonist. Neuropsychopharmacol. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 1986;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesove V, Votava M, Palenicek T, Horacek J. The opposite effect of a low and a high dose of serotonin-1A agonist on behavior induced by MK-801. Neuropharmacol. 2007;52:1071–1078. doi: 10.1016/j.neuropharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chang CP, Pearse RV, 2nd, O'Connell S, Rosenfeld MG. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Conti LH. Characterization of the effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in Brown Norway and Wistar-Kyoto rats. Eur. J. Pharmacology. 2005;507:125–134. doi: 10.1016/j.ejphar.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Conti. LH, Murry JD, Ruiz MA, Printz MP. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- Davis M, Cassella DS, Wrean WH, Kehne JH. Serotonin receptor subtype agonists. Differential effects on sensorimotor reactivity measured with acoustic startle. Psychopharmacol. Bull. 1986;22:837–843. [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Melon C, Dalmus M, Jentzsch KR. 5-HT1A receptors are differentially involved in the anxiolytic- and antidepressant-like effects of 8-OH-DPAT and Fluoxetine in the rat. Eur. J. Neuropsychopharmacol. 2004;14:487–495. doi: 10.1016/j.euroneuro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Dirks A, Groenink L, Schipholt MI, van der Gugten J, Hijzen TH, Geyer MA, Olivier B. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol. Psychiat. 2002;51:583–590. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- Dirks A, Pattij T, Bouwknecht JA, Westphal TT, Hijzten TH, Groenink L, van der Gugten J, Oosting RS, Hen R, Geyer MA, Olivier B. 5-HT1B receptor knockout, but not 5-HT1A receptor knockout mice, show reduced startle reactivity and footshock-induced sensitization, as measured with the acoustic startle response. Behav. Brain Res. 2001;118:169–178. doi: 10.1016/s0166-4328(00)00326-0. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacol. 2000;39:2170–2179. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharm. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm. Beh. 1987;21:193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- Gogos A, Kwek P, Chavez C, van den Busse M. Estrogen treatment blocks 8-hydroxy-2-dipropylaminotetralin- and apomorphine-induced disruptions of prepulse inhibition: Involvement of dopamine D1 or D2 or serotonin 5-HT1A, 5-HT2A, or 5-HT7 receptors. J. Pharmacol. Exper. Ther. 2010;333:218–227. doi: 10.1124/jpet.109.162123. [DOI] [PubMed] [Google Scholar]
- Gogos A, van den Busse M. The importance of baseline in identifying 8-OH-DPAT-induced effects on prepulse inhibition in rats. Brit. J. Pharmacol. 2007;150:750–757. doi: 10.1038/sj.bjp.0707148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann. NY. Acad. Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Risbrough VB. Corticotropin-releasing factor and noradrenergic signaling exert reciprocal control over startle reactivity. Inter. J. Neuropsychopharmacol. 2010;21:1–16. doi: 10.1017/S1461145710001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Bijlsma EY, van Bogaert MJV, Oosting RS, Olivier B. Serotonin1A receptor deletion does not interact with maternal separation-induced increases in startle reactivity and prepulse inhibition deficits. Psychopharmacol. 2011;214:353–365. doi: 10.1007/s00213-010-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Dirks A, Verdouw PM, de Graaff M, Peeters BW, Millan MJ, Olivier B. CRF1 not glucocorticoid receptors mediate prepulse inhibition deficits in mice overexpressing CRF. Biol. Psychiat. 2008;63:360–368. doi: 10.1016/j.biopsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol. Psychiat. 1998;44:1027–2036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Macadam DW, Ison JR. Effects of prestimulation on the electromyographic response associated with the acoustic startle reaction in rats. Physiol. Behav. 1972;8:535–537. doi: 10.1016/0031-9384(72)90341-1. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like response in serotonin 5-HT1A receptor mutant mice. Proc. Nat. Acad. Sci. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaycox LH, Foa EB, Morral AR. Influence of emotional engagement and habituation on exposure therapy for PTSD. J. Consult. Clin. Psychol. 1998;66:185–192. doi: 10.1037//0022-006x.66.1.185. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of Crhr2 reveals an anxiolytic role of corticotropin-releasing hormone receptor-2. Nat. Gen. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats – circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacol. 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol. Psychiat. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lazosky AJ, Britton DR. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacol. 1991;104:132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J. Neurosci. 1992a;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Campeau S, Falls WA, Miserendino MJ, Davis M. Lesions of the central nucleus of the amygdala, but not the paraventricular nucleus of the hypothalamus, block the excitatory effect of corticotropin-releasing factor on the acoustic startle reflex. J. Neurosci. 1992b;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Dep. Anxiety. 2002;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SSG. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat. Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res. Bull. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MSD, Droste SK, Kuhn R, Reul JMHM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Brit. J. Psychiat. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety in mice lacking the serotonin1A receptor. Proc. Nat. Acad. Sci. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Palomo J, Carasso BS, Bakshi VP, Geyer MA. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not α2-adrenoceptors. Psychopharmacol. 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacol. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Proc. Nat. Acad. Sci. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Kallman MJ, Helton DR. Serotonin-1A antagonists attenuate the effects of nicotine withdrawal on the auditory startle response. Synapse. 1997;27:145–152. doi: 10.1002/(SICI)1098-2396(199710)27:2<145::AID-SYN5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J. Pharmacol. Exper. Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pellymounter MA, Geyer MA. Role of corticotropin-releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacol. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm. Behav. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J. Neurosci. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, Mcgeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacol. 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbacher B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol. Psychiat. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc. Nat. Acad. Sci. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes TA, Geyer MA. 8-OH-DPAT disruption of prepulse inhibition in rats: reversal with (+) WAY 100,135 and localization of site of action. Psychopharmacol. 1995;117:41–48. doi: 10.1007/BF02245096. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spina MG, Merlo-Pich E, Akwa Y, Balducci C, Basso AM, Zorrilla EP, Britton KT, Rivier J, Vale WW, Koob GF. Time-dependent induction of anxiogenic-like effects of central infusion of urocortin or corticotropin-releasing factor. Psychopharmacol. 2002;160:113–121. doi: 10.1007/s00213-001-0940-y. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J. Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Conti LH. Restraint stress-induced reduction in prepulse inhibition in Brown Norway rats: Role of the CRF2 receptor. Neuropharmacol. 2011;60:561–571. doi: 10.1016/j.neuropharm.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Page ME, Conti LH. The effect of corticotropin-releasing factor on prepulse inhibition is independent of serotonin in Brown Norway and Wistar-Kyoto rats. Pharmacology Biochemistry and Behavior. 2008;89:324–337. doi: 10.1016/j.pbb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinol. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of startle by corticotropin-releasing factor and fear are both reversed by alpha-helical CRF. Neuropsychopharmacol. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacol. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci. Biobehav. Rev. 2001;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Chefer VI, Zapata A, Shippenberg TS. The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacol. 2010;210:231–240. doi: 10.1007/s00213-010-1799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JMHM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Gen. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behavior. Eur.J. Neurosci. 2002;15:2007–2015. doi: 10.1046/j.1460-9568.2002.02040.x. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Hagenaars M. Fear activation and habituation patterns as early process predictors of response to prolonged exposure treatment in PTSD. J. Traum. Stress. 2002;15:359–367. doi: 10.1023/A:1020177023209. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000.;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Risbrough VB, Geyer MA, Caldwell S, Low MJ, Hauger RL. Role of dopamine D1 and D2 receptors in CRF-induced disruption of sensorimotor gating. Pharmacol. Biochem. Behav. 2007;86:550–558. doi: 10.1016/j.pbb.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Collateralized dorsal raphe nucleus projections: A mechanism for the integration of diverse functions during stress. J. Neurochem. 2011 doi: 10.1016/j.jchemneu.2011.05.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]