Abstract
Schizophrenia patients exhibit perceptual and cognitive deficits, including in visual motion processing. Given that cognitive systems depend upon perceptual inputs, improving patients’ perceptual abilities may be an effective means of cognitive intervention. In healthy people, motion perception can be enhanced through perceptual learning, but it is unknown whether this perceptual plasticity remains in schizophrenia patients. The present study examined the degree to which patients’ performance on visual motion discrimination can be improved, using a perceptual learning procedure. While both schizophrenia patients and healthy controls showed decreased direction discrimination thresholds (improved performance) with training, the magnitude of the improvement was greater in patients (47% improvement) than in controls (21% improvement). Both groups also improved moderately but non-significantly on an untrained task—speed discrimination. The large perceptual training effect in patients on the trained task suggests that perceptual plasticity is robust in schizophrenia and can be applied to develop bottom-up behavioral interventions.
Keywords: Cognitive, neuroscience, motion processing, neuroplasticity, perception, schizophrenic, perceptual learning
1. Introduction
Cognitive impairment is a core dysfunction in schizophrenia. Research in the past few decades has indicated that cognitive deficits extend to certain early perceptual processes (Green et al., 2009; Javitt, 2009). Given that many cognitive processes and daily behaviors rely on perceptual processing, the improvement of deficient perception represents a potential primary step in behavioral intervention for schizophrenia patients; and the emerging evidence is promising. For example, perceptual training in the auditory domain has led to improvement of verbal working memory and cognition in patients (Fisher, Holland, Merzenich, & Vinogradov, 2009). It is unclear, however, whether cognitive improvements as a result of perceptual training are mediated through improvements in basic perceptual domains. To understand the underlying mechanisms, one may need to first examine the effects of perceptual training on perceptual processing itself.
Visual motion processing is deficient in schizophrenia (Chen, 2011). Patients show poor performance discriminating the speed (Brenner, Wilt, Lysaker, Koyfman, & O'Donnell, 2003; Chen, Nakayama, Levy, Matthysse, & Holzman, 1999a; Clementz, McDowell, & Dobkins, 2007; Kim, Wylie, Pasternak, Butler, & Javitt, 2006) as well as the direction of visual motion stimuli (Chen, Nakayama, Levy, Matthysse, & Holzman, 2003; Slaghuis, Holthouse, Hawkes, & Bruno, 2007; Stuve et al., 1997). Brain abnormalities in motion processing have been found at levels ranging from basic neural circuitry (Chen, Norton, & Ongur, 2008; Tadin et al., 2006) to cortical systems (Chen, et al., 2008; Wang, Brown, Dobkins, McDowell & Clementz, 2010). At present, it is unknown whether the visual motion processing deficit in schizophrenia can be ameliorated.
In healthy people, perceptual sensitivity to visual motion can be improved through training that involves extensive exposure to motion stimuli (Ball & Sekuler, 1982; Watanabe, Nanez, & Sasaki, 2001). Enduring and consistent improvement in a perceptual task as a result of such training is known as perceptual learning (Gibson, 1963). Perceptual learning is often specific to the stimulus features used in training (Gilbert, Sigman & Crist, 2001). For example, subjects who have been trained on one direction of motion show increased sensitivity to the trained, but not to other, directions of motion (Ball & Sekuler, 1982). This indicates that the perceptual learning process is primarily mediated by neuroplasticity in the early stages of perceptual systems, where encoding of the stimulus features is precisely localized (Gilbert et al., 2001; Hua et al., 2010). Neuroimaging studies have provided further evidence showing that training-induced performance improvement on a visual discrimination task is associated with increased neural activation in visual processing areas like the striate cortex (Schwartz, Maquet, & Frith, 2002; Yotsumoto, et al., 2009). Single cell recording studies in monkey also indicate that the biological basis of perceptual learning is linked to neuroplasticity in the sensory cortex (e.g., Zohary, Celebrini, Britten & Newsome, 1994).
While the primary perceptual learning mechanisms are specific to the trained domain, non-specific perceptual learning mechanisms may also be at work (Fine & Jacobs, 2002). Perceptual learning seems to be able to transfer from a trained domain (e.g. one direction of motion) to an untrained parallel domain (e.g. another direction of motion) when the task for learning is easy (Liu & Weinshall, 2000) and can also generalize from complex tasks to simpler ones (Fahle, 2005; Green & Bavalier, 2003). In terms of interventions in schizophrenia, the ability of training effects to generalize would mean that bottom-up perceptual learning may potentially produce benefits for other untrained behavioral functions.
In general, improvement in perceptual sensitivity via training requires neuroplasticity mechanisms to be at work (Gilbert et al., 2001). While it is unknown at this point whether and how the mechanisms involved in perceptual learning are affected in schizophrenia, a recent hypothesis posits that neuroplasticity and synaptic functions are altered in this mental disorder (Frankle, Lerma & Laruelle, 2003). Several lines of research seem to be consistent with this hypothesis, including genetic (Balu & Coyle, 2011), molecular (Buckley, Pillai & Howell, 2011; Goto, Yang & Otani, 2010) and behavioral studies (Daskalakis, Christensen, Fitzgerald & Chen, 2008). On the other hand, studies of cortical activations in patients showed intact or even excessive plasticity when cognitive training paradigms were applied (Haut, Lim & MacDonald, 2010; Edwards Barch & Braver, 2010). Similarly, hippocampal volumetric changes in response to exercise, another indicator of neuroplasticity, appear to be normal or even more robust in schizophrenia (Pajonk et al., 2010).
In visual domains, it remains unknown whether the neuroplasticity is impaired in schizophrenia. Given that many cognitive functions rely on visual inputs, it is critical to ascertain whether or not visual plasticity can allow the improvement of abnormal visual processing through perceptual training in this mental disorder. The goal of the present study was to explore the feasibility of improving deficient visual motion perception in schizophrenia using a perceptual learning procedure. Based on robust perceptual learning effects in healthy people, and a mixed set of results regarding neuroplasticity in patients, we hypothesized that patients would show significant perceptual improvement, but to a lesser extent than controls.
2. Methods
2.1. Subjects
Seventeen patients with schizophrenia or schizoaffective disorder and 10 healthy controls participated. General inclusion criteria for both groups of participants were: age between 18 – 65, no drug or alcohol abuse in the six months prior to participation, no neurological problems such as seizure, stroke or major head injury, and IQ > 70.
Patients were recruited from McLean Hospital as well as the Greater Boston area. They were diagnosed using the Structure Clinical Interview for DSM Disorders, 4th edition, (SCID-IV; First et al., 2002a), by independent clinicians who were blind to the purposes of the study, and by a review of all available medical records. Their average Positive, Negative and General Scores on the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein & Opler, 1987) were 14.5 (SD = 5.5), 15.4 (SD = 7.1) and 26.8 (SD = 8.0), respectively. Their average duration of illness was 18.1 years (SD = 12.1 years). The average antipsychotic dose, calculated using chlorpromazine equivalents, was 393.7 mg (SD = 319.2 mg).
Healthy controls were recruited by posting advertisements in the local community. They were screened for exclusion of psychiatric illness using the non-patient version of the SCID (First, Gibbon & William, 2002). The verbal component of the Wechsler Adult Intelligence Scale-Revised (WAIS-R; Wechsler, 1981) was administered to all participants as a measure of general intelligence. Demographic information of the sample is listed in Table 1.
Table.
Demographic information of participants. Means are reported above standard deviations. Education and age are in years. M stands for male and F for female. Verbal IQ was measured with the WAIS–R. T-tests showed that groups did not differ significantly on age, IQ or education (p > .05), and χ2 test of independence showed that the groups did not differ in terms of gender distribution (p >0.05).
| Group | Sex | Age | Verbal IQ | Education |
|---|---|---|---|---|
| Schizophrenia Patient* (n = 16) | M = 8 F = 8 |
41.8 (9.5) | 104.8 (11.4) | 14.5 (1.8) |
| Healthy Control (n = 10) | M = 3 F = 7 |
40.0 (13.5) | 40.0 (13.5) | 16.0 (2.3) |
Values in the patient group reflect the exclusion of the subject who was removed from all analyses.
2.2. Procedures
2.2.1. Direction discrimination: the trained task
Stimuli
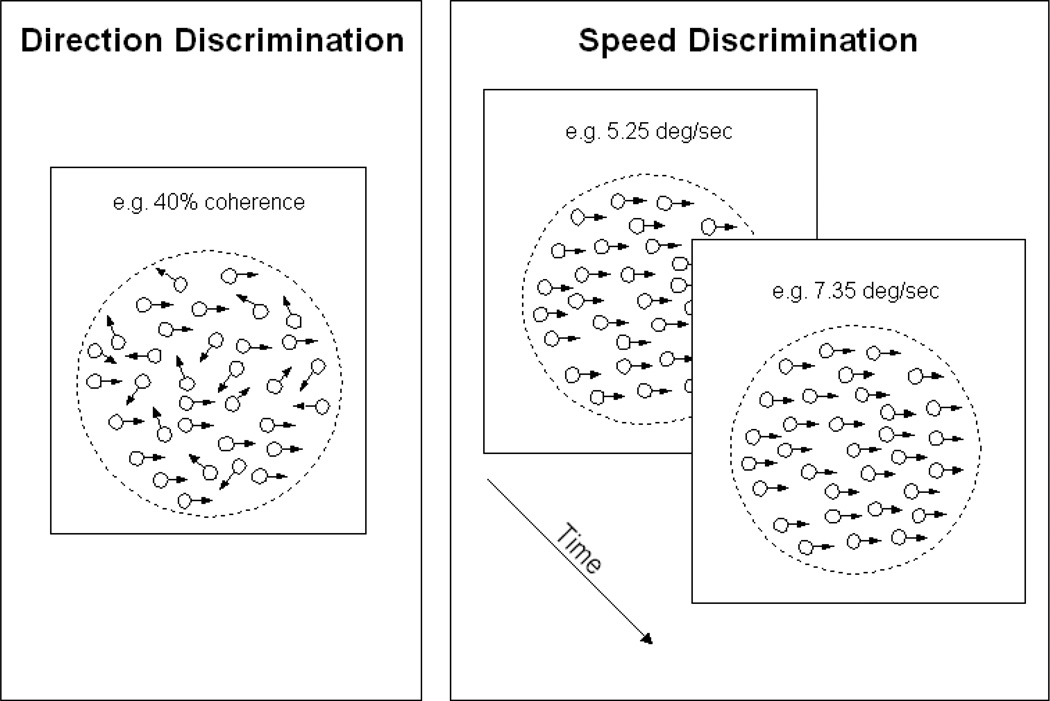
The stimulus for direction discrimination was a random dot pattern (RDP) (Figure 1a). It comprised a portion of dots moving in random directions (noise) and another portion moving coherently to the left or to the right (signal). The stimulus was displayed through a circular window subtending 7 degrees of visual angle. All dots (white) moved at 7 degrees per second against a black background, and had unlimited dot lifetime unless they moved outside of the circular window, in which case they were replaced by another dot that appeared inside the window. The luminance of the dots was 105 cd/m2, and the luminance of the background was 4.05 cd/m2. The size of the dots was 4.2 arc minutes, and the dot density was 3.8 dots per deg2. The presentation time for each stimulus was 400 msec. A fixation dot was present before and during the dot motion. The task was to indicate the direction of the signal dots in each stimulus presentation. The stimulus was generated via VisionShell (Raynald Comtois, Cambridge, MA, 2003) on a Macintosh G4 computer, and was presented on a 17 inch ViewSonic cathode ray tube monitor (refresh rate: 75 Hz). The experiment was done in a dimly lit room, and subjects were positioned 75 cm in front of the monitor (without a chinrest), and instructed to maintain that position during testing.
Figure 1.
Illustration of stimuli and procedures used for direction discrimination (the trained task) and speed discrimination (the untrained task).
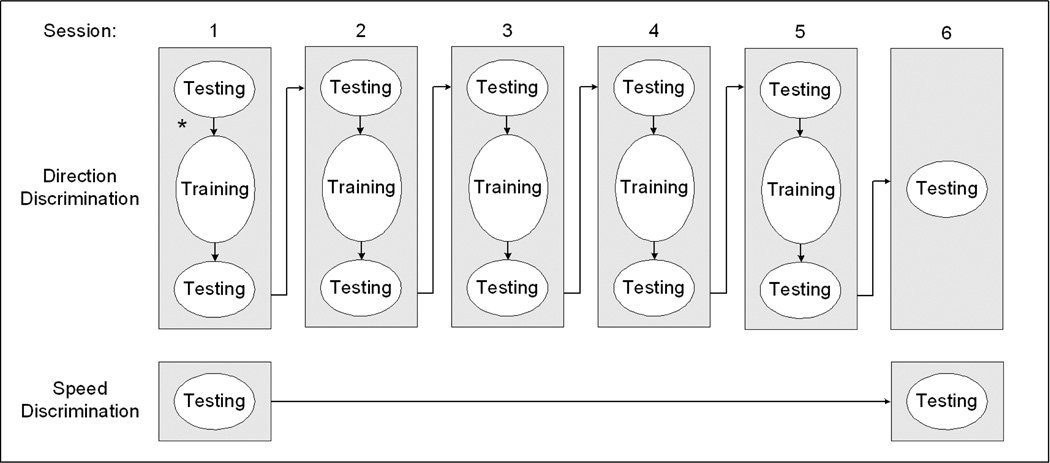
The perceptual learning procedure included two components—testing and training. There were six sessions in total (Figure 2), each occurring on a separate day. Each session included both testing and training, except the sixth session, which only entailed testing. For the first five sessions, testing was conducted before and after the training procedure. The six sessions were completed in two to three weeks, and the between-session intervals were typically two to four days (with no interval greater than one week).
Figure 2.
Illustration of assessment and training during the six sessions of perceptual learning. * Note that speed discrimination testing actually took place between the testing and training for direction discrimination on day one. On day 6, speed discrimination was assessed after the testing on direction discrimination.
Testing
The testing component used RDPs at six motion coherence levels - 0%, 5%, 10%, 20%, 40%, and 100%. The coherence levels of the stimulus were evenly divided but randomly distributed across trials according to the method of constant stimuli. With two directions of motion (left and right), and each of 6 coherence levels being repeated 8 times (2 × 6 × 8), the total number of trials was 96. Prior to the first session of testing, subjects were allowed to practice the task at an easy setting (50% and 100% coherence) until they felt comfortable with it (usually after 8–24 trials). The perceptual threshold was defined by fitting a Weibull function to the accuracy data and determining the minimum coherence level that yielded 80% accuracy (Chen, Bidwell & Holzman, 2005). While the thresholds were measured both before and after training on each session, data analysis was conducted only on those thresholds acquired at the beginning of the day. In doing so, the potential confound of training-related fatigue was eliminated in the perceptual threshold analysis.
Training
The training component used the same direction discrimination task as the testing component, except that the coherence levels of the RDPs were tailored to the individual subjects’ current performance level, so that the task was neither too easy nor too difficult. The coherence levels for each training session were determined using the subject’s current perceptual threshold acquired before training on that day (session). In each session, an average of eight training blocks was administered (minimum: 4 blocks, and maximum: 12 blocks). Each training block consisted of 96 trials (two directions, one coherence level, repeated 48 times). Two coherence levels (twice and 1.5 times that of the person’s threshold for the session) were used for training. A similar number of 2 and 1.5 times threshold level training blocks were administered. Each session thus contained between two and six blocks of training at each coherence level; the actual number of training blocks in a session depended on the duration of time that subjects could remain focused on the training. Breaks were encouraged between training blocks. The training on each day thus consisted of between 384 to 1,152 trials, resulting in a total of 1,920 to 5,760 trials across the five training sessions. As a group, patients completed an average of 1909 trials (SD = 448) at the 2X-threshold level and an average of 1828 trials (SD = 491) at the 1.5X-threshold level. Controls completed an average of 1791 trials (SD = 601) at the 2X-threshold training level, and 1779 trials (SD = 592) trials at the 1.5X-threshold training level. Patients and controls did not significantly differ in the total number of training trials received, F (1,25)= 0.17, p = 0.69, or in the proportion of 1.5X threshold trials to 2X-threshold trials. In order to minimize the influence of the top-down feedback factors, which may be compromised in patients, no feedback on subjects’ responses was given during training (or during testing).
All but one of the subjects completed the five training sessions. This subject, a normal control, completed four, instead of five, training sessions and her data were included in data analysis for direction discrimination. For speed discrimination, her performance was not measured after training, and therefore she was excluded from analyses on this task. One patient was an outlier compared with the rest of the patients; her thresholds increased, rather than decreased, with the training sessions (8.1% on Session 1, 10.3% on Session 2, 24.4% on Session 3, 9.6% on Session 4, 15.9% on Session 5, and 26.1% on Session 6), and this patient was excluded from data analysis.
2.2.2. Speed discrimination: the untrained task
Performance on speed discrimination (Figure 1b) was measured before the training on Session 1 (after the testing on direction discrimination), and again on Session 6 (Figure 2; also after the testing on direction discrimination). Subjects were not trained on this task. Speed discrimination was chosen as a comparison task since it employs motion stimuli similar to those used in the direction discrimination task, but relies on distinct brain mechanisms. Similarly to the direction discrimination task, subjects were allowed to practice the speed discrimination task until they felt comfortable with it (usually 8–24 trials).
Stimuli
The stimulus for speed discrimination was a pair of RDPs sequentially presented in each trial. The RDPs comprised coherently moving dots only (i.e. 100% signal). On a given trial, the two dot patterns moved in the same direction (leftward or rightward). An equal number of rightward and leftward moving dot patterns were presented in a testing block. The size of the RDPs was the same as in direction discrimination (7 degrees in diameter). The RDPs were presented for 667 msec each, with a 667 msec inter-stimulus interval. One of the RDPs traveled at 5.25 deg/sec, and the other traveled at 5.41, 5.51, 5.78, 6.30, 7.35 or 9.45 deg/sec.
Testing
The task was to indicate which of the two RDPs, the first or the second, traveled faster (Figure 1b). The speed differences of the two comparison RDPs, which represent various levels of task difficulty, were evenly divided but randomly distributed across trials according to the method of constant stimuli. The total number of trials was 96 (two directions, six speed differences, and eight repetitions). The perceptual threshold was defined as the minimum speed difference level at which the subject could perform the task with 80% accuracy.
3. Results
3.1. Direction discrimination: the trained task
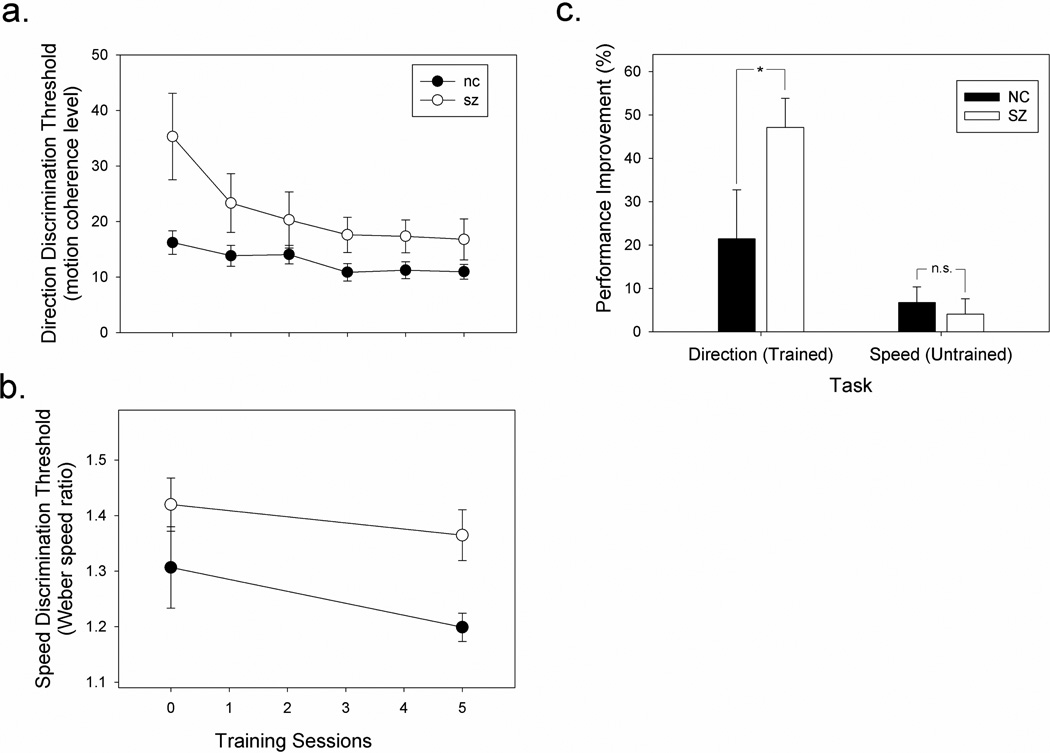
The training on direction discrimination significantly improved the performance on that task (Figure 3a). A two-way ANOVA1 (group × training session) showed a significant main effect for training session, F(5, 120) = 7.02, p < 0.001, η2 = 0.23, but not for group, F(5, 24) = 2.45, p = 0.13, η2 = 0.09. There was a non-significant interaction effect between group and training session, F(5, 120) = 2.57, p = 0.07, η2 = 0.10. Follow-up one-way ANOVAs were performed to see if the main effect for training session was present in both subject groups. The training effect was significant in both controls, F(5, 45) = 2.46, p = 0.047, η2 = 0.22, and patients2, F(5, 75) = 7.90, p = 0.001, η2 = 0.35. Post hoc t-tests revealed that performance levels in patients were significantly worse than in controls before training, t(24) = 2.48, p = 0.02, η2 = 0.20, but not after, t(24) = 1.23, p = 0.23, η2 = 0.06 (Figure 3).
Figure 3.
Summary of performance levels as a function of training sessions. a. Direction discrimination (the trained task). b. Speed discrimination (the untrained task). In both graphs, the abscissa indicates the number of direction discrimination training sessions, and the ordinate indicates subjects’ thresholds. In both tasks, lower thresholds indicate better performance. NC stands for normal control and SZ for schizophrenia. c. Improvement indices for the trained and untrained tasks. In all three graphs, error bars represent 1 standard error. For both tasks, performance improvement was calculated by dividing the difference between the threshold on Session 6 and Session 1 by the threshold for Session 1. Asterisk: p < 0.05; n.s.: non-significant.
Overall, the patient group showed greater improvement (average thresholds were lower by 47% after the training) than the control group (average thresholds were lower by 21% after training). Ten of the 16 patients improved their scores by at least 40%. We computed the proportion of performance improvement by taking the difference in threshold values on Session 1 and Session 6, and dividing by the threshold on Session 1. This provided an index of perceptual improvement (PI) as a result of training. The group difference in PI was marginally significant, t(24) = 2.06, p = 0.05, η2 = 0.15.
Patients had a lower mean verbal IQ score and education level than controls, though neither of these group differences was significant (Table 1). Also, the patient group had a slightly higher number of training trials (the group difference was not statistically significant, t(25) = 0.38, p = 0.69), and significantly higher initial direction discrimination thresholds than controls (as reported above). To ensure that the greater PI in the patient group was not due to these potential confounding variables we performed an analysis of covariance. When verbal IQ, education, original direction discrimination thresholds from day 1, and the number of training trials were simultaneously included in the analysis as covariates, the significance of the group difference in PI remained, F(1, 25) = 4.99, p = 0.037, η2 = 0.20. None of the covariates themselves were significant in determining PI values in the analysis (Day 1 thresholds, F(1, 25) = 2.49, p = 0.13, η2 = 0.11; verbal IQ, F(1, 25) = 1.20, p = 0.29, η2 = 0.06; education F(1, 25) = 1.47, p = 0.24, η2 = 0.07; training trial, F(1, 25) = 1.86, p = 0.18, η2 = 0.085).
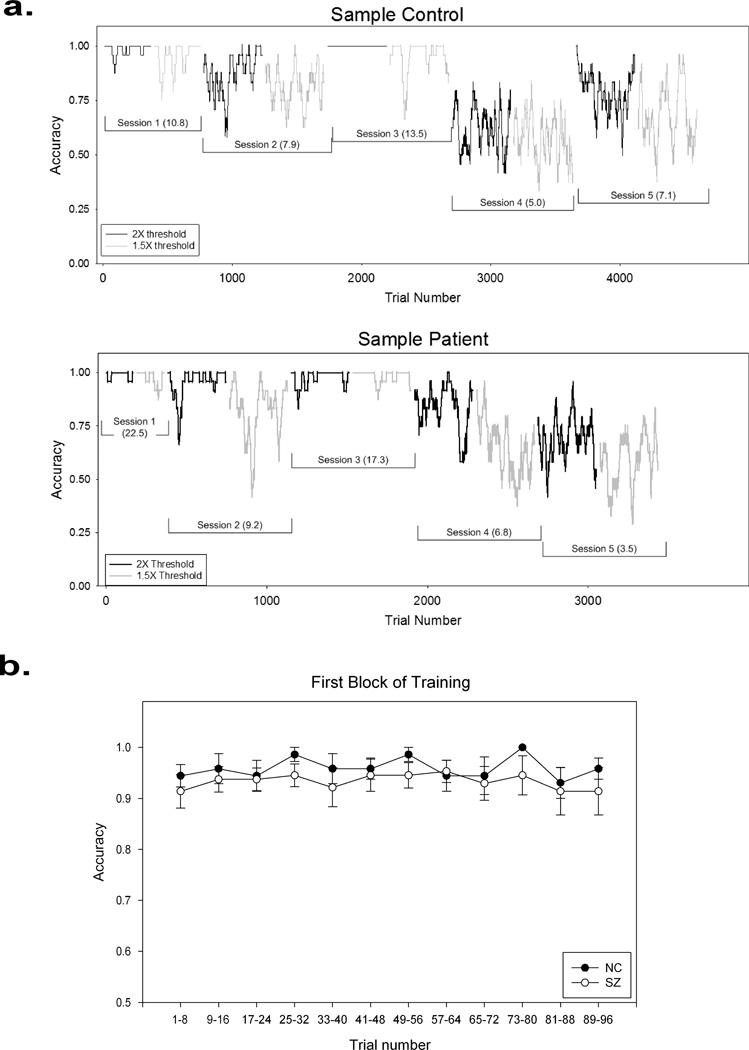
Performance during training was well above the chance level (50% accuracy) and also well below perfection (100% accuracy), which was expected, given that the task difficulty levels for training were tailored to current performance levels of individual subjects. Figure 4a shows typical performance during training for a patient and a control. In the patient group, the average accuracy was 90.5% (SD = 12.5%) when task condition for training was set at the 2X threshold, and 87.6% (SD = 10.8%) when task condition for training was set at the 1.5X threshold. In the control group, the average accuracy was 93.5% (SD = 8.8%) when task condition for training was set at the 2X threshold, and 87.0% (SD = 10.5%) when task condition for training was set at the 1.5X threshold. These results suggest that both groups performed at appropriate task difficulty levels—neither too easy nor too difficult. For both groups, performance on the more challenging training level (1.5X threshold) was slightly worse than on the easier training level (2X threshold), as would be expected. Both groups performed the task accurately at task difficulties tailored to their initial perceptual thresholds (Figure 4b), suggesting that the practice given before the first test was adequate to allow them to master the task procedures.
Figure 4.
a. Performance during training from a sample control (top panel) and patient (bottom panel). Abscissa represents trial number. Ordinate represents accuracy throughout a training session, which was obtained by averaging with a sliding window of 24 trials. Each accuracy point on the graph represents the average of one such window. The first point on the graph is the accuracy of the first 24 trials, and the second point is the accuracy of trials 2 to 25, etc. Normally, the 2X threshold trials (easier) were completed before the 1.5X threshold trials in each session, as was the case for these two examples. The training sessions are marked along with current perceptual thresholds (in parentheses), which were measured at the beginning of the each session. In general, lower thresholds are associated with lower training accuracies. b. Performance accuracy during the first block of training on Session 1. Accuracy was averaged over eight trials at a time, and then across subjects in each group. Error bars represent 1 standard error.
3.2. Speed discrimination: the untrained task
After training on the direction discrimination task, performance on speed discrimination also improved, though not significantly so (Figure 3b and 3c). A two-way ANOVA (group × training day) showed a significant effect for group F(1, 23) = 5.76, p = 0.025, η2 = 0.20, but not for training session, F(1, 23) = 4.14, p = 0.053, η2 = 0.15. The interaction between group and session was not significant, F(1, 23) = 0.15, p = 0.70, η2 = 0.01, indicating that speed discrimination in patients and controls changed similarly as a result of the training on the direction discrimination task. When considering each group separately, neither controls, t(8) = 1.79, p = .11, η2 = .29, nor patients, t(15) = 1.28, p = .22, η2 = .10, showed a significant improvement on speed discrimination. The proportion of improvement in patients (4.0%) was not significantly different from that in controls, 6.8%; t(23) = 0.48, p = 0.63, η2 = 0.01.
3.3. Task specific versus generalized learning
The performance improvements on the trained and untrained tasks were compared in an ANOVA. PI on the trained task was much greater than that on the untrained task, F(1,23) = 18.5, p < 0.001, η2 = 0.45 (as shown in Figure 3c). This effect was greater in patients than in controls, as revealed by a significant task × group interaction F(1,23) = 4.6, p = 0.043, η2 = 0.17. The main effect for group was not significant, F(1,23) = 2.32, p = 0.142, η2 = 0.091.
3.4. Relationship between perceptual learning and other variables
In patients, PI was not correlated with PANSS scores (general subscale: r(14) = −0.27, p = 0.31; positive subscale: r(14) = 0.10, p = 0.72; negative subscale: r(14) = −0.23, p = 0.38) or the level of antipsychotic medication being taken, r(14) = −0.11, p = 0.69. In neither group was PI correlated with verbal IQ scores, patients: r(14) = 0.02, p = 0.93, controls: r(8) = 0.55, p = 0.10, or years of education, patients: r(14) = 0.25, p = 0.38, controls: r(8) = 0.24, p = 0.51. PI on the trained task was not significantly correlated with the degree of improvement on the untrained task in either group, patients: r(14) = 0.20, p = .45), controls r(8) = 0.15, p = 0.70.
4. Discussion
Schizophrenia patients showed significant performance improvement as a result of the perceptual training, as indicated by lowered perceptual thresholds in direction discrimination task after training. Counter to our original hypothesis, the extent of the perceptual improvement in the patient group was greater, not lesser, than that in the control group.
4.1. Greater perceptual improvement in schizophrenia
The significant performance improvement shown here suggests that early perceptual processing is a promising target for intervention in schizophrenia. While cognitive deficits are often difficult to ameliorate in patients, targeting perceptual inputs may be a more feasible approach.
Did the patients’ large improvement on the trained task result from feature-specific perceptual learning, or from a more general sort of implicit learning? Implicit learning refers generally to the acquisition of knowledge, skill and habit that occurs automatically and outside of awareness. Implicit learning in the cognitive domain is moderately impaired in schizophrenia (Horan et al., 2008; Siegert, Weatherall & Bell, 2008), so if it accounted for the improvement in patients in the trained task, one would expect less improvement in patients than in controls. The data show an opposite scenario (larger learning effect in patients), so implicit learning is not likely a primary factor in determining the improvement on the trained task. Also, the training effect was much greater on the trained than on the untrained task, as reported in the results and shown in Figure 3c. Had generalized learning factors produced the improvement on the trained task, one would have expected a similar magnitude of improvement on the trained and untrained tasks, which were procedurally similar. Finally, both patients and controls showed stable and accurate performance during the first training block (Figure 4b), suggesting they all had mastered the task procedure. In other words, if they showed a clear improvement during the first training block, that would suggest that they were still mastering the task at that point. The bulk of the improvement on direction discrimination therefore appears to be indicative of increased perceptual sensitivity rather than procedural learning related to task mastery.
One possible explanation for the greater improvement in patients is that their poor performance prior to training allowed greater room for improvement. There appears to be some truth to this explanation, since baseline performance thresholds were moderately, though non-significantly, related with the extent of perceptual improvement in the analysis of covariance (p = 0.13). However, the greater amount of the improvement in patients cannot be accounted for entirely by this relation, as the ANCOVA on PI remained significant when baseline performance was included. It appears that an increase in perceptual sensitivity, i.e., perceptual learning, accounts for the bulk of the improvement in the patient group. In order to further confirm that perceptual learning underlies the improvement shown here, it would be useful to measure a different direction of motion (e.g. vertical directions) before and after training on horizontal direction discrimination. If the mechanism of improvement is localized early in visual cortex, within direction selective regions, then learning should not, at least not immediately, transfer to untrained directions. The lack of such a condition is a limitation of the present study.
4.2. Neural mechanisms of the improvement on direction discrimination
The stark improvement in the patient group may at first seem counterintuitive, given that perceptual learning relies heavily on neuroplasticity, and that neuroplasticity is hypothesized to be abnormal in schizophrenia (Frankle et al., 2003). However, the abnormality can be in both directions, namely more or less plastic than normal. For example, long-term potentiation (LTP) was augmented in the prefrontal cortex (Goto & Grace, 2006), but impaired in hippocampus (Balu et al., in press). Behaviorally, patients showed reduced plasticity in a motor domain (Daskalikas et al., 2008). The results of the present study show that patients’ performance improvement was substantially greater than that of healthy controls, suggesting that, unlike the motor domain, plasticity is strongly represented in the visual system in schizophrenia. It is possible that the different training effects—increased in the perceptual domain, and decreased in the motor domain—are tied to different mechanisms underlying these two different sorts of learning. Perceptual learning is long lasting, on the order of months at least (Sekuler & Ball, 1982). Motor plasticity in schizophrenia has been studied using a use dependent plasticity paradigm (Daskalakis et al., 2008). Here, spontaneous thumb movements, as a result of transcranial magnetic stimulation (TMS), are recorded before and after 30 minutes or so of training. After training, thumb movements in response to TMS are in the trained direction, with the effect lasting several minutes. The difference in retention length of training effects suggests that different mechanisms are at work in the two types of learning. While perceptual learning, which lasts months, is likely a result of structural reorganization in the synapse, use dependent plasticity in the motor cortex may result from more rapid and transient mechanisms (Butefisch et al., 2000; Zucker and Regehr, 2002).
The Middle Temporal Area (MT) in extrastriate cortex is a key area for processing the motion stimuli used in this task (Newsome & Pare, 1988). In schizophrenia, it has been found that activations in MT were significantly reduced during motion perception (Chen et al., 2008) and eye tracking (Lencer, Nagel, Sprenger, Heide, & Binkofski, 2005). In perceptual training, the improvement in visual sensitivity is often accompanied by an increase in activation of early sensory processing areas (Schwartz et al., 2002; Pourtois et al., 2008). In this context, the results of poor initial sensitivity to motion in patients, combined with the large amount of improvement in the same patients, suggests that while MT functions are disrupted in schizophrenia, they may be able to be augmented through perceptual learning.
4.3. Transferable perceptual improvement?
Patients and controls both showed moderate improvement on speed discrimination after having been trained on direction discrimination, although the training effect did not reach statistical significance (p = 0.053). The non-significance of the effect may be due to the relatively small sample size used in the present study. It is difficult to interpret whether such an improvement is the result of perceptual learning in a specific domain of visual motion (i.e., the direction of motion), a result of general learning factors related to training on direction discrimination, or is a result of having had practice with the speed discrimination task itself (i.e., a test-retest effect). The procedures for the trained and untrained tasks were similar (i.e. making judgments about the physical attributes of random dot patterns by pressing one of two buttons on a keyboard), so learning related to these generalized procedures also may have produced performance improvement on the untrained task (e.g. skill at maintaining attention throughout assessment etc.). At the same time, the velocities used in the direction discrimination task (7 deg/sec) overlapped with those in the speed discrimination task (5.25 deg/sec – 9.4 deg/sec), so some perceptual learning transfer might also be expected between the tasks based on the fact that some of the stimuli in speed discrimination (the untrained task) were close to identical to those used in direction discrimination (the trained task) (Paffen, Verstraten & Vidnyánszky, 2008). In addition, the training on direction discrimination was set at a fairly easy level of task difficulty (accuracy in training hovered around 90%), which has been shown to facilitate transfer of perceptual learning (Liu, 1999; Liu & Weinshall, 2000). It is possible that, had participants not been trained on direction discrimination, they would have still performed better on the second time they took the speed discrimination test, due to increased familiarity with the test, improved strategy, or other such factors. But this test-retest effect seems unlikely to be a major factor, as a previous study showed that schizophrenia patients and controls had stable speed discrimination performances over four separate testing sessions (Chen, Palafox et al.,1999).
In order to clearly demonstrate that the improvement on the untrained task is a result of perceptual learning, rather than procedural learning, it would be useful to test training effects on a similar task (i.e. direction discrimination) using a different stimulus (e.g. static arrows instead of moving dots). If there were improvement of the speed discrimination task (the untrained task here) and not on this direction discrimination task using arrows, then the improvement on the speed discrimination task would have to be stimulus-specific, and therefore perceptual in nature. Future studies should consider such a control task.
One study, comparing training on direction and speed discrimination in healthy participants, did not find a transfer of performance improvement between the trained and the untrained tasks (Saffell & Matthews, 2003). Two factors may have contributed to the difference between the cited study and our study. In the cited study, task difficulty during training was set at 70% accuracy level whereas in our study the task difficulty during training was set at closer to 90% accuracy. Another difference between the two studies was that the cited study’s training comprised moderately fewer trials (2,400) than ours (3,840 on average). The less challenging and more numerous training trials in our study might have been helpful for the training effect to be generalized (Liu & Weinshall, 2000). The transfer of the perceptual training effect to an untrained task, though modest, is potentially important, as it provides a basis for exploring the possibility of improving cognitive functioning via perceptual learning of related basic visual features.
4.4. Neuroplasticity-based intervention
Can perceptual training in schizophrenia actually have a positive and practical impact in patients’ lives? One way to address this question would be to evaluate the effects of the perceptual interventions on the cognitive functions that rely on relevant perceptual signals. Recent studies have found that applying neuroplasticity-based training to basic auditory features can in fact improve a variety of cognitive functions in schizophrenia patients, such as verbal working memory (Fisher et al., 2009; Fisher, Holland, Subramaniam, & Vinogradov, 2010). The cognitive improvement after training on perceptual features suggests the validity of a bottom-up training approach for intervening in cognitive dysfunction.
In principle, improvement in sensory processing should be effective for improving higher order processes in schizophrenia. Low-level sensory abnormalities affect the more complex perceptual processes that require integration of these sensory signals (Kantrowitz et al., 2009; Norton et al., 2008, 2009; McBain et al., 2010). Higher order cognitive processes such as social perception, forming a theory of mind of other people, and emotion perception are also likely affected by abnormalities in earlier stages of perceptual processing (Leitman, Sehatpour, Higgins, et al., 2009; Sergi & Green, 2009; Butler, et al., 2009; Javitt, 2009). Therefore, improving the quality of perceptual signals is highly likely to have a positive effect on higher order functions that are usually referred to as “cognition”. Cognitive training in schizophrenia leads to changes in patterns of brain activity when performing cognitive tasks (Edwards, Barch & Braver, 2010; Haut, Lim & MacDonald, 2010). It will be interesting to see if and how perceptual training leads to similar improvement on cognitive tasks, and to similar changes in associated brain activity.
Perception of movement in particular provides critical signals for a variety of cognitive and social activities. One powerful example of this is biological motion, where the intentions of a visually impoverished human figure are inferred from the motion signals of that figure (Johansson, 1973). Biological motion perception is impaired in schizophrenia (Kim, Doop, Blake & Park, 2005). A recent study found that impairments in basic motion perception and biological motion perception are correlated (Brittain et al., in press). Also, deficient motion perception in patients is associated with deficient social cognitive functioning. Kelemen and colleagues (2005) showed that motion coherence thresholds in schizophrenia patients were strongly correlated with scores on the “Eyes Test”, a measure of Theory of Mind, while performance on form discrimination was not. These intriguing results leave open the possibility that impairments in motion perception and social cognition may be especially related in schizophrenia. In light of this relationship, improvement of deficient motion processing in schizophrenia patients represents a promising bottom-up approach to the remediation of social cognitive dysfunction.
The present study was an exploratory investigation into the integrity of perceptual learning in schizophrenia and did not measure social cognitive status. Future studies would do well to examine this relationship, as well as the relationship of motion perception and other cognitive variables such as those measured in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) tests (Nuechterlein et al., 2008). Such knowledge may shed light on the possibility of transferring perceptual learning to the improvement of quality of life in patients.
4.5. Conclusion
In the present study, patients’ performance in judging the direction of motion improved to such an extent that, after learning, they scored similarly to healthy controls prior to learning. The large training-induced change in patients’ performance suggests the presence of a significant amount of plasticity at the visual processing level. Such intrinsic properties of the visual system can be potential bases for the development of bottom-up behavioral interventions in this mental disorder.
Visual motion processing is deficient in schizophrenia.
Patients received neuroplasticity-based training on direction discrimination.
Performance in patients was improved to a greater extent than in controls.
A robust plasticity is preserved within the visual system in schizophrenia.
Deficient perception may be a promising target for cognitive intervention.
Acknowledgements
This work was supported in part by NIH grant MH R01 61824 and a grant from Harvard University, both to Dr. Chen. We thank the subjects for their extensive participation, as well as Drs. Charles Stromeyer III and Tim Brown, Andrea Cataldo, Stephanie Dibble, Allyson Hodgkins, Grace Masters, Daniel Seichepine and Jenna Glasenberg for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A Huynh-Feldt correction for violation of the sphericity assumption was applied, ε = .51, p < 0.001.
A Huynh-Feldt correction for violation of the sphericity assumption was applied, ε = .54, p < 0 .001.
Contributor Information
Daniel J. Norton, McLean Hospital.
Ryan K. McBain, McLean Hospital.
Dost Ongur, McLean Hospital, Department of Psychiatry, Harvard Medical School.
Yue Chen, McLean Hospital, Department of Psychiatry, Harvard Medical School.
References
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Balu DT, Carlson GC, Talbot K, Kazi H, Hill-Smith TE, Easton RM, Birnbaum MJ, Lucki I. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus. doi: 10.1002/hipo.20887. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neuroscience and Biobehavioral Reviews. 2011;35:848–870. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Wilt MA, Lysaker PH, Koyfman A, O'Donnell BF. Psychometrically matched visual-processing tasks in schizophrenia spectrum disorders. Journal of Abnormal Psychology. 2003;112:28–37. [PubMed] [Google Scholar]
- Brittain P, Ffytche DH, McKendrick A, Surguladze S. Visual processing, social cognition and functional outcome in schizophrenia. Psychiatry Research. doi: 10.1016/j.psychres.2009.09.013. in press. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proceedings of the National Academy of Sciences of the U.S.A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Abeles IY, Weiskopf NG, Tambini A, Jalbrzikowski M, Legatt ME, Zemon V, Loughead J, Gur RC, Javitt DC. Sensory contributions to impaired emotion processing in schizophrenia. Schizophrenia Bulletin. 2009;35:1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Current Opinion in Psychiatry. doi: 10.1097/YCO.0b013e3283436eb7. in press. [DOI] [PubMed] [Google Scholar]
- Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull. 2011;37:709–715. doi: 10.1093/schbul/sbr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophrenia Research. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, et al. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cognitive and Affective Behavioral Neuroscience. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proceedings of the National Academy of Sciences of the U.S.A. 1999;96:4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophrenia Research. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Norton D, Ongur D. Altered center-surround motion inhibition in schizophrenia. Biological Psychiatry. 2008;64:74–77. doi: 10.1016/j.biopsych.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cognitive Affective & Behavioral Neuroscience. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Dobkins KR. Compromised speed discrimination among schizophrenia patients when viewing smooth pursuit targets. Schizophrenia Research. 2007;95:61–64. doi: 10.1016/j.schres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Archives of General Psychiatry. 2008;65:378–385. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- Edwards BG, Barch DM, Braver TS. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Frontiers in Human Neuroscience. 2010;4:32. doi: 10.3389/fnhum.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: specificity versus generalization. Current Opinions in Neurobiology. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Fine I, Jacobs RA. Comparing perceptual learning tasks: a review. Journal of Vision. 2002;2:190–203. doi: 10.1167/2.2.5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders — Patient Edition. Biometrics Research Department, New York. 2002a (SCID — I/P, 11/2002 revision) [Google Scholar]
- First MB, S R, Gibbon M, William JBW. New York, NY Biometric Research Department. New York State Psychiatric Institute; 2002b. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition. (SCID-I/NP, 11/2002b revision) [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia. American Journal of Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophrenia Bulletin. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle W, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Perceptual learning. Annual Review of Psychology. 1963;14:29–56. doi: 10.1146/annurev.ps.14.020163.000333. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biological Psychiatry. 2006;60:1259–1267. doi: 10.1016/j.biopsych.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biological Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, et al. Perception Measurement in Clinical Trials of Schizophrenia: Promising Paradigms From CNTRICS. Schizophrenia Bulletin. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut KM, Lim KO, MacDonald A., 3rd Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35:1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Knowlton BJ, Wynn JK, Mintz J, Nuechterlein KH. Impaired implicit learning in schizophrenia. Neuropsychology. 2008;22:606–617. doi: 10.1037/a0012602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. Perceptual learning improves contrast sensitivity of V1 neurons in cats. Current Biology. 2010;20:887–894. doi: 10.1016/j.cub.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annual Review of Clinical Psychology. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–211. [Google Scholar]
- Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. Seeing the world dimly: the impact of early visual deficits on visual experience in schizophrenia. Schizophrenia Bulletin. 2009;35:1085–1094. doi: 10.1093/schbul/sbp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler L. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia. Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Erdelyi R, Pataki I, Benedek G, Janka Z, Keri S. Theory of mind and motion perception in schizophrenia. Neuropsychology. 2005;19:494–500. doi: 10.1037/0894-4105.19.4.494. [DOI] [PubMed] [Google Scholar]
- Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophrenia Research. 2005;77:299–307. doi: 10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. American Journal of Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. Neuroimage. 2005;24:1256–1259. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Liu Z, Weinshall D. Mechanisms of generalization in perceptual learning. Vision Research. 2000;40:97–109. doi: 10.1016/s0042-6989(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Liu Z. Perceptual learning in motion discrimination that generalizes across motion directions. Proceedings of the National Academy of Sciences of the U.S.A. 1999;96:14085–14087. doi: 10.1073/pnas.96.24.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophrenia Research. 2010;122:151–155. doi: 10.1016/j.schres.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D, Öngür D, Stromeyer C, III, Chen Y. Altered temporal interaction in response to two light pulses in schizophrenia. Schizophrenia Research. 2008;103:275–282. doi: 10.1016/j.schres.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D, McBain R, Holt DJ, Ongur D, Chen Y. Association of impaired facial affect recognition with basic facial and visual processing deficits in schizophrenia. Biological Psychiatry. 2009;65:1094–1098. doi: 10.1016/j.biopsych.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Paffen CL, Verstraten FA, Vidnyánszky Z. Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. Journal of Vision, 8. 2008;25:1–11. doi: 10.1167/8.4.25. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Rauss KS, Vuilleumier P, Schwartz S. Effects of perceptual learning on primary visual cortex activity in humans. Vision Research. 2008;48:55–62. doi: 10.1016/j.visres.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Saffell T, Matthews N. Task-specific perceptual learning on speed and direction discrimination. Vision Research. 2003;43:1365–1374. doi: 10.1016/s0042-6989(03)00137-8. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Green MF. Social perception and early visual processing in schizophrenia. Schizophrenia Research. 2003;59:233–241. doi: 10.1016/s0920-9964(01)00405-4. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proceedings of the National Academy of Sciences of the U.S.A. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Weatherall M, Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain and Cognition. 2008;67:351–359. doi: 10.1016/j.bandc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Holthouse T, Hawkes A, Bruno R. Eye movement and visual motion perception in schizophrenia II: Global coherent motion as a function of target velocity and stimulus density. Experimental Brain Research. 2007;182:415–426. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- Stuve T, Friedman L, Jesberger JA, Gilmore G, Strauss ME, Meltzer H. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychological Medicine. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- Tadin D, Kim J, Doop ML, Gibson C, Lappin JS, Blake R, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. Journal of Neuroscience. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the adult intelligence scale–revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. Journal of Neuroscience. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Sasaki Y, Chan P, Vasios CE, Bonmassar G, Ito N, et al. Location-specific cortical activation changes during sleep after training for perceptual learning. Current Biology. 2009;19:1278–1282. doi: 10.1016/j.cub.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cerebral Cortex. 2010;20:1749–1755. doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Celebrini S, Britten KH, Newsome WT. Neuronal plasticity that underlies improvement in perceptual performance. Science. 1994;263:1289–1292. doi: 10.1126/science.8122114. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual Review of Physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]