Abstract
Zebrafish (Danio rerio) are rapidly emerging as a useful animal model in neurobehavioral research. Mounting evidence shows the suitability of zebrafish to model various aspects of anxiety-related states. Here, we evaluate established and novel approaches to uncover the molecular substrates, genetic pathways and neural circuits of anxiety using adult zebrafish. Experimental approaches to modeling anxiety in zebrafish include novelty-based paradigms, pharmacological and genetic manipulations, as well as innovative video-tracking, 3D-reconstructions and bioinformatics-based searchable databases and omics-based tools. Complementing traditional rodent models of anxiety, we provide a conceptual framework for the wider application of zebrafish and other aquatic models in anxiety research.
Keywords: zebrafish, anxiety, novelty-based paradigms, pharmacological and genetic manipulations, bioinformatics, omics-based tools
1. Introduction
One of the central questions in biological psychiatry is how genes, molecular pathways and patterns of connectivity in the brain produce and modulate anxiety behavior (Bishop 2007; Olivier et al. 1998; Landgraf and Wigger 2002; Suveg et al. 2010; Burgess and Granato 2008). Through the use of numerous behavioral paradigms, genetic/pharmacological screens and neuroimaging, animals have been extensively used to model anxiety pathogenesis (Clement et al. 2002; Conti et al. 2004; de Angelis 1996; Ditzen et al. 2006; Kalueff et al. 2007). The zebrafish (Danio rerio) has emerged as a useful new model for studying the behavioral and molecular mechanisms of brain disorders (Cachat et al. 2010a; Cachat et al. 2011; Stewart et al. 2010a; Stewart et al. 2010b; Blaser et al. 2010; Maximino et al. 2010a; Sackerman et al. 2010).
Although fish behavior was initially presumed to be mostly primitive and instinctively driven (Burt de Perera 2004a; Laland et al. 2003); recent studies have revealed the complexity of zebrafish behavior, and its relevance to modeling fear- and anxiety-like states (Stewart et al. 2010b; Cachat et al. 2011; Bencan et al. 2009; Blaser et al. 2010; Speedie and Gerlai 2008). Mounting evidence also indicates that zebrafish anxiety-like behavior is driven by similar environmental factors as that of rodents (Champagne et al. 2010; Stewart et al. 2010a), and involves evolutionarily conserved circuits (Lee et al. 2010; Amo et al. 2010) that regulate aversive learning and emotionality (Jesuthasan 2011 (in press); Agetsuma et al. 2010). Furthermore, robust anxiogenic endocrine and genomic responses, similar to those seen in humans (Schulkin et al. 1998; Yehuda et al. 1993; Boulom et al. 2002; Zhang et al. 1992) and rodents (Yau et al. 1997; Roy et al. 2001; Hale et al. 2008; Kung et al. 2010), have also been established in zebrafish (Cachat et al. 2010a; Williams et al. 2011 (in press); Wong et al. 2010a; Baraban et al. 2005; Barcellos et al. 2007). Additionally, like rodents (Dvorkin et al. 2008; Eilam and Golani 1989), fish form spatial memories (Burt de Perera 2004b; Riedel 1998), and use them to orient in novel environments, establishing “safe zones” (homebases) where they frequently dwell and return to (Stewart et al. 2010c; Stewart et al. 2010d; Rosemberg et al. 2011). Moreover, zebrafish also display a robust habituation responses (Wong et al. 2010b), which in addition to cognitive map formation (O’keefe 1978), may represent deep neurobiological constructs, such as adaptive processing of sensory information (Eisenstein and Eisenstein 2006).
While affective disorders research has traditionally utilized rodent paradigms (Blanchard et al. 2003; de Mooij-van Malsen et al. 2010 (in press); Hefner and Holmes 2007), interest is growing in the use of zebrafish as a novel and high-throughput model of anxiety (Champagne et al. 2010; Stewart et al. 2010a; Stewart et al. 2010b; Blaser et al. 2010). For example, numerous paradigms have been adapted from rodents and applied to zebrafish (Table 1), showing a striking similarity in reduced exploration, and increased thigmotaxis or freezing (Cachat et al. 2010b; Maximino et al. 2010a; Maximino et al. 2010b; Gaikwad et al. 2011; Levin et al. 2007). In addition, zebrafish anxiety research has the advantage of using both adults and larvae, complementing the throughput of larval models with the complexity of neural phenotypes of adult animals (Norton and Bally-Cuif 2010a; Stewart et al. 2010a; Egan et al. 2009; Grossman et al. 2010; Burne et al. 2011; Cachat et al. 2010b; Webb et al. 2009). Zebrafish also display robust behavioral phenotypes, which are exhibited through overt and easily quantified behavioral endpoints. Finally, the high-throughput nature of zebrafish models also makes them an excellent species for studying various experimental, genetic, and pharmacological factors in anxiety (Stewart et al. 2010a; Chakraborty et al. 2009; Burgess and Granato 2008; Dlugos and Rabin 2003). Here, we discuss several current and emerging approaches that use zebrafish to uncover the neurobiological mechanisms that control animal anxiety behavior.
Table 1.
Summary of several commonly used paradigms for assessing anxiety-related behavior in adult zebrafish.
| Test rationale | Test description and major endpoints | Key references |
|---|---|---|
| Novel tank test* | ||
| Exposure to a novel arena, similar to rodent open field test* | After a period of acclimation, zebrafish are placed individually in a narrow tank (e.g., 1.5-L) maximally filled with water and divided into two (or three) equal virtual horizontal sections, demarcated with a line on the outside walls. The following endpoints are typically recorded in the novel tank test for 5–6 min: the latency to reach the upper portion of the tank (s), time spent in the upper portion of the tank (s), number of transitions (entries) into the upper portion of the tank, number of erratic movements, number of freezing bouts and time spent frozen (s). Increased anxiety is typically accompanied with reduced exploratory behavior, thigmotaxis and geotaxis. In addition to manual recording, video-recording can be used in this test, assessing distance traveled, average velocity, turning angle, and angular velocity. | (Egan et al. 2009; Wong et al. 2010b; Stewart et al. 2010e; Levin et al. 2007; Bencan et al. 2009) |
| Open field test* | ||
| Exposure to a novel arena, similar to the rodent open field test* | After a period of acclimation, zebrafish are placed individually in a novel arena filled with water to the level of about 12 cm. Apparatuses of differing size, color, shape, and texture can be used. Due to the nature of the experimental set-up, manual observation may be precluded. However, video-tracking can be used to measure relevant endpoints, including those associated with thigmotaxis and exploration. Other analyses of anxiety may include assessments of spatiotemporal patterning and/or lateral swimming in response to a stressor. Parameters may be defined to divide the arena into separate zones to record behavior in specific regions of the tank. The following endpoints are typically recorded in the tank for 6 or 30 min: time spent in the periphery or center of the tank (s), distance traveled in each zone in the tank (m), velocity in each zone of the tank (m/s), number of transitions (entries) between zones, number of freezing bouts and time spent frozen (s). | (Stewart et al. 2010c; Stewart et al. 2010d; Grossman et al. 2010) |
| Light-dark box | ||
| Rodent light-dark box as a measurement of scototaxis | After a period of acclimation, zebrafish are placed individually in the light-dark box filled with water (e.g., to a height of 12 cm) and representing a rectangular tank divided into two equal vertical portions by black and white coloration. The following endpoints are typically recorded in this test: latency to enter the white half (s), time spent in the white half (s), the number of entries to the white half of the apparatus. White:total time spent ratios can be calculated to assess scototactic behavior. In addition to manual recording, video-recording can be used in this test, assessing distance traveled, average velocity, turning angle, and angular velocity in the white compartment. | (Maximino et al. 2010b; Maximino et al. 2010c; Stewart et al. 2010f; Serra et al. 1999) |
| Social preference test | ||
| Measures social behavior | After a series of habituation and training trials, zebrafish are placed in groups in a tank (e.g., 40-L) resting on level surface and maximally filled with water. The paradigm has been use to assess how zebrafish respond to conspecific, heterospecific, or changes in coloration and patterning. Prospective shoaling partners can be separated by transparent Plexiglass, with variation in preference exhibited by the subject fish then observed. With the addition of an anxiety-inducing stressor (e.g., anxiogenic or anxiolytic drug, predator) or memory modulating agent, social preference may also be altered. | (Saverino and Gerlai 2008; Engeszer et al. 2004) |
| Shoaling | ||
| Measures the effects of anxiety on social behavior | Zebrafish are placed in groups in a tank (e.g., 40-L) resting on level surface and maximally filled with water. The fish may be exposed to pharmacological manipulation or other type of stressor to evoke anxiety. The cohesion of their shoal is then assessed, with an anxiogenic response leading to an increase in shoal cohesion. Alternatively, with lower anxiety, fish have a greater tendency to break away from the group, and shoal cohesion is lessened. To assess shoaling, still images are obtained for every 10 s via video- recording, and the average distance among all members of the experimental zebrafish shoal, the average distance among all members of the stimulus group, and the average distance between all experimental and stimulus fish can be quantified. The fish coloration and the “nearest neighbor” distance can also be assessed in this test | (Engeszer et al. 2004; Miller and Gerlai 2007; Wright et al. 2003; Ruhl and McRobert 2005) [May be add some m ore refs on fish coloration and nearest neighbor distance in the shoaling test – can you find them?] |
| Boldness and novel object approaching | ||
| A novelty-based paradigm that can also be adapted to assess predator avoidance or social behavior | Boldness has been assessed in zebrafish, such as through latency to feed after a disturbance and biting to a mirror stimulus. The novel object test is based on placing fish in a cylindrical tank devoid of visual cues, either individually or in a group, and after a period of acclimation, a novel stimulus in introduced. Video-aided analysis can be used to segregate the tank into concentric rings centered around the object, with the following endpoints typically recorded for 10 min: latency to approach the object (s), frequency of approach, time spent near the object (s), number of freezing bouts, and time spent frozen (s). | (Wright et al. 2003; Wright et al. 2006; Ogwang 2008; Moretz et al. 2007) |
| Predator avoidance | ||
| Assesses fear- and anxiety-like behavior in the presence of a natural stressor | The tendency for zebrafish to avoid an inherent stressor, such as the natural predator Indian leaf fish, is assessed. Zebrafish are place in the center of a two-compartment choice test with a predator placed in one of the apparatus arms. Avoidance or willingness to approach the predator is then measured. The following endpoints are typically recorded: the latency to enter the same arm as the predator (s), time spent in the same arm as the predator (s), time spent in the arms without the predator (s) number of transitions (entries) into the same arm as the predator, and number of transitions (entries) into the arms without the predator. | (Bass and Gerlai 2008; Gerlai 2010) |
While the zebrafish novel tank and open field tests are both based conceptually on the rodent open field paradigm, they differ in several key aspects, as the novel tank test mainly assesses geotaxic “vertical” behavior, and the zebrafish open field test mainly measures thigmotaxis and locomotion in the horizontal direction.
2. Behavioral modulation and analyses of zebrafish anxiety
Among several zebrafish anxiety paradigms (Table 1), the novel tank test has become one of the most popular tests (Cachat et al. 2010c; Stewart et al. 2010b; Wong et al. 2010b; Egan et al. 2009). Anxiety in this test is reflected in reduced exploration (i.e., longer latency to reach the top, fewer entries to the top, longer and more frequent freezing) together with elevated erratic movements and freezing (Levin et al. 2007; Barcellos et al. 2007; Egan et al. 2009; Cachat et al. 2010a; Stewart et al. 2010b).
While the link between anxiety and memory is well-recognized, the neurobiology of their interplay remains poorly understood, and has only recently been examined in zebrafish (de Castro et al. 2009; Gaikwad et al. 2011; Piato et al. 2011). Notably, the memory-impairing effects of acute stress on zebrafish strikingly parallels both rodent and clinical data (Morrow et al. 2000; Yun et al. 2010; Chen et al. 2010; Wright and Conrad 2005; Hu and Wang 2006; Hardison and Purcell 1959; Gaikwad et al. 2011; Sandi et al. 2005; Diamond et al. 2006; Diamond et al. 1999; Park et al. 2008; El Hage et al. 2006). Specifically, chronic restraint stress (Yun et al. 2010; Chen et al. 2010; Wright and Conrad 2005; Hu and Wang 2006) and exposure to a predator or its odors (Sandi et al. 2005; Diamond et al. 1999; Morrow et al. 2000; Park et al. 2008; Diamond et al. 2006; El Hage et al. 2006; Cohen et al. 2009; Kozlovsky et al. 2008; Woodson et al. 2003) impairs memory in rodents, while acute psychological stress affects cognitive functions in humans (Hardison and Purcell 1959). In such paradigms, ecologically relevant stressors (e.g., predator exposure) are especially useful for eliciting an innate anxiety response, and have also been applied to zebrafish (Gerlai et al. 2009; Bass and Gerlai 2008; Egan et al. 2009). For example, examining the effects of 24- and 72-h exposure to the Indian leaf fish on zebrafish exploration, we found that novel tank testing does not reflect the traditional indices of anxiety per se, but triggers the apparent escape-like hypervigilance, as evidenced by an observed increase in locomotion and erratic movements (own unpublished data) similar to those evoked in rodents by predator stress (Blanchard et al. 1990; Blanchard et al. 2001). In contrast, chronic unpredictable mild stress reduced zebrafish exploratory behavior, but decreased shoal cohesion, suggesting a lower energy and/or decreased novelty seeking associated with an extended stress period (Piato et al. 2011) – a phenotype resembling rodent chronic unpredictable stress, with anhedonia and reduced self-care behavior and exploration (Isingrini et al. 2010; Pandey et al. 2010; Kazlauckas et al. 2011).
In addition to the high sensitivity of zebrafish anxiety-related behaviors, they benefit from being three-dimensional (3D) due to an additional vertical dimension (Cachat et al. 2010c; Cachat et al. 2011; Grossman et al. 2010; Rosemberg et al. 2011). Thus, while rodent models are traditionally studied in 2D coordinates, zebrafish paradigms offer an enhanced dimensionality for phenotyping anxiety (Grossman et al. 2010; Cachat et al. 2011) profiles. As a more “realistic” assessment of zebrafish behavior, 3D analyses can also be used to globally assess behavioral profiles while mapping individual endpoints to the spatiotemporal reconstructions. Cluster analyses may also complement 3D reconstructions to identify informative subgroups within a large data set, categorize experimental manipulations or behavioral endpoints based on the similarity of their alterations, consolidate behavioral data and increased their density (Cachat et al. 2011).
3. Pharmacological modulation of zebrafish anxiety
In addition to behavioral manipulations, zebrafish anxiety can be challenged pharmacologically, as recently comprehensively evaluated in (Stewart et al. 2010b). To assess pharmacogenic anxiety, fish are usually treated with an anxiogenic or anxiolytic agent in exposure beakers (acute treatment) or home tanks (chronic treatment) prior to testing (Cachat et al. 2010a; Cachat et al. 2011; Grossman et al. 2010; Stewart et al. 2010b; Stewart et al. 2011a). Several compounds recently tested in adult zebrafish will be used as examples here, to illustrate fish sensitivity to various anxiotropic drugs. Tranylcypromine (TCP) blocks the degradation of serotonin (Jie et al. 2009), lysergic acid diethylamide (LSD) is a potent hallucinogen that acts on several serotonin receptors (Backstrom et al. 1999; Wing et al. 1990), and dizocilpine (MK-801) is an antagonist of N-methyl-D-aspartate (NMDA) receptors (Del Pozo et al. 1996; Layer et al. 1993).
Monoamine oxidase inhibitors (MAOIs) are widely used clinically to treat anxiety (Ballenger 1999; Mallinger et al. 2009) and yield similar results in rodents for both chronic (Crawley 1985; Maki et al. 2000; Takamori et al. 2001) and acute (Maki et al. 2000; de Angelis 1996; Freund et al. 1979) treatments. Similar to rodents, the MAOI agent TCP reduced anxiety in the novel tank test in zebrafish both acutely (as assessed by shorter latency to enter the top, increased number of top transitions and reduced freezing duration) (Stewart et al. 2010b) and chronically (as reflected in fewer erratic movements; Fig. 1). LSD has been extensively tested in rodents, and exhibits a biphasic action with initial anxiety/hypoactivity followed by hyperlocomotion (Mittman and Geyer 1991; Adams and Geyer 1985; Adams and Geyer 1982; Marona-Lewicka et al. 2005). The effects of LSD have recently been studied in zebrafish, demonstrating anxiolytic-like action for both acute administration (e.g., shorter latency to enter the top, increased number of top transitions, top duration, and reduced freezing duration in the novel tank, as well as more center entries in the open field test) (Grossman et al. 2010; Stewart et al. 2010b) and repeated treatment (e.g., increased top duration in the novel tank test; Fig. 2).
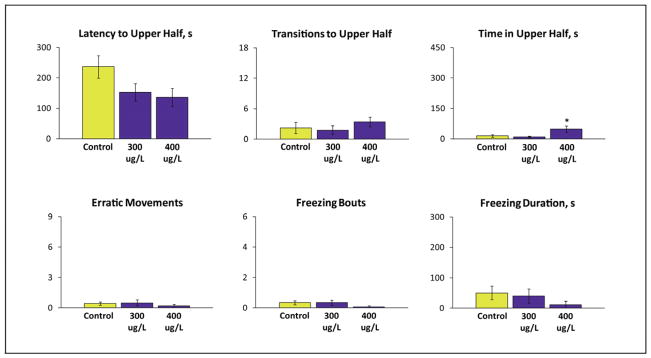
Figure 1.
Effects of tranylcypromine (TCP) (300–400 μg/L) exposure on adult wild type (short-fin) zebrafish behavior in the 6 min novel tank test. TCP was administered for 30 min followed by testing 1 week later. A one-way ANOVA test (factor: dose) revealed that the drug significantly affects the number of erratic movements (F(2, 41) = 5.699, P < 0.05) in adult wild type (short-fin) zebrafish. Data are presented as mean ± SEM (n = 14 per group), *P<0.05 vs. control; post-hoc Tukey test for significant ANOVA data.
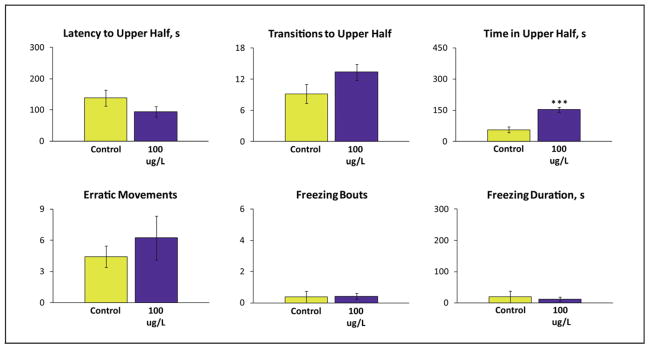
Figure 2.
Effects of repeated lysergic acid diethylamide (LSD, 100 μg/L) exposure on adult wild type (short-fin) zebrafish behavior in the 6 min novel tank test. Zebrafish were exposed to LSD twice a day for one week. Wilcoxon U-test revealed that the drug significantly affects the time spent in the top in adult wild type (short-fin) zebrafish. Data are presented as mean ± SEM (n = 14 per group), ***P<0.005 vs. control, U-test.
In rodents, the NMDA receptor antagonist MK-801 evokes hyperlocomotion, place preference, reduced predator avoidance, and increased exploratory activity (Layer et al. 1993; Del Pozo et al. 1996; Adamec et al. 1999; Jessa et al. 1996; Sharma and Kulkarni 1991; Rikuko and Akemi 1998). Similarly, MK-801 also produces hyperlocomotion and circling behavior in zebrafish (Seibt et al. 2010; Swain et al. 2004). With the growing use of MK-801 in modeling behavioral disorders, we have further examined its behavioral and endocrine effects, showing anxiolytic-like effects such as increased top duration, decreased latency to top entry and lower cortisol levels in the novel tank test (Fig. 3). With the growing use of MK-801 in modeling behavioral disorders, we have further examined its behavioral and endocrine effects, showing anxiolytic-like effects such as increased top duration and decreased latency to top entry (Fig. 3) as well as lower cortisol levels in the novel tank test (data not shown). Interestingly, an increase in erratic movements was also observed here. While heightened erratic behavior can reflect increased anxiety in zebrafish (Egan et al. 2009; Cachat et al. 2010a), its appearance together with the other anxiolytic behaviors is in line with the hyperlocomotion, demonstrated for MK-801 in previous rodent_studies (Layer et al. 1993; Martin et al. 1997; Mathe et al. 1996) as well in some zebrafish models (Ewald 2009).
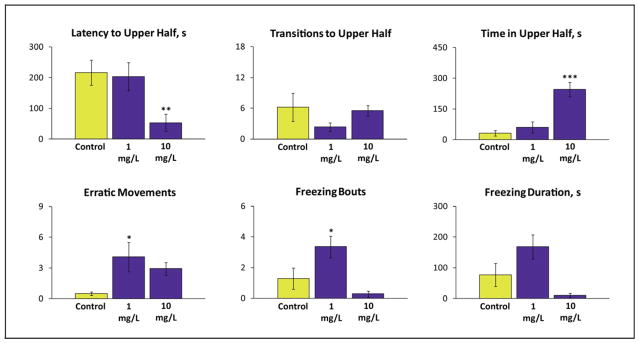
Figure 3.
Effects of acute 20-min dizocilpine (MK-801, 1–10 mg/L) exposure on adult wild type (short-fin) zebrafish behavior in the 6 min novel tank test. A one-way ANOVA test (factor: dose) revealed that the drug significantly affects the latency to enter the top (F(3, 55) = 9.315, P < 0.005), time spent in the top (F(3, 55) = 20.834, P < 0.005), number of erratic movement (F(3, 55) = 4.563, P < 0.005), and number of freezing bouts (F(3, 55) = 8.255, P < 0.005) in adult wild type (short-fin) zebrafish. Data are presented as mean ± SEM (n = 14 per group), *P<0.05, **P < 0.01, ***P < 0.005 vs. control; post-hoc Tukey test for significant ANOVA data.
Notably, studies outlined here were performed at different times, using different cohorts, and evaluated by different experimenters, thereby leading to some data variability (e.g., Fig. 1–3) not uncommon for both zebrafish (Sackerman et al. 2010; Bencan et al. 2009; Echevarria et al. 2008; Levin et al. 2007) and rodent (Cryan et al. 2003; Klenerova et al. 2009; Eckerman et al. 1980) models. While similar baseline behaviors are the ideal situation in psychopharmacology research, this is not always the case since behavioral endpoints are highly sensitive to procedural/environmental factors. However, it is important to ensure that the controls and experimental fish are always tested under the same conditions within each experiment. In this situation, while a variance among controls could lead to variable numerical values for a specific endpoint, the overall relationship between groups should be maintained (Fig. 1–3).
While conventional assays for assessing zebrafish behavior like novel tank test remain prevalent, new measures of anxiety-like behavior are emerging. For example, paradigms for screening classic anxiolytic agents through evaluating color, shoal cohesion, and position relative to tank height have recently been developed (Gebauer et al. 2011 (in press); Miller and Gerlai 2007; Saverino and Gerlai 2008) (also see Table 1 for details). Taken together, this confirms the high sensitivity of adult zebrafish to various pharmacological manipulations that affect clinical or rodent anxiety-like behavior. In combination with high throughput, phenotypical robustness, low cost and enhanced behavioral dimensionality, this makes zebrafish an excellent model for anxiolytic drug screening.
4. Genetic manipulation and zebrafish anxiety
The ease of genetic manipulations in zebrafish contributes to their utility for studying anxiety disorders. The isolation of multiple zebrafish behavioral mutants has allowed researchers to uncover the genetic pathways and neural circuits underlying behaviors (Norton and Bally-Cuif 2010b). For example, as the habenula area appears to play a role in experience-dependent fear-like behaviors (Hauptman 2011), the genetic disruption of its afferents in zebrafish prevents normal stress avoidance responses (Lee et al. 2010). Moreover, transgenic zebrafish lines have been developed to differentially express colored fluorescent proteins in the neurons of habenula subnuclei to help confirm their putative targeting (Hauptman 2011). Overall, research using zebrafish anxiety models has increasingly incorporated mutant zebrafish models to assess the genetic factors that precipitate abnormal neurobiological, physiological and behavioral phenotypes (Gerlai et al. 2000; Hogan et al. 2008; Key and Devine 2003) (see (Bergner et al. 2009) for a recent review).
5. Modeling zebrafish anxiety in the age of omics
One of the key challenges in neuroscience is to decipher the functional and structural layout of the brain at the neuronal level (Stewart et al. 2011b). The connectome, reflecting the development of a highly organized connection matrix of the brain (DeFelipe 2010), offers the possibility to elucidate the pathogenesis of anxiety disorders based on identified quantitative or qualitative defects in circuitry (Lichtman et al. 2008). While efforts have been primarily focused on the human brain, animal models of anxiety also benefit from functional connectivity mapping. With a relatively limited number axons, the zebrafish brain is a good object to investigate its connectivity (Friedrich et al. 2010). For example, synaptic output has been suppressed in zebrafish by tetanus toxin light chain (TeTxLC), a permanent blocker of synaptic vesicle release, which can be used to identify subsets of neurons involved in various behaviors (Friedrich et al. 2010; Asakawa et al. 2008; Koide et al. 2009; Hauptman 2011). Brainbow technology is another promising connectomic approach, in which neurons are labeled in varying hues, and used for the multicolor labeling and axonal tracing of the zebrafish sensory system (Lichtman et al. 2008; Pan et al. 2011). Since Brainbow labeling facilitates the surveying of quantitative and qualitative aspects of circuitry in diverse brain regions, it may be a useful approach for assessing the “connectopathology” of anxiety in zebrafish.
Notable advances have also been achieved at a higher level – specifically with the recent comprehensive mapping of all zebrafish dopaminergic axon projections. For example, injected anterograde and retrograde tracers to examine the projections and subnuclei of the habenula and associated brain regions, enable using pathway-specific manipulations to examine their involvement in affective behaviors (Hauptman 2011; Agetsuma et al. 2010). Furthermore, by combining the selective genetic marking of individual nerve cells with high resolution microscopy, researchers have assembled a 3D projectome map for zebrafish (Tay et al. 2011).
Another approach in modeling affective disorders is assessing the differential engagement of neuronal pathways. For example, mapping neuronal activity by assessing the expression of immediate early gene (e.g., c-fos) is increasingly used in zebrafish (Lau et al. 2011; Baraban et al. 2005; Wong et al. 2010a; Williams et al. 2011 (in press); Stewart et al. 2011a). Genetically encoded calcium indicators also show promise of monitoring neuronal activity in zebrafish (Fetcho 2007; Higashijima et al. 2003; Muto et al. 2011). Further research is needed to harness the noninvasiveness of in vivo analysis, while combining it with the single cell resolution offered by other techniques.
Efforts to dissect interconnected physiological pathways underlying anxiety also apply innovative bioinformatics tools to identify behavioral patterns and phenotypes. For example, while the Zebrafish Information Network (ZFIN (Zebrafish_Information_Network_(ZFIN) 2011)) is the main searchable database of zebrafish genetic, genomic and developmental data (Sprague et al. 2008), a recently launched Zebrafish Neurophenome Project (ZNP (Zebrafish_Neuroscience_Research_Consortium_(ZNRC) 2011)) is an interactive searchable database of behavioral and related phenotypes in zebrafish. It allows investigators to rapidly search previously published zebrafish data, refine their research using these models and share their findings. Further ZNP development, based on published information and curated data deposited by established zebrafish investigators, will enable sophisticated data-mining and complex data analyses, to foster our understanding of affective pathogenesis in zebrafish.
6. Further directions: using other aquatic models and cross-species comparisons
Comparison between various species is crucial for uncovering conserved and divergent mechanisms underlying pathogenic mechanisms (Signore et al. 2009; Furutani-Seiki and Wittbrodt 2004). As one such step, comparing related fish species (e.g., zebrafish and medaka, provides an excellent means to unravel the similarities and differences in their behavioral phenotypes. Other aquatic species, such as guppies, are used to model pharmacogenic anxiety, and demonstrate some similarity to zebrafish responses (Hallgren et al. 2011 (in press)). Clearly, a widening of spectrum of model species to study anxiety behavior by including zebrafish and other aquatic models remains an important strategy for translational biological psychiatry (Kalueff et al. 2007). For example, the role of sex determinants in behavior has emerged as significant area of investigation (Lopez Patino et al. 2008; Ruhl and McRobert 2005; Piyapong et al. 2010). While zebrafish have little sex-linked genetic markers, medaka have an XX, XY sex-determination system, like mammals (Furutani-Seiki and Wittbrodt 2004). Therefore, complementary use of zebrafish and other aquatic model species may be particularly beneficial in the field of affective research.
Furthermore, while complex behavioral phenotypes may be closely connected with the genotype, they are not necessarily dependent upon it alone. Gene expression profiles instead represent the primary level of integration between environmental factors and the genome, ultimately guiding complex trait behaviors such as anxiety. Thus, in order to elucidate the molecular basis of phenotypic variation, cross-species comparisons of gene expression profiles are necessary (Renn et al. 2004).
In conclusion, anxiety is a complex and multifaceted neurobehavioral disorder, and its full understanding can only be achieved through different coordinated approaches. Zebrafish models strongly parallel animal and clinical evidence, further supporting their validity and translatability for identifying pathways involved in anxiety regulation, and discovering potential new classes of psychotropic drugs. Conceptual innovations in this field, including sophisticated video-imaging and bioinformatics/omics tools (Stewart et al. 2011b; Kalueff et al. 2007), will further foster pre-clinical anxiety research using zebrafish and other aquatic models.
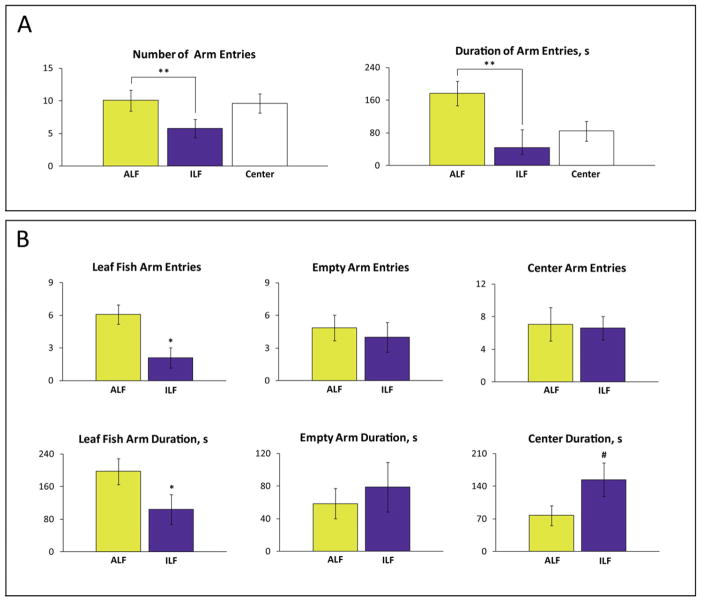
Figure 4.
Behavioral effects of the predator avoidance in adult wild type (short-fin) zebrafish in the two-compartment choice test. Zebrafish were placed in the center of the tank and the exposed to a predator (placed in an adjacent arm) for 6 min. The number or entries to, and the time spent in, the predator vs. non-predator containing arms was assessed. (A) In this experiment, zebrafish were exposed to two different predators simultaneously, the Indian leaf fish (ILF) and African leaf fish (ALF), with each placed in separate, opposite arms of the tank. Zebrafish generally exhibit stronger avoidance toward the open center arm and the ILF (indigenous to their natural environment in the wild) compared to the ALF. (B) In this experiment, zebrafish were exposed to either the ILF or ALF on alternating trials, during which the single predator was placed into one arm of the test apparatus. The number of arm entries and duration is significantly affected by ILF vs. ALF predator exposure. Data are presented as mean ± SEM (n = 15 per group), *P<0.05, **P < 0.005, #P = 0.05–0.09 vs. control, U-test
Highlights.
Zebrafish are useful for studying the underpinnings of neurobehavioral disorders
Current and emerging approaches used to uncover anxiety mechanisms are discussed
Pharmacological and genetic manipulations have validated zebrafish anxiety models
New and innovative bioinformatic approaches are emerging tools in anxiety research
Inter-species comparisons are key for uncovering pathogenic mechanisms
Acknowledgments
The study was supported by Tulane University Intramural funds, Tulane Neurophenotyping Platform, Tulane University Pilot grant and Newcomb Fellows grant. This article is dedicated to the memory of Eli Utterback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamec RE, Burton P, et al. NMDA Receptors Mediate Lasting Increases in Anxiety-Like Behavior Produced by the Stress of Predator Exposure—Implications for Anxiety Associated with Posttraumatic Stress Disorder. Physiol Behav. 1999;65(4/5):723–737. doi: 10.1016/s0031-9384(98)00226-1. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. LSD-induced alterations of locomotor patterns and exploration in rats. Psychopharmacology (Berl) 1982;77(2):179–85. doi: 10.1007/BF00431945. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci. 1985;99(5):881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Agetsuma M, Aizawa H, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13(11):1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- Amo R, Aizawa H, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30(4):1566–74. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa K, Suster ML, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A. 2008;105(4):1255–60. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom JR, Chang MS, et al. Agonist-directed signaling of serotonin 5-HT2C receptors: differences between serotonin and lysergic acid diethylamide (LSD) Neuropsychopharmacology. 1999;21(2 Suppl):77S–81S. doi: 10.1016/S0893-133X(99)00005-6. [DOI] [PubMed] [Google Scholar]
- Ballenger JC. Current treatments of the anxiety disorders in adults. Biol Psychiatry. 1999;46(11):1579–94. doi: 10.1016/s0006-3223(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, et al. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131(3):759–68. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Barcellos LJG, Ritter F, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272(1–4):774–778. [Google Scholar]
- Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186(1):107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, et al. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94(1):75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergner C, Egan C, et al. Mutant and Transgenic Zebrafish in Modeling Neurobehavioral Disorders. In: Kalueff AV, Bergner C, editors. Transgenic and Mutant Models of Brain Disorders. New York: Humana Press; 2009. [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11(7):307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, et al. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, et al. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463(1–3):97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, et al. The Characterization and Modelling of Antipredator Defensive Behavior. Neurosci Biobehav Rev. 1990;14:463–472. doi: 10.1016/s0149-7634(05)80069-7. [DOI] [PubMed] [Google Scholar]
- Blaser RE, Chadwick L, et al. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208(1):56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Boulom V, Lee HW, et al. Stimulation of DNA synthesis, activation of mitogen-activated protein kinase ERK2 and nuclear accumulation of c-fos in human aortic smooth muscle cells by ketamine. Cell Prolif. 2002;35(3):155–65. doi: 10.1046/j.1365-2184.2002.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. The neurogenetic frontier--lessons from misbehaving zebrafish. Brief Funct Genomic Proteomic. 2008;7(6):474–82. doi: 10.1093/bfgp/eln039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne T, Scott E, et al. Big ideas for small brains: what can psychiatry learn from worms, flies, bees and fish? Mol Psychiatry. 2011;16(1):7–16. doi: 10.1038/mp.2010.35. [DOI] [PubMed] [Google Scholar]
- Burt de Perera T. Fish can encode order in their spatial map. Proc Biol Sci. 2004a;271(1553):2131–4. doi: 10.1098/rspb.2004.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt de Perera T. Fish can encode order in their spatial map. Proc R Soc Lond B. 2004b;272:4. doi: 10.1098/rspb.2004.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachat JM, Canavello PR, et al. Modeling Stress and Anxiety in Zebrafish. In: Kalueff AV, Cachat J, editors. Zebrafish Models in Neurobehavioral Research. New York: Humana Press; 2010b. [Google Scholar]
- Cachat J, Stewart A, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Prot. 2010a;5(11):1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- Cachat J, Stewart A, et al. Deconstructing Adult Zebrafish Behavior with Swim Trace Visualizations. In: Kalueff AV, Cachat J, editors. Zebrafish Neurobehavioral Protocols. New York: Humana Press; 2010c. [Google Scholar]
- Cachat J, Stewart A, et al. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One. 2011;6(3):e17597. doi: 10.1371/journal.pone.0017597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C, Hsu CH, et al. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10(2):116–24. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Hoefnagels CC, et al. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav Brain Res. 2010;214(2):332–42. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mao Y, et al. Environmental enrichment and chronic restraint stress in ICR mice: effects on prepulse inhibition of startle and Y-maze spatial recognition memory. Behav Brain Res. 2010;212(1):49–55. doi: 10.1016/j.bbr.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Clement Y, Calatayud F, et al. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57(1):57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liberzon I, et al. Exposure to extreme stress impairs contextual odour discrimination in an animal model of PTSD. Int J Neuropsychopharmacol. 2009;12(3):291–303. doi: 10.1017/S146114570800919X. [DOI] [PubMed] [Google Scholar]
- Conti L, Jirout M, et al. Identification of Quantitative Trait Loci for Anxiety and Locomotion Phenotypes in Rat Recombinant Inbred Strains. Behavior Genetics. 2004;34(1):93–103. doi: 10.1023/B:BEGE.0000009479.02183.1f. [DOI] [PubMed] [Google Scholar]
- Crawley JN. A monoamine oxidase inhibitor reverses the ‘separation syndrome’ in a new hamster separation model of depression. Eur J Pharmacol. 1985;112(1):129–33. doi: 10.1016/0014-2999(85)90250-x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, et al. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168(3):347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- de Angelis L. Experimental anxiety and antidepressant drugs: the effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn Schmiedebergs Arch Pharmacol. 1996;354(3):379–83. doi: 10.1007/BF00171072. [DOI] [PubMed] [Google Scholar]
- de Castro MR, Lima JV, et al. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae) Comp Biochem Physiol C Toxicol Pharmacol. 2009;150(3):337–42. doi: 10.1016/j.cbpc.2009.05.017. [DOI] [PubMed] [Google Scholar]
- de Mooij-van Malsen AJ, Vinkers CH, et al. Cross-species behavioural genetics: A starting point for unravelling the neurobiology of human psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.10.003. in press. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. From the connectome to the synaptome: an epic love story. Science. 2010;330(6008):1198–201. doi: 10.1126/science.1193378. [DOI] [PubMed] [Google Scholar]
- Del Pozo E, Barrios M, et al. The NMDA receptor antagonist dizocilpine (MK-801) stereoselectively inhibits morphine-induced place preference conditioning in mice. Psychopharmacology (Berl) 1996;125(3):209–13. doi: 10.1007/BF02247330. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, et al. Influence of predator stress on the consolidation versus retrieval of long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16(7):571–6. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, et al. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9(5):542–52. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ditzen C, Jastorff AM, et al. Protein biomarkers in a mouse model of extremes in trait anxiety. Mol Cell Proteomics. 2006;5(10):1914–20. doi: 10.1074/mcp.M600088-MCP200. [DOI] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacol Biochem Behav. 2003;74(2):471–80. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- Dvorkin A, Benjamini Y, et al. Mouse cognition-related behavior in the open-field: emergence of places of attraction. PLoS Comput Biol. 2008;4(2):e1000027. doi: 10.1371/journal.pcbi.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria DJ, Hammack CM, et al. A Novel Behavioral Test Battery to Assess Global Drug Effects Using the Zebrafish. Int J Compar Psychol. 2008;21(1):19–34. [Google Scholar]
- Eckerman DA, Gordon WA, et al. Effects of scopolamine, pentobarbital, and amphetamine on radial arm maze performance in the rat. Pharmacol Biochem Behav. 1980;12(4):595–602. doi: 10.1016/0091-3057(80)90194-x. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205(1):38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Golani I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34(3):199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Eisenstein EM, Eisenstein D. A behavioral homeostasis theory of habituation and sensitization: II. Further developments and predictions. Rev Neurosci. 2006;17(5):533–57. doi: 10.1515/revneuro.2006.17.5.533. [DOI] [PubMed] [Google Scholar]
- El Hage W, Griebel G, et al. Long-term impaired memory following predatory stress in mice. Physiol Behav. 2006;87(1):45–50. doi: 10.1016/j.physbeh.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Ryan MJ, et al. Learned social preference in zebrafish. Curr Biol. 2004;14(10):881–4. doi: 10.1016/j.cub.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Ewald HS. PhD. Louisville: University of Louisville; 2009. A zebrafish model of schizophrenia and sickness behavior: MK-801 and endogenous NMDAR antagonism; p. 107. [Google Scholar]
- Fetcho JR. Imaging neuronal activity with calcium indicators in larval zebrafish. CSH Protoc 2007. 2007 doi: 10.1101/pdb.prot4781. pdb prot4781. [DOI] [PubMed] [Google Scholar]
- Freund JL, Freund D, et al. The open-field, non-stressed behavior of rats under the acute and chronic effect of imipramine and tranylcypromine, depending on the individual reaction type (emotional and non-emotional) Arzneimittelforschung. 1979;29(8):1150–4. [PubMed] [Google Scholar]
- Friedrich RW, Jacobson GA, et al. Circuit Neuroscience in Zebrafish. Current biology: CB. 2010;20(8):R371–R381. doi: 10.1016/j.cub.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Furutani-Seiki M, Wittbrodt J. Medaka and zebrafish, an evolutionary twin study. Mech Dev. 2004;121(7–8):629–37. doi: 10.1016/j.mod.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Gaikwad S, Stewart A, et al. Acute stress disrupts performance of zebrafish in the cued and spatial memory tests: the utility of fish models to study stress-memory interplay. Behav Processes. 2011;87(2):224–30. doi: 10.1016/j.beproc.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Gebauer DL, Pagnussat N, et al. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.04.021. in press. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Zebrafish antipredatory responses: a future for translational research? Behav Brain Res. 2010;207(2):223–31. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, et al. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67(4):773–82. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Fernandes Y, et al. Zebrafish (Danio rerio) responds to the animated image of a predator: towards the development of an automated aversive task. Behav Brain Res. 2009;201(2):318–24. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman L, Utterback U, et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res. 2010;214(2):277–84. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, et al. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155(3):659–72. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren S, Volkova K, et al. Anxiogenic behaviour induced by 17alpha-ethynylestradiol in male guppies (Poecilia reticulata) Fish Physiol Biochem. 2011 doi: 10.1007/s10695-011-9488-x. in press. [DOI] [PubMed] [Google Scholar]
- Hardison J, Purcell K. The effects of psychological stress as a function of need and cognitive control. J Pers. 1959;27(2):250–8. doi: 10.1111/j.1467-6494.1959.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Hauptman JS. From the bench to the bedside: Breaking down the blood-brain barrier, decoding the habenula, understanding hand choice, and the role of ketone bodies in epilepsy. Surg Neurol Int. 2011;1:86. doi: 10.4103/2152-7806.74143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176(2):210–5. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Masino MA, et al. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J Neurophysiol. 2003;90(6):3986–97. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Verkade H, et al. Manipulation of gene expression during zebrafish embryonic development using transient approaches. Methods Mol Biol. 2008;469:273–300. doi: 10.1007/978-1-60327-469-2_19. [DOI] [PubMed] [Google Scholar]
- Hu XY, Wang DX. Effects of lidocaine on learning and memory dysfunction as well neuropathologic change induced by chronic stress: experiment with mice. Zhonghua Yi Xue Za Zhi. 2006;86(47):3335–9. [PubMed] [Google Scholar]
- Isingrini E, Camus V, et al. Association between Repeated Unpredictable Chronic Mild Stress (UCMS) Procedures with a High Fat Diet: A Model of Fluoxetine Resistance in Mice. PLoS ONE. 2010;5(4):e10404. doi: 10.1371/journal.pone.0010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessa M, Nazar M, et al. The effects of repeated administration of diazepam, MK-801 and CGP 37849 on rat behavior in two models of anxiety. Euro Neuropsychopharm. 1996;6:55–61. doi: 10.1016/0924-977x(95)00068-z. [DOI] [PubMed] [Google Scholar]
- Jesuthasan S. Fear, anxiety and control in the zebrafish. Dev Neurobiol. 2011 doi: 10.1002/dneu.20873. in press. [DOI] [PubMed] [Google Scholar]
- Jie Z, Li T, et al. Trans-2-phenylcyclopropylamine induces nerve cells apoptosis in zebrafish mediated by depression of LSD1 activity. Brain Res Bull. 2009;80(1–2):79–84. doi: 10.1016/j.brainresbull.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Wheaton M, et al. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179(1):1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Kazlauckas V, Kalininea E, et al. Distinctive effects of unpredictable subchronic stress on memory, serum corticosterone and hippocampal BDNF levels in high and low exploratory mice. Behav Brain Res. 2011;218:80–86. doi: 10.1016/j.bbr.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Key B, Devine CA. Zebrafish as an experimental model: strategies for developmental and molecular neurobiology studies. Methods Cell Sci. 2003;25(1–2):1–6. doi: 10.1023/B:MICS.0000006849.98007.03. [DOI] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, et al. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60(2):57–62. [PubMed] [Google Scholar]
- Koide T, Miyasaka N, et al. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc Natl Acad Sci U S A. 2009;106(24):9884–9. doi: 10.1073/pnas.0900470106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, et al. The immediate early gene Arc is associated with behavioral resilience to stress exposure in an animal model of posttraumatic stress disorder. Eur Neuropsychopharmacol. 2008;18(2):107–16. doi: 10.1016/j.euroneuro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Kung JC, Chen TC, et al. Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci. 2010;17:29. doi: 10.1186/1423-0127-17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN, Brown C, et al. Learning in fishes: from three-second memory to culture. Fish and Fisheries. 2003;4(3):199–202. [Google Scholar]
- Landgraf R, Wigger A. High vs low anxiety-related behavior rats: an animal model of extremes in trait anxiety. Behav Genet. 2002;32(5):301–14. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- Lau BY, Mathur P, et al. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci U S A. 2011;108(6):2581–6. doi: 10.1073/pnas.1018275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer RT, Kaddis FG, et al. The NMDA receptor antagonist MK-801 elicits conditioned place preference in rats. Pharmacol Biochem Behav. 1993;44(1):245–7. doi: 10.1016/0091-3057(93)90306-e. [DOI] [PubMed] [Google Scholar]
- Lee A, Mathuru AS, et al. The habenula prevents helpless behavior in larval zebrafish. Curr Biol. 2010;20(24):2211–6. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, et al. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90(1):54–8. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Livet J, et al. A technicolour approach to the connectome. Nat Rev Neurosci. 2008;9(6):417–22. doi: 10.1038/nrn2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Patino MA, Yu L, et al. Gender differences in zebrafish responses to cocaine withdrawal. Physiol Behav. 2008;95(1–2):36–47. doi: 10.1016/j.physbeh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki Y, Inoue T, et al. Monoamine oxidase inhibitors reduce conditioned fear stress-induced freezing behavior in rats. Eur J Pharmacol. 2000;406(3):411–8. doi: 10.1016/s0014-2999(00)00706-8. [DOI] [PubMed] [Google Scholar]
- Mallinger AG, Frank E, et al. Revisiting the effectiveness of standard antidepressants in bipolar disorder: are monoamine oxidase inhibitors superior? Psychopharmacol Bull. 2009;42(2):64–74. [PMC free article] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, et al. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology (Berl) 2005;180(3):427–35. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, et al. MK-801-induced hyperlocomotion: Differential effects of M100907, SDZ PSD 958 and raclopride. Euro J Pharm. 1997;335:107–116. doi: 10.1016/s0014-2999(97)01188-6. [DOI] [PubMed] [Google Scholar]
- Mathe JM, Nomikos GG, et al. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Euro J Pharm. 1996;309:1–11. doi: 10.1016/0014-2999(96)00315-9. [DOI] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, et al. Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010a;214(2):157–71. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, et al. Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res. 2010b;210(1):1–7. doi: 10.1016/j.bbr.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Maximino C, Marques de Brito T, et al. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010c;5(2):209–16. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184(2):157–66. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Mittman SM, Geyer MA. Dissociation of multiple effects of acute LSD on exploratory behavior in rats by ritanserin and propranolol. Psychopharmacology (Berl) 1991;105(1):69–76. doi: 10.1007/BF02316866. [DOI] [PubMed] [Google Scholar]
- Moretz JA, Martins ElP, et al. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behavioral Ecology. 2007;18(3):556–562. [Google Scholar]
- Morrow BA, Roth RH, et al. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000;52(6):519–23. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Muto A, Ohkura M, et al. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1000887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010a;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neuroscience. 2010b;11(1):90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogwang SP. MS in Fisheries Biology and Management. Department of Biology. Bergen, University of Bergen; 2008. Inspection of a novel object by wild and laboratory Zebrafish (Danio rerio H.) in the presence and absence of alarm substance; p. 68. [Google Scholar]
- O’keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Olivier B, Molewijk HE, et al. Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci Biobehav Rev. 1998;23(2):215–27. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Pan YA, Livet J, et al. Multicolor Brainbow imaging in zebrafish. Cold Spring Harb Protoc. 2011 doi: 10.1101/pdb.prot5546. pdb prot5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey DK, Pati D, et al. Chronic Unpredictable Stress: Possible Animal Model of Comorbid Depression. Int JPreclin Pharm Res. 2010;1(1):54–63. [Google Scholar]
- Park CR, Zoladz PR, et al. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 2008;15(4):271–80. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piato AL, Capiotti KM, et al. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):561–7. doi: 10.1016/j.pnpbp.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Piyapong C, Krause J, et al. Sex matters: a social context to boldness in guppies (Poecilia reticulata) Behavioral Ecology. 2010;21(1):3–8. [Google Scholar]
- Renn SC, Aubin-Horth N, et al. Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. BMC Genomics. 2004;5(1):42. doi: 10.1186/1471-2164-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G. Long-term habituation to spatial novelty in blind cave fish (Astyanax hubbsi): role of the telencephalon and its subregions. Learn Mem. 1998;4(6):451–61. doi: 10.1101/lm.4.6.451. [DOI] [PubMed] [Google Scholar]
- Rikuko S, Akemi K. The effect of MK-801 on openfield activities and object exploration and its sex difference. Jpn J Anim Psy. 1998;48(2):161–176. [Google Scholar]
- Rosemberg DB, Rico EP, et al. Differences in Spatio-Temporal Behavior of Zebrafish in the Open Tank Paradigm after a Short-Period Confinement into Dark and Bright Environments. PLoS One. 2011;6(5):e19397. doi: 10.1371/journal.pone.0019397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V, Belzung C, et al. Environmental enrichment in BALB/c mice: effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav. 2001;74(3):313–20. doi: 10.1016/s0031-9384(01)00561-3. [DOI] [PubMed] [Google Scholar]
- Ruhl N, McRobert SP. The effect of sex and shoal size on shoaling behaviour in Danio rerio. Journal of Fish Biology. 2005;67(5):1318–1326. [Google Scholar]
- Sackerman J, Donegan JJ, et al. Zebrafish Behavior in Novel Environments: Effects of Acute Exposure to Anxiolytic Compounds and Choice of Danio rerio Line. Int J Comp Psychol. 2010;23(1):43–61. [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, et al. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiatry. 2005;57(8):856–64. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191(1):77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, et al. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23(3):219–43. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, Oliveira Rda L, et al. Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK-801 in zebrafish (Danio rerio) Behav Brain Res. 2010;214(2):417–22. doi: 10.1016/j.bbr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Serra EL, Medalha CC, et al. Natural preference of zebrafish (Danio rerio) for a dark environment. Braz J Med Biol Res. 1999;32(12):1551–3. doi: 10.1590/s0100-879x1999001200016. [DOI] [PubMed] [Google Scholar]
- Sharma AC, Kulkarni SK. MK-801 produces antianxiety effect in elevated plus-maze in mice. Drug Dev Res. 1991;22:251–258. [Google Scholar]
- Signore IA, Guerrero N, et al. Zebrafish and medaka: model organisms for a comparative developmental approach of brain asymmetry. Philos Trans R Soc Lond B Biol Sci. 2009;364(1519):991–1003. doi: 10.1098/rstb.2008.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188(1):168–77. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, et al. The Zebrafish Information Network: the zebrafish model organism database provides expanded support for genotypes and phenotypes. Nucleic Acids Res. 2008;36(Database issue):D768–72. doi: 10.1093/nar/gkm956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Maximino C, et al. Neurophenotyping of adult zebrafish using the light/dark box paradigm. In: Kalueff AV, Cachat J, editors. Zebrafish Neurobehavioral Protocols. New York: Humana Press; 2010f. [Google Scholar]
- Stewart A, Kadri F, et al. The Developing Utility of Zebrafish in Modeling Neurobehavioral Disorders. Int J Comp Psychol. 2010a;23(1):104–121. [Google Scholar]
- Stewart A, Cachat J, et al. Homebase behavior of zebrafish in novelty-based paradigms. Behav Processes. 2010c;85(2):198–203. doi: 10.1016/j.beproc.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Stewart A, Cachat J, et al. Phenotyping of zebrafish homebase behaviors in novelty-based tests. In: Kalueff AV, Cachat J, editors. Zebrafish Neurobehavioral Protocols. New York: Humana Press; 2010d. [Google Scholar]
- Stewart A, Wong K, et al. Zebrafish models to study drug abuse-related phenotypes. Revs in Neurosci. 2010e;22(1):95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- Stewart A, Wu N, et al. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog Neuropsychopharmacol Biol Psychiatry. 2010b doi: 10.1016/j.pnpbp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Stewart A, Riehl R, et al. Behavioral effects of MDMA (“Ecstasy”) on adult zebrafish. Behav Pharmacol. 2011a;22(3):275–80. doi: 10.1097/FBP.0b013e328345f758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Gaikwad S, et al. Experimental models for anxiolytic drug discovery in the era of omes and omics. Expert Opin Drug Discov. 2011b;6(1–15) doi: 10.1517/17460441.2011.586028. [DOI] [PubMed] [Google Scholar]
- Suveg C, Morelen D, et al. The Emotion Dysregulation Model of Anxiety: A preliminary path analytic examination. J Anxiety Disord. 2010;24(8):924–30. doi: 10.1016/j.janxdis.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, et al. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26(6):725–9. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Takamori K, Yoshida S, et al. Repeated treatment with imipramine, fluvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sci. 2001;69(16):1919–26. doi: 10.1016/s0024-3205(01)01279-6. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, et al. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb KJ, Norton WH, et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biol. 2009;10(7):R81. doi: 10.1186/gb-2009-10-7-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Wong K, et al. Behavioral and physiological effects of RDX on adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. 2011 doi: 10.1016/j.cbpc.2011.02.010. in press. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, et al. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100(3):417–25. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Wong K, Stewart A, et al. Modeling seizure-related behavioral and endocrine phenotypes in adult zebrafish. Brain Res. 2010a;1348:209–15. doi: 10.1016/j.brainres.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Wong K, Elegante M, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010b;208(2):450–7. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, et al. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10(5):326–36. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, Rimmer LB, et al. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio) Naturwissenschaften. 2003;90(8):374–7. doi: 10.1007/s00114-003-0443-2. [DOI] [PubMed] [Google Scholar]
- Wright D, Nakamichi R, et al. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behav Genet. 2006;36(2):271–84. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8(2):151–4. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, et al. Site-specific regulation of corticosteroid and serotonin receptor subtype gene expression in the rat hippocampus following 3,4-methylenedioxymethamphetamine: role of corticosterone and serotonin. Neuroscience. 1997;78(1):111–21. doi: 10.1016/s0306-4522(96)00497-6. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, et al. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biological psychiatry. 1993;34(1):18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yun J, Koike H, et al. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: Possible involvement of a brain-specific transcription factor Npas4. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06893.x. [DOI] [PubMed] [Google Scholar]
- Zebrafish Information Network (ZFIN) Zebrafish Model Organism Database. 2011 doi: 10.1093/nar/gkg027. Retrieved 5/18/11, from http://zfin.org. [DOI] [PMC free article] [PubMed]
- Zebrafish Neuroscience Research Consortium (ZNRC) Zebrafish Neurophenome Project (ZNP) 2011 Retrieved 5/18/2011, from http://www.kaluefflab.com/znpindex.
- Zhang P, Hirsch EC, et al. c-fos protein-like immunoreactivity: distribution in the human brain and over-expression in the hippocampus of patients with Alzheimer’s disease. Neuroscience. 1992;46(1):9–21. doi: 10.1016/0306-4522(92)90004-l. [DOI] [PubMed] [Google Scholar]