Abstract
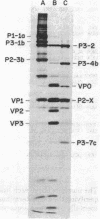
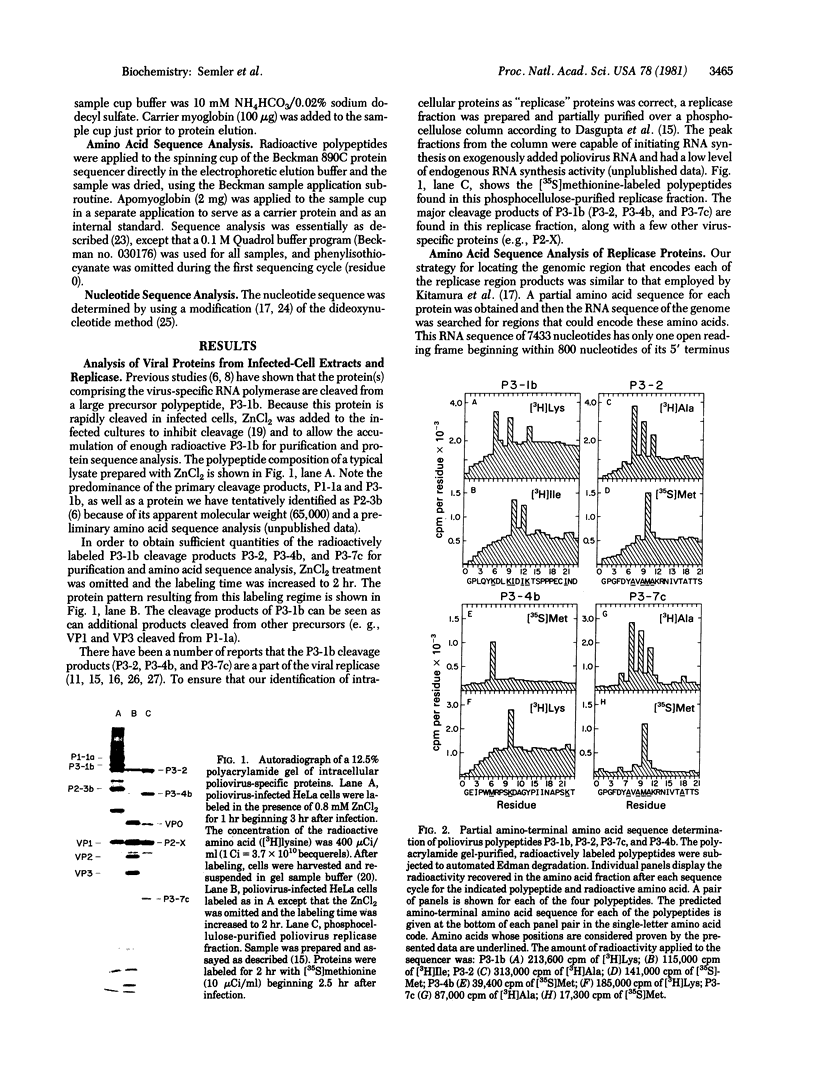
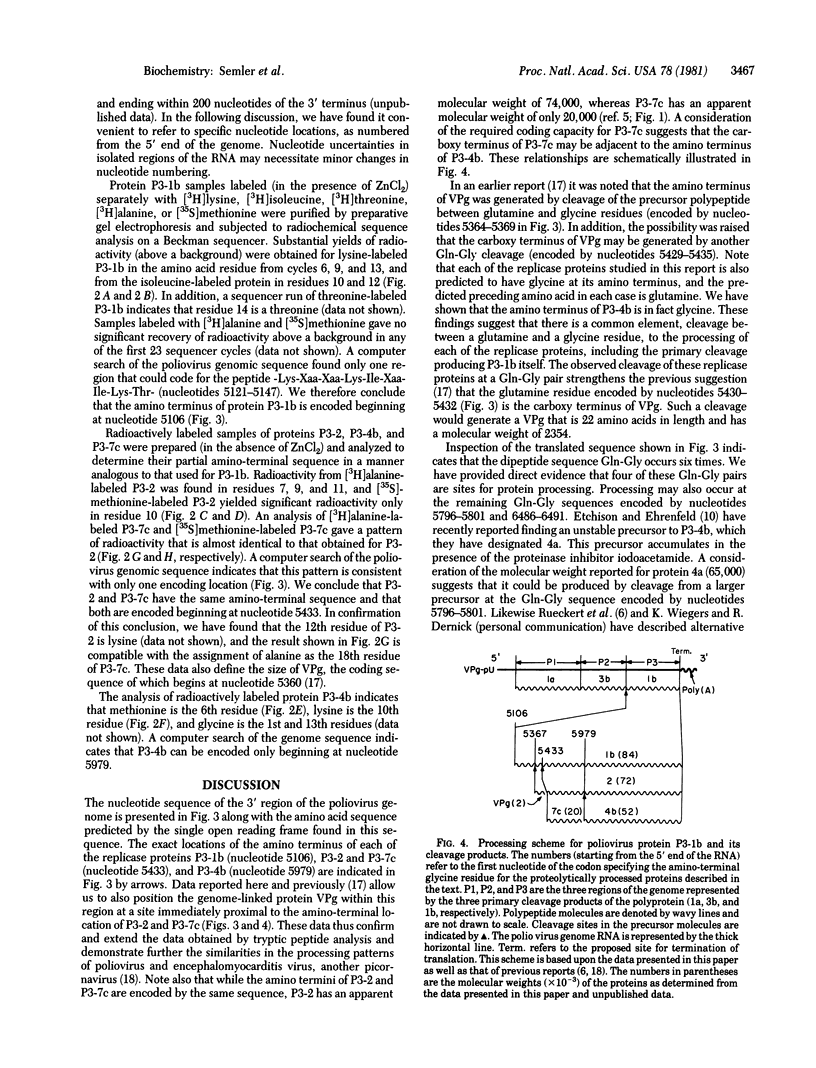
A partial amino-terminal amino acid sequence of each of the major proteins encoded by the replicase region (P3) of the poliovirus genome has been determined. A comparison of this sequence information with the amino acid sequence predicted from the RNA sequence that has been determined for the 3' region of the poliovirus genome has allowed us to locate precisely the proteolytic cleavage sites at which the initial polyprotein is processed to create the poliovirus products P3-1b (NCVP1b), P3-2 (NCVP2), P3-4b (NCVP4b), and P3-7c (NCVP7c). For each of these products, as well as for the small genome-linked protein VPg, proteolytic cleavage occurs between a glutamine and a glycine residue to create the amino terminus of each protein. This result suggests that a single proteinase may be responsible for all of these cleavages. The sequence data also allow the precise positioning of the genome-linked protein VPg within the precursor P3-1b just proximal to the amino terminus of polypeptide P3-2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhown A. S., Mole J. E., Hunter F., Bennett J. C. High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem. 1980 Mar 15;103(1):184–190. doi: 10.1016/0003-2697(80)90254-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowles S. A., Tershak D. R. Proteolysis of noncapsid protein 2 of type 3 poliovirus at the restrictive temperature: breakdown of noncapsid protein 2 correlates with loss of RNA synthesis. J Virol. 1978 Aug;27(2):443–448. doi: 10.1128/jvi.27.2.443-448.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E. A comparison of the virus-specific polypeptides of encephalomyocarditis virus, human rhinovirus-1A, and poliovirus. Virology. 1973 Dec;56(2):439–453. doi: 10.1016/0042-6822(73)90048-2. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Baron M. H., Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Viral polypeptides associated with the RNA replication complex in poliovirus-infected cells. Virology. 1980 Nov;107(1):135–142. doi: 10.1016/0042-6822(80)90279-2. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Van Dyke T. A. Isolation of a soluble and template-dependent poliovirus RNA polymerase that copies virion RNA in vitro. J Virol. 1979 Oct;32(1):155–161. doi: 10.1128/jvi.32.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Adler C. J., Rothberg P. G., Martinko J., Nathenson S. G., Wimmer E. The genome-linked protein of picornaviruses. VII. Genetic mapping of poliovirus VPg by protein and RNA sequence studies. Cell. 1980 Aug;21(1):295–302. doi: 10.1016/0092-8674(80)90137-3. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Adler C., Wimmer E. Structure and expression of the picornavirus genome. Ann N Y Acad Sci. 1980;354:183–201. doi: 10.1111/j.1749-6632.1980.tb27967.x. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Wimmer E. Sequence of 1060 3'-terminal nucleotides of poliovirus RNA as determined by a modification of the dideoxynucleotide method. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3196–3200. doi: 10.1073/pnas.77.6.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B., Chow N., Lively M., Powers J. Virus-specified protease in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2992–2995. doi: 10.1073/pnas.76.6.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundquist R. E., Ehrenfeld E., Maizel J. V., Jr Isolation of a viral polypeptide associated with poliovirus RNA polymerase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4773–4777. doi: 10.1073/pnas.71.12.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Palmenberg A. C., Golini F., Wimmer E., Rueckert R. R. Picornaviral VPg sequences are contained in the replicase precursor. J Virol. 1980 Aug;35(2):414–419. doi: 10.1128/jvi.35.2.414-419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]