Abstract
The Error Related Negativity (ERN) is thought to index a neural behavior monitoring system with its source in anterior cingulate cortex (ACC). While ACC is involved in a wide variety of cognitive and emotional tasks, there is debate as to what aspects of ACC function are indexed by the ERN. In one model the ERN indexes purely cognitive function, responding to mismatch between intended and executed actions. Another model posits that the ERN is more emotionally driven, elicited when an action is inconsistent with motivational goals. If the ERN indexes mismatch between intended and executed action, then it should be insensitive to motivational valence, e.g. reward or punishment; in contrast if the ERN indexes the evaluation of responses relative to goals, then it might respond differentially under different motivational valence. This study used a flanker task motivated by potential reward and potential punishment on different trials and also examined the N2 and P3 to the imperative stimulus, the response Pe, and the FRN and P3 to the outcome feedback to assess the impact of motivation valence on other stages of information processing in this choice reaction time task. Participants were slower on punishment motivated trials and both the N2 and ERN were larger on punishment-motivated trials, indicating that loss aversion has an impact on multiple stages of information processing including behavior monitoring.
Keywords: Error-related Negativity, Motivation, Reward, Punishment
1. Introduction
Effective behavior monitoring is critical for the efficient generation of goal-directed behavior. An organism must continually evaluate its actions in the context of motivational goals to determine if those actions are effective. Recent work has identified the anterior cingulate cortex (ACC) as a nexus in the neural network of behavior monitoring, and the Error Negativity (Ne) or Error-Related Negativity (ERN) component of the response-related event-related potential (ERP) as an index of that system (Carter, et al., 1998; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993; Gehring & Knight, 2000). The ERN has its onset immediately following a behavioral response, reaching peak amplitude within 100 ms of the response, is larger following incorrect responses, has a medial prefrontal scalp distribution, and modeling places its source in the ACC (Dehaene, Posner, & Tucker, 1994; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1990; Gehring, Goss, Coles, Meyer, & Donchin, 1993).
Anterior cingulate cortex is involved in a wide variety of functions, including attention selection (Posner & Petersen, 1990), pain perception (Talbot, et al., 1991), decision making (Bush, et al., 2002), emotion regulation (Davidson, Putnam, & Larson, 2000), concentration or mental effort (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001), conflict monitoring or mediation (Botvinick, Cohen, & Carter, 2004; Carter, et al., 1998), and error detection (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993). Some of these functions are ‘cold cognitive’ with little relation to affective processes (e.g. attention control or conflict monitoring) while others are explicitly emotional (e.g. emotion regulation). One influential perspective is that the ACC is not homogenous in function but contains functional subdivisions, with a ventral inferior subdivision devoted to emotional functions and a dorsal superior portion specialized for cognitive functions (Bush, Luu, & Posner, 2000). Recent reviews have returned to more integrative perspectives, positing that the ACC serves as an interface between cognition and emotion (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001), e.g. integrating negative affect, pain and cognitive control (Shackman, et al., 2011). These integrative accounts draw on evidence from multiple assessment modalities, including functional MRI and PET, human EEG/ERP and MEG, and monkey single- and multi-unit recording. The theoretical questions addressed, and the experimental designs employed, in these different neuroimaging modalities do not completely overlap, and the results are not fully consistent, so it is not clear what specific ACC functions are indexed by each assessment modality. The current study specifically addresses the question of which functions are indexed by the ERN.
Reflecting the dichotomous perspective on ACC function, there are two primary classes of theory of the function of the ACC-based behavior monitoring system as indexed by the ERN: theories that are primarily cognitive and theories that are primarily motivational/affective. The cognitive theories include the detection of behavioral errors (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Gehring, Goss, Coles, Meyer, & Donchin, 1993) or mediation between conflicting response options (Carter, et al., 1998), while the motivational/affective theories generally posit that the monitoring system responds to an action that fails to meet motivational goals or a negative affective response to an ineffective action (e.g. Phan, Don, & Scott, 2004; Suchan, Zoppelt, & Daum, 2003). The cognitive models are linked to the motor system (Holroyd, 2001; Holroyd & Coles, 2002) while the motivational models are more closely tied to affective systems (Hajcak, Moser, Yeung, & Robert F. Simons, 2005; Luu, Collins, & Tucker, 2000)). While the ERN is independent of the specific motor response (e.g. an equivalent ERN is produced by finger versus hand responses or hand versus foot responses; Holroyd, Dien, & Coles, 1998), the error detection and response conflict theories explicitly link the monitoring system’s response to either a mismatch between intended and executed motor actions or high activation levels among multiple motor programs vying for access to effectors thus requiring some mediation between those motor programs (Carter, et al., 1998; Holroyd & Coles, 2002). However, some studies have shown that motivational state (Boksem, Meijman, & Lorist, 2006; Hajcak, Moser, Yeung, & Robert F. Simons, 2005) or trait (Luu, Collins, & Tucker, 2000; Pailing & Segalowitz, 2004) can impact the ERN, suggesting that the behavior monitoring system performs a function other than the simple detecting behavioral errors or mediation between competing response options, perhaps performing some evaluation of executed actions in the context of motivational goals (Phan, Don, & Scott, 2004; Suchan, Zoppelt, & Daum, 2003).
The behavior monitoring system is not equally engaged by all errors, rather the system is differentially engaged by errors with greater motivational value (Hajcak, Moser, Yeung, & Robert F. Simons, 2005). For example, the ERN is larger when motivation is increased by telling participants that their performance is being evaluated or compared with the performance of others (Boksem, Meijman, & Lorist, 2006; Hajcak, Moser, Yeung, & Simons, 2005). The ERN is also larger when there is a monetary incentive (appetitive motivation) for accurate performance (Boksem, Meijman, & Lorist, 2006; Hajcak, Moser, Yeung, & Simons, 2005). In addition to experimental manipulation, a number of studies have examined how individual differences in motivation impact the ERN. Depressed individuals and individuals with borderline personality disorder have a reduced ERN amplitude to errors (Ruchsow, et al., 2006a; Ruchsow, et al., 2006b) while patients with obsessive-compulsive disorder and individuals who score high on self-reported obsessive-compulsive personality traits have larger ERNs compared to control participants (Gehring, Himle, & Nisenson, 2000) (Hajcak & Simons, 2002), presumably because the former care less and the latter care more about the consequences of their actions. Luu, Collins, and Tucker (2000) found that individuals scoring high on self-reported negative affect had larger ERNs on early trials and smaller ERNs on later trials within an experimental session and interpreted this as indicating that these individuals were more motivated to avoid the aversive affect associated with mistakes early in the experiment session but later, once they discovered that they were unable to completely avoid making mistakes, they motivationally disengaged from the task (i.e. stopped caring). Other personality characteristics associated with poor self-regulation have been linked to reduced efficiency in the behavior monitoring system via the ERN. Individuals who self-report as more externalizing, a personality construct associated with reduced impulse control, and individuals who are behaviorally impulsive, also show a reduced ERN to errors (Hall, Bernat, & Patrick, 2007; Ruchsow, Spitzer, Grön, Grothe, & Kiefer, 2005). Pailing & Segalowitz (2004) found that individuals high on neuroticism had a larger ERN when their task performance was motivated by external rewards as opposed to the internal motivation to perform well.
The above results indicate that motivation has an impact on behavior monitoring. There are two basic motivation valences: appetitive and aversive (Gray, 1981). The behavior monitoring system has been theoretically linked to appetitive motivation via the mesotelencephalic dopaminergic reward system (Holroyd & Coles, 2002), however the ERN has also been described as reflecting negative affect that results from behaviors that fail to meet outcome expectation (Hajcak, McDonald, & Simons, 2004; Luu, Collins, & Tucker, 2000). Thus it is not clear if the behavior monitoring system is engaged preferentially by appetitive or aversive motivation or is equally engaged by both. Individuals who score high on the punishment-related BIS scale on the Carver & White BIS/BAS self-report inventory of Gray’s Behavioral Inhibition System (BIS: aversive motivation) and Behavioral Activation System (BAS: appetitive motivation) systems (Carver & White, 1994; Gray, 1981) have a larger ERN to errors, but there is no relationship between ERN and BAS score, suggesting a preferential link between behavior monitoring and the aversive motivation system (Boksem, Tops, Wester, Meijman, & Lorist, 2006). Few studies have directly contrasted the impact of positive and negative motivation on the ERN. Dikman and Allen (2000) used appetitive motivation (money for correct responses) and aversive motivation (unpleasant tones to errors) and found that appetitive and aversive motivation produced equivalent ERN amplitude in highly socialized individuals but participants that scored low on self-reported socialization had a smaller ERN when motivated by punishment, indicating less punishment sensitivity in these individuals. However the rewarding and punishing motivators in that study differed on several dimensions other than valence: the rewards were signaled visually, the punishments auditorially, and the rewards were a secondary motivator (symbol indicating that money would be given later) while the punishments were primary (unpleasant sounds), making interpretation difficult. Additionally the rewarding and punishing motivations took place in different blocks, allowing other processes, like arousal, to have an impact. Potts, George, Martin, & Barratt (2006) used a task with trial-by-trial interspersed monetary gain and loss trials, with motivation type indicated by stimulus identity, and found that individuals scoring low on self-reported impulsivity had larger ERNs on punishment motivated trials but that high impulsives had equivalent ERN amplitude across motivation type, indicating that threat of punishment increased behavior monitoring, but only in low impulsive individuals. Thus in both the Dikman and Allen (2000) and Potts et al. (2006) studies aversive motivation enhanced the ERN, but only in subsets of the participants, leaving unclear whether this punishment enhancement is a general characteristic of the ERN or rather reflects aspects of the individual.
Much of the work relating the behavior monitoring system to explicit reward and punishment has used an ERP component related to the ERN, the feedback-related negativity (FRN). If the participant does not know which response is correct at response execution, then there is no response-related ERN. However, if the participant is provided with informative feedback as to the correctness of their response, then an FRN is elicited to feedback indicating that their response was incorrect (Miltner, Braun, & Coles, 1997). Subsequent studies have shown than an FRN is elicited to stimuli signaling events other than simple behavioral errors; an FRN is also elicited in monetarily motivated choice option tasks to selections that result in an outcome that is not the best available on that trial (Gehring & Willoughby, 2002; Hajcak, Holroyd, Moser, & Simons, 2005; Holroyd, Larsen, & Cohen, 2004; reviewed in Nieuwenhuis, Holroyd, Mol, & Coles, 2004). Thus is it clear that the FRN responds differentially to losses and gains, rather than to simple behavioral error, particularly since an FRN can be elicited to reward expectation violations in the absence of any motor action ( e.g. Potts, Martin, Burton, & Montague, 2006). However there is not complete agreement to what extent the ERN and FRN reflect the same, different, or partially overlapping cognitive operations and neural systems. For example, Miltner, Braun, & Coles (1997) reported that the response- and feedback-related negativities had the same medial frontal scalp distribution, concluding that they reflected activity in the same neural system, while Gehring & Willoughby (2004) and Potts, Martin, Kamp, & Donchin (2011) reported that the reward outcome-related FRN had a more anterior distribution than the response-related ERN, indicating at least some difference in neural systems reflected by the components (see review in Nieuwenhuis, Holroyd, Mol, & Coles (2004) for a more complete discussion). Potts et al. (2011), using principal components analysis (PCA), found that the response-locked ERN was described by a single ‘ERN’ principal component while the FRN had two subcomponents, an ‘ERN’ ACC subcomponent and a more frontal subcomponent, perhaps reflecting activity in orbitofrontal cortex. Thus the cognitive operations and neural systems indexed by the ERN and FRN may only partially overlap, and theoretical conclusions drawn from FRN findings may not fully generalize to the ERN.
The current study examined the impact of motivation on the ERN in a modification of the Eriksen flanker task (Eriksen & Eriksen, 1979) with punishment and reward incentives, in a sample of 64 participants. Our prior study (Potts, George, Martin, & Barratt, 2006) used a similar design and found an impact of motivation but only in the 18 out of the 37 total participants that scored low on impulsivity. That study used non-standard flanker stimuli (T’s and N’s), and also the rewarding stimulus was always the ‘T’ and always required a right-hand response, confounding stimulus identity and response hand with motivation type. The current design used the letters ‘S’ and ‘H’ as stimuli and counterbalanced rewarding and punishing stimuli and response hand. If the behavior monitoring system performs a purely cognitive operation, as proposed by the cognitive/motor theories, i.e. detecting mismatch between intended and executed motor response or mediation between conflicting response options, then motivation type should have no impact on the ERN. However, if the motivation type has an impact on the ERN, that would be more consistent with the motivational/affective theories that posit that the behavioral monitoring system, as indexed by the ERN, is performing an evaluative operation, evaluating actions in the context of motivational goals.
Response monitoring is only one of the cognitive operation involved in this choice reaction time task; others include formation of the stimulus percept and attention to the task-relevant features, activating the correct stimulus-response mapping in the presence of a conflicting information, activating and executing the correct response option, monitoring response execution for error, evaluating the outcome of the executed action, and updating internal models of the task context when the outcome violates expectation. Many of these operations also have ERP indices and we examined some of those indices for effects of motivational valence, specifically the medial frontal N2 to the stimulus and FRN to the outcome feedback, and the centroparietal P3 to the stimulus, error positivity (Pe) to the response, and P3 to the feedback.
2. Methods
2.1 Participants
Seventy-seven undergraduates at the University of South Florida participated for psychology course credit. Seven participants performed at less that 50% accuracy in any condition and were eliminated from further analysis. Data from seven participants was eliminated from the stimulus-locked, six participants from the response-locked, and nine from the response-locked analyses because of excessive artifact (fewer than 20 clean trials in one or more conditions) in their EEG leaving 63 participants in the response-locked and 64 participants in the response-locked, and 61 participants in the feedback-locked datasets; for the 64 participants set: 47 females, 58 right handed, mean age 20.27 (SD = 2.27, range 18 – 27).
2.2 Stimuli and Task
This study used a flanker task, in which one of two critical central letters, an S or an H, was flanked to the right and left by distracter letters (Eriksen & Eriksen, 1979). In some cases the flankers matched the central letter (HHHHH or SSSSS), sometimes they matched the other letter (HHSHH, SSHSS). Subjects responded in a two-choice, forced-alternative manner, pressing a left-hand response-pad key to one central letter, a right-hand key to the other. One letter was potentially rewarding in that participants received a monetary reward for correct responses to that stimulus but no consequence for incorrect responses, while the other letter was potentially punishing in that participants lost money for incorrect responses to that letter while receiving nothing for correct responses (rewarding stimulus and response hand mappings were counterbalanced across subjects).
A trial began with a fixation cross (which was onscreen any time another stimulus was not) being replaced by a asterisk warning symbol, followed 800 ms later by the five-letter string that remained onscreen for 100 ms. Subjects had 600 ms from stimulus onset to respond (late responses were counted as errors to the participant; the restricted response window was included to place time pressure on the participants to make the error rate on incongruent trials approximately 80%, based on our prior studies). Two hundred and thirty ms after the end of the response period a feedback appeared for 1000 ms reporting the outcome of the current trial and the running total of money in the subject’s “bank”, replaced with the fixation cross for the inter-trial interval which varied randomly between 500 – 1500 ms. Participants started with $5 (US) and each correct response on a reward-motivated trial was rewarded with $.05 while each incorrect response on a punishment-motivated trial lost $.05. There were 12 blocks of 80 trials each for 960 trials total with congruent and incongruent and reward and punishment trials distributed randomly and equiprobably across the experiment (240 each condition crossing congruence with motivation). Participants were told they would at least leave with the initial $5 regardless of performance. Participants were paid their winnings in cash at the end of their session.
2.3 Behavioral Analysis
Behavior was analyzed for accuracy and speed within each condition: congruent and incongruent stimuli, reward and punishment motivated trials, and, for reaction time (RT), correct and error responses, using repeated-measures ANOVAs with paired t-tests to test individual contrasts within significant interactions. Responses with reaction times less than 100 ms (including no-response trials) or over 1000 ms were considered invalid trials and not included in the behavioral analysis.
2.4 ERP Acquisition and Analysis
The EEG was acquired continuously using a 128 channel Electrical Geodesics System 250 using Geodesic Sensor Nets with built-in electrodes at the supra- and sub-orbital locations and near the external canthi, employing Netstation software for acquisition and signal processing (EGI, Eugene, OR). Electrode impedance was maintained below 50 k-Ohm as per the manufacturer’s recommendation for the high input stage impendence of the system amplifier. The EEG recorded referenced to the vertex with .01 Hz highpass and 100 Hz lowpass analog filtering, and digitized at 250 Hz. The EEG was then digitally filtered at 20 Hz lowpass, and segmented into epochs spanning 200 ms before and 800 ms after the imperative and feedback stimuli and 100 ms before to 500 ms after the keypress response. The segments screened for artifact using Netstation automated artifact rejection (e.g. eye blinks and lateral eye movements and absolute excursions of more than 200 µV), and the artifact-free segments averaged for each subject into four conditions: Reward Correct, Reward Error, Punish Correct, Punish Error to create the ERPs (correct trials were those in which the participant pressed the correct key and the RT was faster than the 600 ms limit, incorrect trials were when the participant pressed the incorrect key but within the time limit; ERPs from the slow trials in which the participant pressed either key with an RT of longer than the 600 ms time limit are not presented here). The EEG from the congruent and incongruent trials were averaged together as there were few errors in the congruent trials. The ERPs were baseline corrected over the 200 ms pre-stimulus period for the stimulus-related ERP and the 100 ms pre-response period for the response-related ERP and rereferenced into an average reference representation (Dien, 1998).
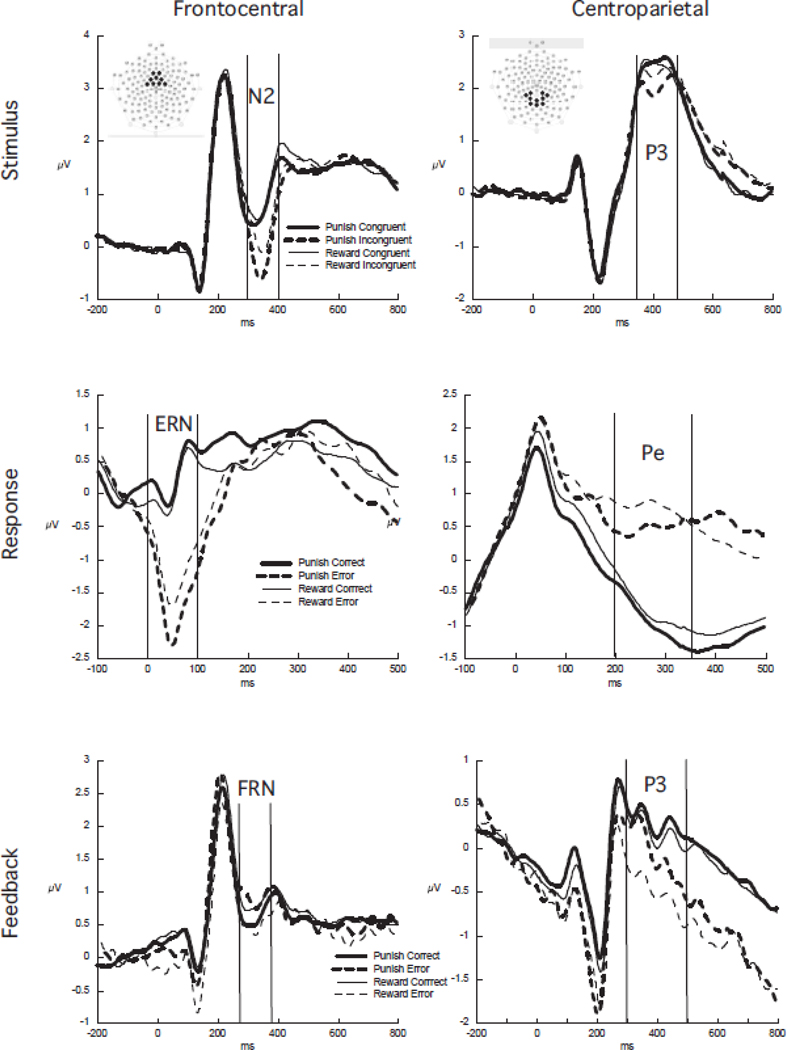
In the stimulus-related ERP the N2 was extracted from 300 – 400 ms post-stimulus from a frontocentral region of interest (ROI) consisting of 8 electrodes surrounding FCz in the Geodesic Sensor Net and the P3 from 350 – 480 ms from a centroparietal ROI consisting of 10 electrodes near Pz. In the response-related ERP the ERN was extracted from 0 – 100 ms post-response from the same frontocentral ROI as the N2 and the error positivity (Pe) was extracted from 200 – 350 ms post-response from the same centroparietal ROI as the P3. In the feedback-related ERP the FRN was extracted from 275 – 375 ms from the frontocentral ROI and the P3 from 300 – 500 ms from the centroparietal ROI (see Figure 1). The stimulus-related N2 and P3 were cast into repeated-measures ANOVAs with Motivation (Reward, Punishment) and Stimulus (Congruent, Incongruent) as factors. The ERN, Pe, FRN, and feedback P3 were cast into repeated-measures ANOVAs with Motivation (Reward, Punish) and Response (Correct, Error) as factors.
Figure 1.
Waveform plots of the stimulus, response, and feedback-locked ERPs averaged across all participants and across the frontocentral and centroparietal electrodes in the Geodesic Sensor Net. The analysis windows for the stimulus N2 and P3, the response ERN and Pe, and the feedback FRN and P3 are delimited. The insets in the top row plots show the location of the ROIs in the Sensor Net, looking down on the top of the head with the nose at the top of the inset. Note that the x- and y-axis scaling differs between plots.
3. Results
3.1 Behavioral Results
Five of the participants were 100% accurate in one of the congruent flanker conditions thus with no reaction time data for the congruent error condition in the analysis model. Therefore only data from the 59 participants who had no missing cells were included in the reaction time analysis.
Mean reaction times by condition are shown in Table 1. Participants were slower to respond when the flankers were incongruent with the critical central stimulus, F(1, 58) = 6.63, p < .05. Participants were slower on punishment motivated trials, F(1, 58) = 15.72, p < .001, and when making correct responses, F(1, 58) = 37.89, p < .0001. Stimulus congruence interacted with response correctness, F(1, 58) = 32.01, p < .0001, indicating that participants were slower on the incongruent flanker trials but only when responding correctly. The Motivation x Response interaction was marginally significant, F(1, 58) = 3.65, p = .062, indicating that the RT on punishment motivated trials was slower when the response was correct. The three-way Flanker x Motivation x Response interaction was also significant, F(1, 58) = 5.99, p < .05, indicating that the motivation and flanker effects combined to make the slowest responses on punishment motivated incongruent trials but only when the participants were responding correctly.
Table 1.
| Reward | Punish | |||
|---|---|---|---|---|
| Correct | Error | Correct | Error | |
| Congruent | 373 (27.3) | 362 (44.0) | 384 (25.9) | 379 (46.7) |
| Incongruent | 393 (107.7) | 361 (31.6) | 427 (29.7) | 361 (34.6) |
Participants were more accurate on the congruent (94.7% correct responses, SD 5.2) than incongruent (82.2%, SD = 9.7) trials, F(1, 63) = 271.41, p < .0001. Participants were marginally more accurate on reward (89.0%, SD = 9.7) than punishment (87.9, SD = 10.2) motivated trials, F(1, 63) = 3.13, p = .082. The Stimulus x Motivation interaction also approached significance, F(1, 63) = 3.42, p = .07, suggesting that the motivation difference in accuracy was only present on incongruent trials.
3.2 ERP Results
The waveforms for the stimulus, response, and feedback ERPs from the medial frontal and centroparietal ROIs are show in Figure 1 with the analysis windows delimited.
3.2.1 Stimulus-related ERP
For the N2 there were main effects for Stimulus, F(1, 62) = 79.96, p < .001, with more negativity on incongruent than congruent trials, and Motivation, F(1, 62) = 23.97, p < .001, with more negativity on punishment motivated trials. While the waveform plots suggest that the motivation effect was more pronounced on incongruent trials, this was not a significant interaction (p > .2).
For the P3 there was a main effect for Stimulus, F(1, 62) = 3.33, p < .05, with less positivity on the incongruent trials. This effect was modified by interaction with Motivation, F(1, 62) = 1.33, p < .05, showing that the reduced positivity on incongruent trials was only present when the trials were punishment motivated.
3.2.2 Response-related ERP
There was a main effect for Response, F(1, 63) = 98.00, p < .001 on the ERN, showing that the ERN to the error responses was more negative. Response was modified by interaction with Motivation, F(1, 63) = 6.67, p < .05, indicating that the ERN was more negative to errors resulting in monetary loss compared to failure to achieve monetary gain. This was supported by post-hoc paired t-tests showing that the ERN difference on correct trials was not different between the reward and punishment conditions, t(63) = 1.30, p = .20, but that the ERN on error trials did differ between punishment and reward conditions, t(63) = 2.39, p < .05.
For the Pe there was a main effect for Response, F(1, 63) = 48.49, p < .001, showing more positivity on error trials. There was a trend for Motivation, F(1, 63) = 3.02, p = .09, suggesting more positivity on reward trials. The interaction did not approach significance.
3.2.3 Feedback-related ERP
For the FRN, neither main effect was significant, but the Motivation x Response interaction was, F(1, 60) = 7.60, p < .01, indicating more negativity to error feedback on the reward trials only. On the punishment motivated trials the ERP was more negative to the correct feedback.
For the feedback P3 there were main effects for response, with a larger P3 to correct response feedback, F(1, 60) = 8.23, p < .01, and motivation, with a larger P3 on punishment motivated trials, F(1, 60) = 6.11, p < .05.
4. Discussion
Participants were both fast and accurate when the stimuli were congruent, placing little challenge on the neural systems of stimulus conflict mediation and response monitoring, and there was no impact of motivation valence on behavior in the absence of challenge. However, when the stimuli were incongruent participants were less accurate and slower, at least when making correct responses, reflecting the challenge placed on the systems supporting choice response by the competing flankers. Motivation type had an impact on responding when the flankers were incongruent with marginal decreased accuracy and significantly longer reaction times to make a correct response when the trial was punishment motivated. This indicates that attempting to avoid loss places a greater challenge on the neural systems involved in choice response under conflict than attempting to achieve an equivalent amount of gain. The ERPs show that this risk aversion, expressed behaviorally, affects multiple stages of information processing.
The first place in the ERP where these was a visible difference between the congruent and incongruent conditions was in the N2 component, after the early P1 and N1 components associated with percept formation and spatial attention. The N2 was more negative to the incongruent stimuli and on punishment motivated trials, perhaps reflecting the early signal of stimulus-response mapping conflict requiring additional cognitive control (Donkers & van Boxtel, 2004; Folstein & Van Petten, 2008; Van Veen & Carter, 2002). The larger N2 under punishment motivation suggests that additional cognitive control is engaged when the goal is to avoid loss, consistent with the subsequent slower reaction times indicating more careful responding.
The P3 was essentially equivalent in all conditions except incongruent trials that were punishment motivated where the P3 amplitude was reduced. The P3 (or P300) has been associated with multiple cognitive operations including attention orienting and/or allocation, novelty detection, task relevance evaluation, and working memory updating (see review in (Polich, 2007)). Perhaps the most prominent theory of the cognitive operation indexed by the P300 is the context updating theory that posits that the P300 indexes the degree to which the individual’s internal representation of the external milieu or context (Donchin & Coles, 1988; Donchin & Coles, 1998). This updating occurs when a presented stimulus fails to fit into the participant’s expectations, i.e. the event is to some degree surprising. The P3 is also associated with task demands, being reduced when the task becomes more difficult or when a secondary task draws cognitive resources away from the primary task (Polich, 1987; Wickens, Kramer, L, & Donchin, 1983). The P3 is also larger as performance motivation on the task increases (Carrillo-de-la-Peoa & Cadaveira, 2000). The reduced P3 to incongruent stimuli in the punishment condition therefore could reflect this condition being the most expected one, the one that the participant held as the default condition (despite the conditions being equiprobable), or that the incongruent punishment condition was the most cognitively demanding. This latter interpretation is perhaps more defensible in light of the behavioral data which indicated slowest reaction times and worst accuracy in this condition.
It is possible that some part of the P3 effect is due to component overlap with either the stimulus-related N2 or the response-related ERN. However, the N2 peaks substantially earlier than the P3 (see Figure 1), so N2 overlap is unlikely. The mean reaction time across conditions is 380 ms and the ERN peaks about 50 ms after the response, putting the average ERN peak in the stimulus-locked ERP at about 430 ms, at roughly the peak of the P3. However, no ERN effect is apparent in the fronto-central ROI stimulus-locked waveform where the ERN would be the largest. Also the ERN effect would likely be much more temporally smeared in the stimulus-locked ERP due to latency jitter, and the pattern of effects is different between the ERN and the P3 with the ERN being much larger on errors (which occur much more frequently on the incongruent trials), while the P3 is least positive only on punishment motivated incongruent trials. Thus the effect appears to be a P3 effect, not due to component overlap with the N2 or ERN.
The current results showed a larger ERN to errors committed under punishment motivation, indicating that appetitive and aversive motivation differentially impact the neural system of behavior monitoring. Punishment motivation resulted in a larger ERN to errors than reward motivation, demonstrating a differential impact of motivation type on the ERN. This result is mores consistent with the motivational or affective theories that posit that the ERN reflects an evaluative process applied to an action that failed to meet motivational goals and/or negative affect associated with committing an error (e.g. Hajcak, Moser, Yeung, & Robert F. Simons, 2005; Luu, Collins, & Tucker, 2000) than with the purely cognitive theories that posit that the ERN reflects the detection of a behavioral error or some compensatory operation following the error, like the updating of motor plans (e.g. Gehring, Goss, Coles, Meyer, & Donchin, 1993). If the ERN responds only to mismatch between intended and executed motor responses or response conflict, then the type of motivation would not have had an impact on the ERN, since the mismatch and the response options did not vary between reward and punishment motivated trials. However, in the current results, the ERN was larger to errors on punishment motivated trials, in which the error resulted in monetary loss, compared to reward motivated trials, where the error led to failure to acquire a monetary reward. Thus the neural system indexed by the ERN must perform more than the simple detection of or compensation for a behavioral error; it must perform some evaluation of that error within the motivational context.
Previous studies supporting the motivational model of the ERN have relied largely on manipulation of overall motivation (Boksem, Meijman, & Lorist, 2006; Hajcak, Moser, Yeung, & Robert F. Simons, 2005), or on individual differences for support, with findings indicating that participants more motivated to perform accurately, e.g. participants motivated by money, anxious individuals, or individuals with obsessive-compulsive disorder, have larger ERN amplitude to errors, while participants less motivated, e.g. motivated simply by experimenter instruction, or externalizing or depressed individuals, have smaller ERN amplitude. However, these most of these studies did not examine the impact of explicit appetitive versus aversive motivation manipulation the ERN. Two that did, Dikman & Allen (2000) and Potts et al. (2006), found that low socialized and impulsive individuals a had smaller ERN on punishment motivated trials, indicating that these individuals were less responsive to aversive motivation. Similarly, Pailing & Segalowitz (2004) found that high neuroticism individuals had a larger ERN when motivated by extrinsic rewards (i.e. cash) than simply by the desire to perform well, while low neuroticism individuals had equivalent ERN amplitude under external and internal motivation. All of these studies support a roll of motivation on the behavior monitoring system, with personality factors interacting with motivation type to produce differential ERN response to errors under different motivation conditions. However, the exact nature of this motivation impact has not been fully described.
One explanation for the larger ERN on punishment motivated trials in the current study is that punishment threat provides greater overall motivation than the potential for reward. Hajcak, Moser, Yeung, & Robert F. Simons (2005) demonstrated that experimentally manipulating overall motivation by informing participants that their performance was being evaluated or by providing financial incentive for accurate performance increased ERN amplitude, although the study did not explicitly contrast appetitive and aversive motivation. An alternative hypothesis is that the larger ERN on punishment motivated trials is outcome related, reflecting a larger discrepancy between the outcome associated with the punishment trial error response (with is the correct response for a reward trials) and the delivered outcome. For each participant, one response is always correct on reward trials, the other on punishment trials. Thus a reward response, when correct, results in monetary gain, while that same response when incorrect, i.e. on a punishment trial, results in monetary loss, which is a large outcome discrepancy associated with that response. In contrast, the punishment response, when correct, results in no loss or gain, and that same response when made erroneously, i.e. on a reward trial, results in no loss or gain, thus there is no outcome discrepancy associated with the punishment response. The FRN is larger to greater, compared to lesser outcome discrepancies (Holroyd, Larsen, & Cohen, 2004), and to the extent that the ERN and FRN reflect the same processes, the response-related outcome discrepancy is greater on punishment trials and this greater discrepancy could elicit a larger ERN.
The impact of choice or action outcome discrepancy has been more studied using the ERP response to negative outcome feedback, the FRN. Studies using the FRN have show that its amplitude is related to trial outcome with larger FRN negativity elicited to worse outcomes. For example, if there are three possible outcomes, one of which is winning or losing no money (a zero change outcome) and the other two outcomes are winning money, then the zero outcome is the worst and has the largest FRN (Holroyd, Larsen, & Cohen, 2004). However, if the other two outcomes are monetary losses, then the zero outcome is the best possible and the FRN is smallest to that outcome (Holroyd, Larsen, & Cohen, 2004). In the current design making and error on a punishment motivated trial resulted in monetary loss while an error on a reward motivated trial resulted in a zero outcome, thus the punishment motivated error outcome was objectively worse than the reward motivated error outcome and thus would be expected to elicit a larger FRN, and to the extent that the ERN and FRN reflect activity in the same monitoring or evaluative system (an unresolved question, see Gehring & Willoughby, 2004; Miltner, Braun, & Coles, 1997), a larger ERN. However, the same relationship was true on correct trials: the outcome to a correct response on a punishment motivated trial was objectively worse than to a correct response on a reward motivated trial, but there was no greater ERN to correct responses on punishment motivated trials compared to correct responses on reward motivated trials. Thus the ERN was not indexing overall trial outcome, but only a differential response to punishing and non-rewarding errors – an error was required to elicit a differential motivation effect on the ERN. Thus the ERN motivation effect appears to be specifically indexing loss aversion.
We did assess the FRN to the outcome feedback in the current study and found a a larger FRN to feedback indicating that the participant made an incorrect response on the trial but only on reward motivated trials; on punishment motivated trials the FRN actually appeared to be more negative following correct feedback. The feedback here was either the addition or subtraction of money from the total ‘pot’, presented as numbers on the screen. When participants responded correctly on punishment trials or incorrectly on reward trials there was no change to the totals, and this produced a larger FRN. Both the conditions that failed to change the participant’s total winnings, either by not adding to (reward error) nor subtracting from (punish correct) the total, produced the largest FRN. This is contrary to the generally established response pattern for the FRN which usually responds in a valenced manner, more negative to losses, less negative to wins (Gehring & Willoughby, 2002; Yeung & Sanfey, 2004). This effect may be due, in part, to the imperative flanker stimulus containing all the information necessary for the participant to assess the outcome of the trial. Prior studies on the relationship between the ERN and FRN using designs in which the participants learn the correct stimulus-response mappings across time show that on early trials, when the participants do not know the correct response, the ERP error effect is elicited to the performance feedback in the FRN, not to the behavioral response itself. On later trials, after participants know the correct response, the error effect is elicited to the behavioral response and the feedback no longer elicits an error effect (Holroyd & Coles, 2002; Nieuwenhuis, et al., 2002). Thus the error effect moves to the event that carries the information about the correctness of the trial. In the current design, the participants were informed about as to which stimuli were rewarding and which were punishing and had several practice trials. Thus they had all the information about the nature of the trial at the response, and the feedback contained only redundant or confirmatory information. There may also have been a methodological contribution to this non-standard effect; the current experiment was designed to study the ERN, not the FRN, thus there was no latency jitter introduced between the response and the feedback, therefore the baseline correction violated the assumption of the baseline period, that the baseline EEG is random with respect to the event of interest. In the current data the baseline contained ERN and or Pe effects that may have influenced the effects in the feedback-locked ERP.
The feedback P3 was larger to the feedback signaling correct trials and to feedback on punishment trials, but there was no interaction. Since the P3 is larger to more relevant stimuli (e.g. Courchesne, Hillyard, & Galambos, 1975), this indicates that correct response feedback and feedback signaling the outcome on punishment motivated trials was more relevant.
These findings of increased effort, instantiated in neural systems of cognitive control, resource allocation, and behavioral monitoring, under the threat of monetary loss, are consistent with the well-described endowment effect, in which individuals place greater value on things they already possess than on something they have the potential to acquire (Kahneman, Knetsch, & Thaler, 1990; Thaler, 1980). Individuals appear more motivated to avoid losing what they already have than by the opportunity for additional gain, and this bias may be reflected behaviorally in slower reaction times and in the larger N2 and ERN on punishment motivated trials, reflecting the greater motivation to avoid loss and greater engagement of cognitive resources under loss threat. This suggests that neuroeconomic principles, usually related to complex decision-making tasks (Loewenstein, Rick, & Cohen, 2008; Trepel, Fox, & Poldrack, 2005) may also apply to the brain’s internal resource allocation mechanisms in simple reaction time tasks.
Highlights.
We contrasted two theories of the error-related negativity (ERN), one purely cognitive/motor, one emotion/motivational by using gain and loss motivation on different trials in a response-conflict (flanker) task. If the cognitive/motor theory is correct, then motivation type would have no effect on the ERN.
We also examined event-related potential (ERP) indices of other cognitive operations involved in choice-response under conflict.
Participants were slower and both the stimulus-related N2 and response-related ERN were larger on punishment trials. The stimulus P3, the response error positivity, and the feedback-related negativity and feedback P3 were also all influenced by motivational valence.
The ERN is influenced by motivation type, consistent with motivational theories.
Loss aversion, reflected behaviorally in slower reaction times on punishment motivated trials, influences multiple stages of information processing, before and after response execution.
Acknowledgements
This study was funded by NIH DA023273. The author thanks Melissa Silva for her contributions to the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex: The Evolution of an Interface between Emotion and Cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Boksem MAS, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006;72:123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, B R. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-de-la-Peoa MT, Cadaveira F. The effect of motivational instructions on P300 amplitude. Neurophysiologie Clinique/Clinical Neurophysiology. 2000;30:232–239. doi: 10.1016/s0987-7053(00)00220-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography & Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the Neural Circuitry of Emotion Regulation--A Possible Prelude to Violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behavior Research Methods, Instruments, & Computers. 1998;30:34–43. [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behavioral & Brain Sciences. 1988;11:357–427. [Google Scholar]
- Donchin E, Coles MGH. Context updating and the P300. The Behavioral and Brain Sciences. 1998;21:152–153. [Google Scholar]
- Donkers FCL, van Boxtel GJM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain & Cognition. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Eriksen C, Eriksen B. Target redundancey in visual search: Do repetitions of the target within the display impair processing? Perception and Psychophysics. 1979;26:195–205. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological Brain Research. Tilburg: Tilburg Univesity Press; 1990. pp. 192–195. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography & Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain. Current Opinions on Performance Monitoring. Leipzin: Max Planck Institute of Cognitive Neuroscience; 2004. pp. 14–20. [Google Scholar]
- Gray JA. A critique of Eysenck's theory of personality. In: Eysenck HJ, editor. A model for personality. Berlin: Springer; 1981. pp. 246–277. [Google Scholar]
- Hajcak G, Holroyd CB, Moser JS, Simons RF. Brain potentials associated with expected and unexpected good and bad outcomes. Psychophysiology. 2005;42:161–170. doi: 10.1111/j.1469-8986.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons Robert F. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing Psychopathology and the Error-Related Negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB. Reinforcement learning and the error-related negativity: A computational and neurophysiological investigation. US: U Illinois At Urbana-Champaign; 2001. p. 1. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MGH. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neuroscience Letters. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004;41:245–253. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch JL, Thaler RH. Experimental tests of the endowment effect and the Coase theorem. Journal of Political Economy. 1990;98:1325–1348. [Google Scholar]
- Loewenstein G, Rick S, Cohen JD. Neuroeconomics. Annual Review of Psychology. 2008;59:647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a "generic" neural system for error detection. Journal of Cognitive Neuroscience. 1997;9 doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, Coles MGH. Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neuroscience & Biobehavioral Reviews. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof K, Talsma D, Coles MG, Holroyd CB, Kok A, Van der Molen MW. A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cognitive, Affective & Behavioral Neuroscience. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Phan L, Don MT, Scott M. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Polich J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalography and clinical Neurophysiology/Evoked Potentials Section. 1987;68:311–320. doi: 10.1016/0168-5597(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention systems of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Potts GF, George MRM, Martin LE, Barratt ES. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Potts G, Martin L, Burton P, Montague P. When things are better or worse than expected: Medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1–8. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Kamp S-M, Donchin E. Neural response to action and reward prediction errors: Comparing the error-related negativity to behavioral errors and the feedback-related negativity to reward prediction violations. Psychophysiology. 2011;48:218–228. doi: 10.1111/j.1469-8986.2010.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M. Error processing in major depressive disorder: Evidence from event-related potentials. Journal of Psychiatric Research. 2006a;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Spitzer M, Grön G, Grothe J, Kiefer M. Error processing and impulsiveness in normals: Evidence from event-related potentials. Cognitive Brain Research. 2005;24:317–325. doi: 10.1016/j.cogbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Walter H, Buchheim A, Martius P, Spitzer M, K‰chele H, Gr^n G, Kiefer M. Electrophysiological correlates of error processing in borderline personality disorder. Biological Psychology. 2006b;72:133–140. doi: 10.1016/j.biopsycho.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchan B, Zoppelt D, Daum I. Frontocentral negativity in electroencephalogram reflects motor response evaluation in humans on correct trials. Neuroscience Letters. 2003;350:101–104. doi: 10.1016/s0304-3940(03)00879-6. [DOI] [PubMed] [Google Scholar]
- Talbot J, Marrett S, Evans A, Meyer E, Bushnell M, Duncan G. Multiple representations of pain in human cerebral cortex. Science. 1991;251:1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Thaler R. Toward a positive theory of consumer choice. Journal of Economic Behavior and Organization. 1980;1:39–60. [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Cognitive Brain Research. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. Special Issue: The Pittsburgh special issue. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wickens C, Kramer A, L V, Donchin E. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent Coding of Reward Magnitude and Valence in the Human Brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]