Abstract
Background
Small intestinal submucosa (SIS) is a porcine-derived, acellular, collagen-based matrix that has been tested without seeded smooth muscle cells (SMCs) for intestinal tissue engineering. We examined the expression patterns of contractile proteins of SIS with SMCs implanted in an in vivo rodent model.
Materials and methods
Intestinal SMCs were isolated from Lewis rat pups. Four-ply tubular SMCs-seeded SIS or blank SIS scaffolds were implanted in an adult rat jejunal interposition model. Recipients were sacrificed at 2, 4, and 8 weeks following the implantation. The retrieved specimens were examined using antibodies against contractile proteins of SMCs.
Results
Cultured intestinal SMCs expressed α-smooth muscle actin (α-SMA), calponin, and less smooth muscle myosin heavy chain (SM-MHC) in vitro. Cell-seeded SIS scaffolds contracted significantly over 8 weeks of implantation but were comparable to SIS scaffolds without cell seeding. Implanted cell-seeded SIS scaffolds at 2 weeks expressed extensive α-SMA, some calponin, and minimal SM-MHC. At 4 weeks, α-SMA-expressing cells decreased significantly, whereas calponin or SM-MHC expressing cells were rarely detected. A small number of α-SMA-expressing cells were present at 8 weeks, whereas more calponin or SM-MHC expressing cells emerged in proximity with the anastomotic interface.
Conclusions
Cell-seeded SIS contracted significantly after implantation, but the expressions of contractile proteins were present at the site of SIS interposition. No organized smooth muscle was formed at the site of implantation. A better scaffold design is needed to produce structured smooth muscle.
Keywords: small intestinal submucosa, smooth muscle cells, intestine, contractile proteins
INTRODUCTION
Short bowel syndrome occurs following the massive loss of diseased or injured small intestines, such as Crohn’s disease and necrotizing enterocolitis [1]. Chronic parenteral nutrition, serial transverse enteroplasty, and small intestinal transplantation have been employed to compensate for the malabsorption [2, 3]. Intestinal tissue engineering has emerged as a promising alternative for the treatment of short bowel syndrome [4]. The tissue-engineered gastrointestinal tract, from esophagus to large intestine, has been experimented in a number of animal studies [5–8]. In intestinal tissue engineering, intestinal cells, biomaterial scaffolds, and various signaling molecules have been used [9]. The neomucosa has been regenerated from organoid units [10]. However, the intestinal smooth muscle layers were not regenerated in these studies [11, 12].
Multiple synthetic or naturally derived scaffolds have been utilized in combination with or without seeding intestinal smooth muscle cells (SMCs) to create a neointestine mimicking the native intestine [13]. Among them, small intestinal submucosa (SIS) is an FDA-approved product that contains the extracellular matrix of the small intestine. SIS is a porcine-derived, acellular, collagen-based matrix that has been widely used for the tissue engineering of skin, body wall, myocardium, blood vessel, valve, trachea, bone, cartilage, joint, intervertebral disk, tendon, urinary bladder, urethra, vagina, and gastrointestinal tract [14–16]. Previous reports using SIS did not document the expression of contractile proteins in SMCs although they claimed the presence of smooth muscular regeneration by histological criteria [16].
In our previous study, we noted minimally regenerated mucosal epithelia or smooth muscle in implanted SIS scaffolds and significant shrinkage of both 1-ply and 4-ply SIS [17]. In this study, we examined the presence of seeded intestinal SMCs in SIS and characterized the expression of contractile proteins in a rodent model of jejunal interposition.
MATERIALS AND METHODS
Reagents and Media
Antibiotic-antimycotic (ABAM), Hank’s balanced salt solution (HBSS), fetal bovine serum (FBS), Dulbecco’s modified eagle medium (DMEM), trypsin, phosphate buffered solution (PBS), and Dulbecco’s phosphate buffered solution (D-PBS) were purchased from Invitrogen (Carlsbad, CA). Collagenase IV, dimethyl sulfoxide (DMSO), paraformaldehyde (PFA), and Triton X-100 were from Sigma (St Louis, MO). Bovine serum albumin (BSA) and buffered formalin phosphate were from Fisher Scientific (Fair Lawn, NJ). Normal goat serum (NGS) and DAPI mounting medium were from Vector (Burlingame, CA).
Laboratory Animals
All the animal care and use complied with the institutional regulations established and approved by the Animal Research Committee at University of California, Los Angeles. Female adult Lewis rats (Charles River Laboratories, Wilmington, MA) weighing 200–220g were maintained in a temperature-regulated environment (24°C) on a 12-hour light/dark cycle, housed in individual cages with soft bedding and a microisolator cover, and given access to standard rat chow (Harland Teklad, Madison, WI) and tap water ad libitum.
Primary Harvest of Intestinal SMCs
Postnatal day 2–4 Lewis rat pups (Charles River Laboratories, Wilmington, MA) were sacrificed through the inhalational isoflurane (Abbott Laboratories, North Chicago, IL). The whole small intestines were dissected from the ligament of Treitz to ileum sterilely, which were subsequently rinsed in iced HBSS. Muscular strips were teased off (free of mucosa) and collected into iced HBSS. After being spinned down at 1,000 RPM and at 4°C for 5 min, muscular strips were digested in 1 mg/ml collagenase IV in HBSS at 37°C for 30 min. After being inactivated by 10% FBS/DMEM, cell pellets were spinned down at 1,000 RPM for 5 min and resuspended in 10% FBS plus 1 × ABAM and incubated in 75-cm2 cell culture flasks (Corning Inc, Corning, NY) at a density of 2 × 106 cells per ml at 37°C and in an atmosphere of 10% CO2. The culture media were refreshed every 3 days.
Characterization of Intestinal SMCs in vitro
At 80% confluency, intestinal SMCs were trypsinized (1 ml of trypsin per well) and replated onto 48-well tissue culture plate (Corning Inc, Corning, NY) for further immunofluorescent staining. After being fed with 10% FBS for 3 days, cells were fixed in 4% iced PFA for 20 min, permeabilized with 0.1% Triton X-100 for 15 min, and blocked with 4% NGS for 30 min at 37°C. Cytoskeletal proteins of SMCs were detected by the incubation with mouse-anti-human monoclonal antibodies (Dako, Capinteria, CA) diluted in 4% BSA at 4°C overnight: 1:100 anti-α-smooth muscle actin (α-SMA), 1:100 anti-calponin (CALP), and 1:100 anti-smooth muscle myosin heavy chain (SM-MHC), whereas primary antibodies were omitted as negative control. Cells were incubated with 1:200 FITC-conjugated goat-anti-mouse IgG (Molecular Probes, Eugene, OR) for 60 min before being counterstained with DAPI mounting medium.
Seeding of SIS scaffolds
Four-ply SIS sheets (Surgisis® ES™ Soft Tissue Grafts, Cook Medical Inc., Bloomington, IN) were fashioned into rectangular-shaped sheets (2.0 cm × 1.5 cm). Intestinal SMCs were resuspended in 10% FBS at a density of 20 × 106 cells per ml, and 1 ml cell suspension (20 × 106 cells) was seeded onto the rough side of each SIS. Seeded scaffolds were incubated at 37°C and 10% CO2 for 3 hr before another one ml of 10% FBS was added onto each sheet for overnight incubation, whereas SIS grafts rehydrated with 10% cell-free FBS served as negative control. The seeded SMCs on SIS scaffolds were fixed in 10% buffered formalin phosphate, embedded in paraffin and sectioned vertically to determine the efficiency of cell seeding.
Surgical Procedure
A modified jejunal interposition model previously described was employed for the implantation [15]. Briefly, a 3-cm well-vascularized jejunal loop 10 cm distal to Treitz ligament was exteriorized. The interrupted intestinal tract was anastomosed end-to-end by interrupted 6-0 Prolene sutures to restore the anatomical continuity. The isolated jejunal segment was further divided into two 1.5-cm segments. The cell-seeded SIS sheet was rolled with the cell-seeded side facing outward (n = 9) over a 5-cm silicone tube (10F Red Robinson catheter; Bard, Covington, GA) to create a 2-cm tubular SIS that was interposed between the two jejunal segments in an end-to-end fashion using interrupted 6-0 Prolene sutures. The isolated jejunal segments were fixed to the silicone stent by using 3-0 silk sutures (Ethicon, Somerville, NJ). Both ends of the jejunal segment were brought out of the abdominal wall as double permanent ostomies on each side symmetrically (1 cm from the median incision). Non-seeded SIS grafts (n = 6) were implanted as controls using the same protocol. Postoperative care regimens included 0.4 mg/0.1 mg per ml of sulfamethoxazole/trimethoprim (High-Tech Pharmacal, Amityville, NY) was added into drinking water (1:100) for 5 consecutive days. From postoperative day 7 onwards, jejunostomies were flushed with sterile saline every 3 days.
Histology and Immunofluorescence Microscopy
Animals were sacrificed at 2, 4, and 8 weeks as scheduled (n = 3 per time point). Retrieved tissues including native intestine and neointestine were fixed in 10% buffered formalin phosphate, dehydrated in graded ethanol, embedded in paraffin, and sectioned in 5 µm for hematoxylin and eosin and immunofluorescent staining. Briefly, sections were deparaffinized in xylene, rehydrated in graded ethanol, autoclaved in antigen retrieval citra solution (BioGenex, San Ramon, CA) for 30 min, and blocked with 4% NGS. Sections were incubated with primary mouse-anti-human monoclonal antibodies (anti-α-SMA, anti-CALP, and anti-SM-MHC) in a humidified chamber at 4°C overnight and subsequently detected with 1:200 FITC-conjugated goat-anti-mouse IgG (Molecular Probes, Eugene, OR) at 37°C for 1 hr. Normal goat serum rather than primary antibodies was used as negative control, whereas native small intestines were stained by using the same protocol as positive control. Sections were mounted and counterstained with DAPI mounting medium. Photomicrographs were captured by using a standard light and fluorescent microscope (Leica, Bannockburn, IL) equipped with SPOT image collection system (Diagnostic Instruments, Sterling Heights, MI). The length of SIS graft at each time point was measured in triplicates and was expressed as the percentage of its length at the time of implantation (16 mm).
Statistical Analysis
All data were expressed as mean ± standard deviation. The inter-group or inter-timepoint difference in relative SIS scaffold length was compared by using the analysis of variance or Student’s –t-test. A P-value less than 0.05 was considered statistically significant.
RESULTS
Expression of Contractile Proteins by Intestinal SMCs in vitro
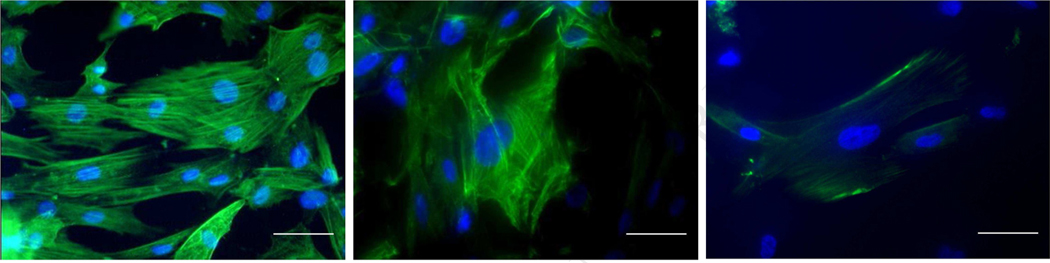
Intestinal SMCs reached confluency on day 7 of culture and exhibited immunoreactivity of intracellular contractile filamentous proteins including α-SMA (Fig.1A), CALP (Fig.1B), and SM-MHC (Fig.1C). Cross sections of cell-seeded SIS scaffolds showed that intestinal SMCs were present throughout the four layers of the four-ply SIS with more cells present on the rough side.
FIG. 1.
In vitro expression patterns of α-smooth muscle actin (A), calponin (B), and myosin heavy chain (C) in primary culture of intestinal smooth muscle cells (green fluorescence indicated stained contractile cytoskeletons and blue fluorescence indicated DAPI-counterstained nuclei) (400X, scale bar = 50 μm).
Histology of Implanted Scaffolds
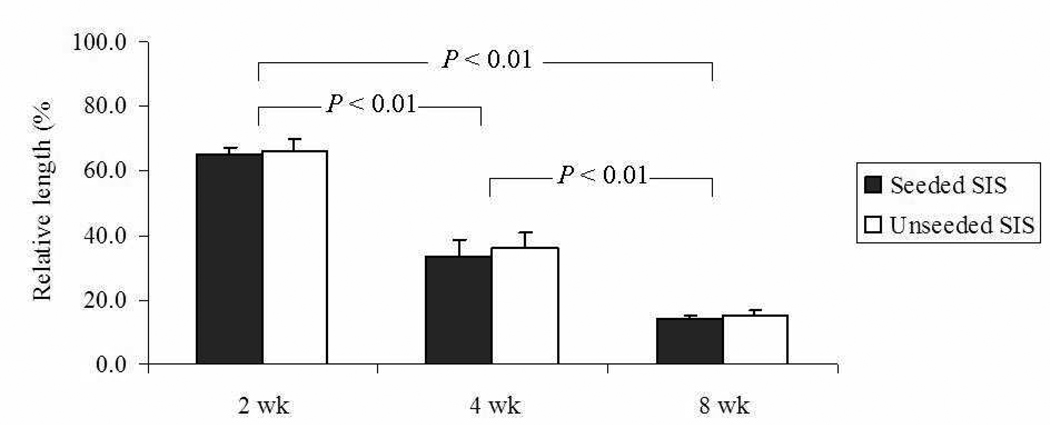
The length of the cell-seeded SIS tubular scaffolds decreased significantly over the 8 weeks (P < 0.01) but was comparable to that of SIS scaffolds without cell seeding (FIG. 3). The implanted scaffolds remained without mucosa through the fourth week (FIG. 4A and B), whereas scaffolds were partially lined with epithelia by week 8 (FIG. 4C). Non-specific inflammation was noted throughout 8 weeks. Neovascualrization at the site of implantation was found as early as 2 weeks.
FIG. 3.
Lengths of cell-seeded (n = 9) and non-seeded (n = 6) SIS scaffolds over 8 weeks of implantation versus original length.
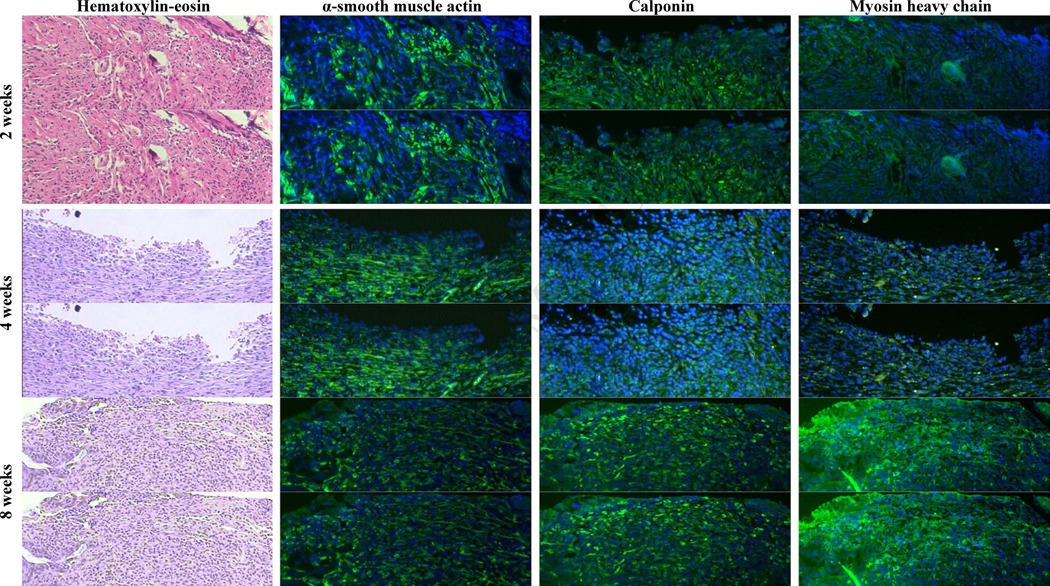
FIG. 4.
Hematoxylin-eosin and immunofluorescent staining of contractile proteins (green fluorescence against DAPI-counterstained nuclei) in regenerated tissues between the jejunal interposition over 8 weeks of implantation (200X, scale bar = 100 μm).
In vivo Contractile Protein Expression of Seeded Intestinal SMCs
The contractile protein expressions of cell-seeded SIS scaffolds following the implantation are shown in Figure 4. At 2 weeks, implant site exhibited extensive α-SMA expression, less CALP expression, and minimal SM-MHC expression. At 4 weeks, α-SMA-expressing cells decreased significantly, and CALP-expressing and SM-MHC-expressing cells were rarely seen in the implant site. A small number of α-SMA-expressing cells were noted at 8 weeks, but more cells expressing CALP or SM-MHC were observed in the proximity of the anastomotic interface.
DISCUSSION
Similar to skeletal muscle cells, intestinal SMCs are able to contract using myosin and actin filaments. Alpha-SMA belongs to the α-actin protein family, one of six subtypes of actin [18]. As a non-specific maker of SMCs, α-SMA is also abundantly expressed by non-muscle cells such as myofibroblasts [19]. Alpha-SMA is thought to be an early differentiation marker of SMCs. SM-MHC is a cytoplasmic structural protein and a major component of the contractile apparatus in SMCs. The expression of SM-MHC is developmentally regulated and emerges during smooth muscle development [20]. As a binding protein to calmodulin, F-actin and tropomyosin, calponin is thought to be also involved in the regulation of smooth muscle contraction. Calponin expression is specific to and representative of the differentiated (contractile) phenotype of developing smooth muscle [21]. Postnatal intestinal SMCs are expected to express α-SMA, calponin, and SM-MHC. In vitro, the isolated SMCs tended to lose differentiation, as shown by our immunostaining results. Moreover, the variation in SM-MHC expression demonstrates the heterogeneity of our primary seeded cells, some of which are less mature and with a further differentiation potential after the implantation.
SIS scaffold is reported to be biocompatible with multiple cell types, including fibroblasts, epithelial cells, epidermal cells, endothelial cells, mesenchymal stem cells, Schwann cells, and bladder SMCs [22]. SIS is a bioscaffold composed of extracellular matrix thought to be useful for tissue regeneration. SIS supports cell adhesion, survival, migration, and proliferation with the deposition of basement membrane components. Nevertheless, the biological properties of SIS as scaffold are less defined in the regeneration of smooth muscle. It has been shown that human bladder SMCs culture in SIS scaffolds exhibit normal cell viability, apoptosis profile, metabolic activity, and DNA synthesis [23]. The seeded SMCs in our experiments penetrated into the four layers of SIS scaffold. Additionally, SIS is thought to be non-toxic and non-immunogenic [24]. As porcine-derived SIS is a xenograft for rodent recipients, a chronic post-implantation foreign body inflammation was noted in our implants, consistent with previous reports [25]. Such reaction is deemed to be remodeling of the scaffolds [26].
As shown in our previous study [17], SIS demonstrates a less favorable biomechanical property when being exposed to the intestinal environment. SIS scaffold is reported to shrink remarkably in vivo [27]. The shrinkage was no different when intestinal SMCs were seeded in the SIS prior to implantation. The shrinkage is primarily attributed to the wound contraction that occurs at the implant site. The extracellular matrix content of the SIS is likely degraded by endogenous proteinases at the implant site [28].
Seeding of intestinal SMCs failed to support sufficient intestinal smooth muscle regeneration in this study. It is known that differentiated SMCs have a restricted capability of regeneration [29]. Other studies reported well-formed smooth muscle layers by using SIS scaffolds without seeded cells [14–16]; however, the observed smooth muscle is likely derived from the adjoining native smooth muscle rather than newly formed tissue.
Nakase et al. [27] implanted gastric SMCs-seeded collagen sponge as a patch to cover ileal wall defect. Seeded cells exhibited a transition from synthetic to contractile states over 12 weeks of implantation as shown by ultrastructural features. The expression of contractile protein was verified by the immunoreactivity to calponin. Striking shrinkage was also observed in the collagen sponge. Our experimental model is different from the report of Nakase et al. [27] in several respects: intestinal SMCs, scaffold shape (tubular versus patch), defect size (1.6 cm × 1.2 cm for rat versus 1.0 cm × 1.0 cm for dog), and implant site (excluded segment versus in-continuity). In our model, intestinal SMCs lost calponin and SM-MHC expression within a short period of implantation. Three growth factors, basic fibroblast growth factor (bFGF), transforming growth factor β (TGF-β), and vascular growth factor (VEGF) have been identified in SIS that may stimulate DNA synthesis and proliferation [30]. The importance of these factors in our model remains to be determined.
The major limitations of our model include less biomechanically favorable scaffold, minimally regenerated epithelia, and low survival of seeded cells following implantation. Synthetic biomaterial may be an alternative scaffold to guide muscularis regeneration. A ‘sandwich’ seeded cell/scaffold graft can be beneficial for synchronous epithelial and smooth muscle regeneration [31]. Scaffolds coated with microsphere-encapsulated growth factor, such as bFGF, are likely to be useful for intestinal tissue engineering [32].
In conclusion, our in vivo study suggests that SIS allows some SMCs to maintain their contractile protein expressions after implantation, but SIS seeded with SMCs still contracted significantly. The regenerated tissue also lacked orientation and organization of the SMCs. The incorporation of physicochemical signals to guide muscularis regeneration into the design of scaffolds may improve intestinal smooth muscle regeneration.
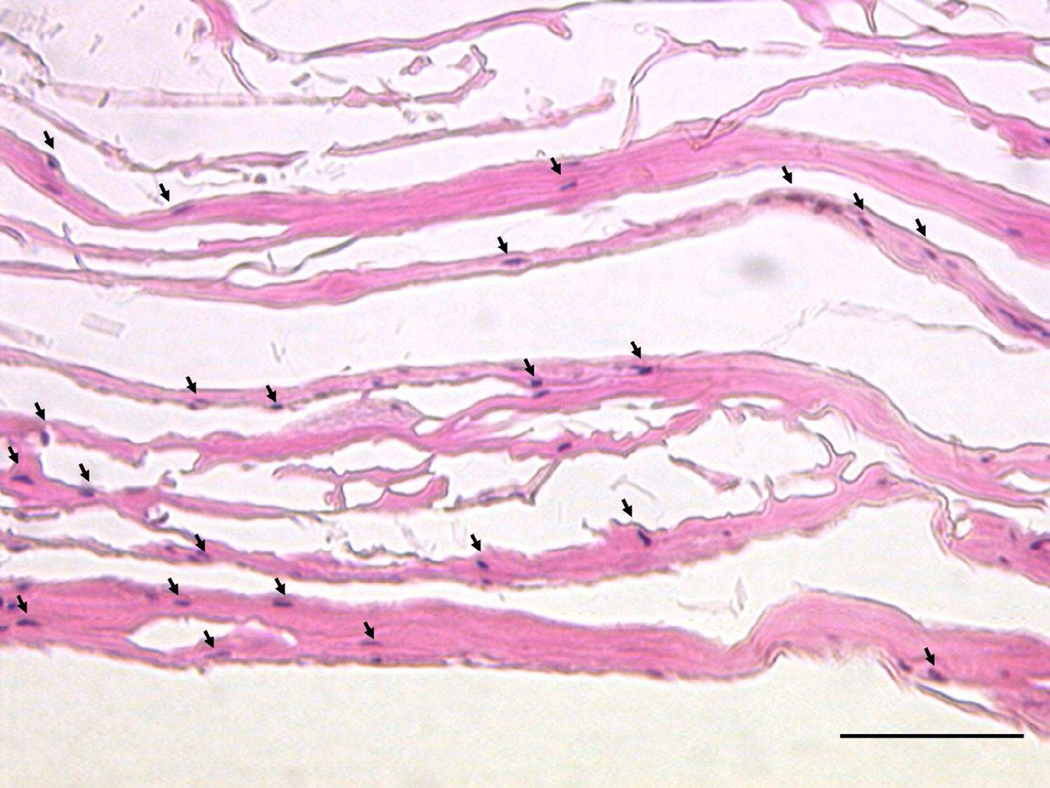
FIG. 2.
Seeding of intestinal smooth muscle cells (arrows, hematoxylin-stained nuclei) onto four-ply acellular small intestinal submucosa scaffold (eosin-stained layers) with the smooth side on the top and the rough side on the bottom (400X, scale bar = 50 μm).
ACKNOWLEDGEMENT
This work was supported by the Fubon Foundation and the National Institutes of Health R01 DK083319.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wales PW, Christison-Lagay ER. Short bowel syndrome: epidemiology and etiology. Semin Pediatr Surg. 2010;19:3. doi: 10.1053/j.sempedsurg.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaji T, Tanaka H, Wallace LE, et al. Nutritional effects of the serial transverse enteroplasty procedure in experimental short bowel syndrome. J Pediatr Surg. 2009;44:1552. doi: 10.1016/j.jpedsurg.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Nayyar N, Mazariegos G, Ranganathan S, et al. Pediatric small bowel transplantation. Semin Pediatr Surg. 2010;19:68. doi: 10.1053/j.sempedsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Dunn JC. Is the tissue-engineered intestine clinically viable? Nat Clin Pract Gastroenterol Hepatol. 2008;5:366. doi: 10.1038/ncpgasthep1151. [DOI] [PubMed] [Google Scholar]
- 5.Saxena AK, Baumgart H, Komann C, et al. Esophagus tissue engineering: in situ generation of rudimentary tubular vascularized esophageal conduit using the ovine model. J Pediatr Surg. 2010;45:859. doi: 10.1016/j.jpedsurg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Hori Y, Nakamura T, Kimura D, et al. Functional analysis of the tissue-engineered stomach wall. Artif Organs. 2002;26:868. doi: 10.1046/j.1525-1594.2002.07006.x. [DOI] [PubMed] [Google Scholar]
- 7.Jwo SC, Chiu JH, Ng KK, et al. Intestinal regeneration by a novel surgical procedure. Br J Surg. 2008;95:657. doi: 10.1002/bjs.6069. [DOI] [PubMed] [Google Scholar]
- 8.Grikscheit TC, Ochoa ER, Ramsanahie A, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann Surg. 2003;238:35. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sala FG, Kunisaki SM, Ochoa ER, et al. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J Surg Res. 2009;156:205. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 10.Ramsanahie A, Duxbury MS, Grikscheit TC, et al. Effect of GLP-2 on mucosal morphology and SGLT1 expression in tissue-engineered neointestine. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1345. doi: 10.1152/ajpgi.00374.2002. [DOI] [PubMed] [Google Scholar]
- 11.Nakase Y, Nakamura T, Kin S, et al. Endocrine cell and nerve regeneration in autologous in situ tissue-engineered small intestine. J Surg Res. 2007;137:61. doi: 10.1016/j.jss.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Tahara K, Murakami T, Fujishiro J, et al. Regeneration of the rat neonatal intestine in transplantation. Ann Surg. 2005;242:124. doi: 10.1097/01.sla.0000168089.64630.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansaloni L, Bonasoni P, Cambrini P, et al. Experimental evaluation of Surgisis as scaffold for neointestine regeneration in a rat model. Transplant Proc. 2006;38:1844. doi: 10.1016/j.transproceed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen MK, Badylak SF. Small bowel tissue engineering using small intestinal submucosa as a scaffold. J Surg Res. 2001;99:352. doi: 10.1006/jsre.2001.6199. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZQ, Watanabe Y, Toki A. Experimental assessment of small intestinal submucosa as a small bowel graft in a rat model. J Pediatr Surg. 2003;38:1596. doi: 10.1016/s0022-3468(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZQ, Watanabe Y, Noda T, et al. Morphologic evaluation of regenerated small bowel by small intestinal submucosa. J Pediatr Surg. 2005;40:1898. doi: 10.1016/j.jpedsurg.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Lee M, Chang PC, Dunn JC. Evaluation of small intestinal submucosa as scaffolds for intestinal tissue engineering. J Surg Res. 2008;147(2):168. doi: 10.1016/j.jss.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Zohar R, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp Cell Res. 2006;312:205. doi: 10.1016/j.yexcr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Chaponnier C, Gabbiani G. Pathological situations characterized by altered actin isoform expression. J Pathol. 2004;204:386. doi: 10.1002/path.1635. [DOI] [PubMed] [Google Scholar]
- 20.Eddinger TJ, Wolf JA. Expression of four myosin heavy chain isoforms with development in mouse uterus. Cell Motil Cytoskeleton. 1993;25:358. doi: 10.1002/cm.970250406. [DOI] [PubMed] [Google Scholar]
- 21.Shanahan CM, Weissberg PL, Metcalfe JC. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993;73:193. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- 22.Pariente JL, Kim BS, Atala A. In vitro biocompatibility evaluation of naturally derived and synthetic biomaterials using normal human bladder smooth muscle cells. J Urol. 2002;167:1867. [PubMed] [Google Scholar]
- 23.Feng C, Xu YM, Fu Q, et al. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J Biomed Mater Res A. 2010;94:317. doi: 10.1002/jbm.a.32729. [DOI] [PubMed] [Google Scholar]
- 24.Kim MS, Ahn HH, Shin YN, et al. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials. 2007;28:5137. doi: 10.1016/j.biomaterials.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Ashley RA, Roth CC, Palmer BW, et al. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int. 2010;105:1462. doi: 10.1111/j.1464-410X.2009.08965.x. [DOI] [PubMed] [Google Scholar]
- 26.Allman AJ, McPherson TB, Badylak SF, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 27.Nakase Y, Hagiwara A, Nakamura T, et al. Tissue engineering of small intestinal tissue using collagen sponge scaffolds seeded with smooth muscle cells. Tissue Eng. 2006;12:403. doi: 10.1089/ten.2006.12.403. [DOI] [PubMed] [Google Scholar]
- 28.Hodde JP, Badylak SF, Brightman AO, et al. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2:209. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- 29.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 30.Hurst RE, Bonner RB. Mapping of the distribution of significant proteins and proteoglycans in small intestinal submucosa by fluorescence microscopy. J Biomater Sci Polym Ed. 2001;12:1267. doi: 10.1163/156856201753395798. [DOI] [PubMed] [Google Scholar]
- 31.Nakahara T, Nakamura T, Kobayashi E, et al. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003;9:153. doi: 10.1089/107632703762687636. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Wu BM, Stelzner M, et al. Intestinal smooth muscle cell maintenance by basic fibroblast growth factor. Tissue Eng Part A. 2008;14(8):1395. doi: 10.1089/ten.tea.2007.0232. [DOI] [PubMed] [Google Scholar]