Abstract
Increasing evidence indicates that the medial prefrontal cortex (mPFC) and the amygdala mediate expression and extinction of conditioned fear, but few studies have examined the inputs to these structures. The dorsal part of the midline thalamus (dMT) contains structures such as the mediodorsal nucleus, paraventricular nucleus, and paratenial nucleus that project prominently to mPFC, as well as to basal (BA) and central (Ce) nuclei of the amygdala. Using temporary inactivation with GABA agonist muscimol, we found that dMT was necessary for retrieving auditory fear memory that was 24 h old, but not 2-8 h old. However, pre-training infusions did not impair fear acquisition or extinction. To determine the possible targets of dMT that might modulate fear retrieval, we combined dMT inactivation with Fos immunohistochemistry. Rats with inactivation-induced impairment showed increased Fos in the lateral division of Ce (CeL), and decreased Fos in the medial division of Ce. No differences in Fos expression were observed in the mPFC or BA. We suggest that the projections from the paraventricular nucleus to CeL are involved in retrieval of well consolidated fear memories.
Paré
Keywords: thalamus, central amygdala, Fos, prefrontal cortex, extinction
1. Introduction
In recent years, there has been renewed interest in the neural circuits mediating the regulation of conditioned fear memories. The medial prefrontal cortex (mPFC) has emerged as a key structure in emotion regulation (Etkin et al., 2011; Quirk and Beer, 2006; Sotres-Bayon et al., 2006), and can modulate fear expression bidirectionally via projections to the amygdala (McDonald, 1991; Quirk and Beer, 2006; Sotres-Bayon and Quirk, 2010; Vertes, 2004). Pharmacological inactivation and stimulation studies indicate that the prelimbic (PL) subregion of the mPFC is essential for fear expression (Corcoran and Quirk, 2007; Sierra-Mercado et al., 2011; Vidal-Gonzalez et al., 2006), whereas the infralimbic subregion (IL) is essential for fear extinction (Kim et al., 2009; Laurent and Westbrook, 2009; Milad and Quirk, 2002; Sierra-Mercado et al., 2011). PL and IL both receive a strong input from the dorsal midline thalamic nuclei (dMT), including the mediodorsal nucleus (MD) and paraventricular nucleus (PV) (Hoover and Vertes, 2007; Vertes, 2006). dMT nuclei also project to the basal (BA) and central (Ce) nuclei of the amygdala (Turner and Herkenham, 1991; Vertes and Hoover, 2008). Thus, dMT nuclei are well situated to modulate fear retrieval and/or extinction, either via the mPFC or the amygdala.
Despite anatomical as well as physiological evidence for an influence of dMT on mPFC and amygdala neurons (Ferron et al., 1984; Gigg et al., 1994; Pirot et al., 1994; Vives and Mogenson, 1985), relatively few studies have examined dMT's role in conditioned fear. Regarding fear acquisition, MD lesions reportedly had no effect on acquisition or expression of tone conditioning (Garcia et al., 2006), but impaired acquisition and expression of context conditioning (Li et al., 2004). Regarding extinction, MD lesions did not impair extinction learning or retrieval (Garcia et al., 2006), even though extinction increases mPFC potentials evoked by MD stimulation (Herry and Garcia, 2002; Herry et al., 1999).
The lesion approach often underestimates the role of a given structure, due to recovery of function by other areas (Anglada-Figueroa and Quirk, 2005; Poulos et al., 2010). In light of this, we recently used the GABAA agonist muscimol to evaluate the contributions of prefrontal, hippocampal, and amygdalar areas to the expression and extinction of tone-induced fear (Sierra-Mercado et al., 2011). In the present study, we used the same technique to evaluate the role of the dMT in fear acquisition, retrieval and extinction. We found that while dMT was not necessary for acquisition or extinction, it was necessary for retrieval of fear memories that were 24 h old, but not 2-8 h old. We extended our approach with Fos immunohistochemistry (neural activity marker) to evaluate the effect of dMT inactivation on target areas involved in fear regulation. Impaired fear retrieval was correlated with changes in Fos expression in Ce, but not in PL, IL or BA.
2. Materials and Methods
2.1 Subjects
A total of 108 male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 270-320 g were housed and handled as described previously (Quirk et al., 2000). Rats were restricted to 18 g/day of standard laboratory rat chow, followed by training to press a bar for food on a variable interval schedule of reinforcement (VI-60). Pressing a bar for food ensures a constant level of activity in which freezing behavior can be reliably measured during long training sessions, and provides an additional measure of fear (suppression ratio) that is more sensitive than freezing (Quirk et al., 2000). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico, School of Medicine in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
2.2 Surgery
After bar-press training, rats were anesthetized with intraperitoneal injections of a mixture of ketamine (80 mg/kg)-xylazine (10 mg/kg) and were stereotaxically implanted with a double 26-gauge guide cannula (Plastics One, Roanoke, VA, USA) aiming the medial dorsal nucleus of the thalamus (coordinates: anteroposterior, -2.5 mm from bregma; mediolateral, +/- 0.60 mm from midline; dorsoventral, - 4.5 mm from skull surface) (Paxinos and Watson, 1997). Cannulas were fixed to the skull with dental cement and two stainless steel screws previously fixed with a screwdriver. After surgery, a triple antibiotic was applied and an analgesic (Ketofen; 2 mg/kg) was injected intramuscularly. Stainless steel obturators (33-gauge) were inserted into the guide cannulas to avoid obstructions until infusions were made. After surgery, animals were allowed to recover 5-7 days before initiating experiments.
2.3 Histology
Upon completion of experiments, rats were transcardially perfused with 0.9% saline followed by 10% buffered formalin. Brains were extracted and stored in a 30% sucrose/ 10% formalin solution. Coronal sections were cut 40 μm thick, mounted on slides, and stained for Nissl bodies.
2.4 Drug infusions
On the day prior to commencement of the experiment (Day 0), injectors were briefly inserted without infusion and rats were acclimated for handling. Injector tips extended 1.0 mm beyond the guide cannula. Muscimol (MUS; Sigma Aldrich) was used to enhance GABAA receptor activity, thereby inactivating target structures, and was infused 15 minutes prior to behavioral testing. MUS or saline-vehicle (SAL) was infused at a rate of 0.2 μl/min (0.11 nmol/0.2 μl/per side) (Sierra-Mercado et al., 2011). After infusion, injectors were left in place for 1 min to allow the drug to diffuse.
2.5 Fear Conditioning and Extinction
Rats were conditioned and extinguished in standard operant chambers (Coulbourn Instruments, Allentown, PA) located in sound-attenuating cubicles (Med Associates, Burlington, VT). The floor of the chambers consisted of stainless steel bars that delivered a scrambled electric footshock. Between experiments, shock grids and floor trays were cleaned with soap and water, and chamber walls were cleaned with wet paper towels. The same chamber was used for conditioning, retrieval tests, and extinction training. Rats were conditioned with a pure tone (30 sec, 4 kHz, 77 dB) paired with a shock delivered to the floor grids (0.5 s, 0.52 mA). All trials were separated by a variable interval averaging 3 minutes.
Conditioning was performed on Day 1 and consisted of 5 habituation tones followed immediately by 7 conditioning tones that co-terminated with footshocks. To evaluate the role of dMT in fear acquisition and extinction learning, dMT was inactivated prior to the conditioning session on day 1 or prior to the extinction session on day 2. In both of these experiments, 20 extinction tones were given. Extinction memory was evaluated on day 3, with a 15 tone test. To evaluate the role of dMT in fear retrieval, dMT was inactivated at one of four post-conditioning timepoints: 2, 4, 8 or 24 h postconditioning. Each group was given two tones, 15 min after infusion.
One week after completion of fear experiments, a subset of rats were infused with either MUS or SAL to assess the effects of dMT inactivation on locomotor activity and anxiety levels. An open field square apparatus was used (l: 91.5 cm, w: 91.5 cm, h: 61 cm), which was divided into peripheral (within 15.25 cm of the walls) and central (l: 61 cm, w: 61 cm) regions of equal area. Rats were tested for 10 min each (Walsh and Cummins, 1976).
2.6 Immunohistochemistry
Ninety minutes after fear retrieval, muscimol or saline infused rats were deeply anesthetized with sodium pentobarbital (450 mg/kg i.p.) and perfused transcardially with 100 ml saline (0.9 %), followed by 500 ml of 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were post fixed for 3 h in the same fixative solution and transferred to a solution of 30 % sucrose in 0.1 M phosphate buffer at 4° C for 2 nights. Brains were then frozen and a series of 40 μm sections were cut with a cryostat (Leica, CM 1850) in the frontal plane and collected at different levels of the medial prefrontal cortex and amygdala. One complete series of sections was processed for immunohistochemistry with anti-Fos serum raised in rabbit (Ab-5, Oncogene Science) at a dilution of 1:20.000 overnight. The primary antiserum was localized using a variation of the avidin-biotin complex system. Sections were incubated for 120 minutes at room temperature in a solution of biotinylated goat anti-rabbit IgG (Vector Laboratories) and then placed in the mixed avidin-biotinhorseradish peroxidase complex solution (ABC Elite kit, Vector Laboratories) for 90 minutes. Black immunoreactive nuclei labeled for Fos were visualized after ten minutes of exposure to a chromogen solution containing 0.02 % 3.-30 diaminobenzidinetetrahydrochloride with 0.3 % nickel-ammonium sulfate (DAB-Ni) in 0.05 M Tris buffer (pH 7.6) followed by incubation for 5 min in a chromogen solution with glucose oxidase (10 %) and D-Glucose (10 %). The reaction was stopped using potassium PBS (pH 7,4). Sections were mounted in coated-gelatin slides and then dehydrated and cover slipped. Counter sections were collected, stained for Nissl bodies, cover slipped and examined in an optical microscope to determine the anatomical boundaries of each structure analyzed.
2.7 Immunoreactivity quantification
Counting of Fos positive cells was carried out at 20× magnification of an Olympus microscope (Model BX51) equipped with a digital camera. Images were generated for PL, IL, basal nucleus of the amygdala (BA), lateral/central nucleus of the central amygdala (CeL/CeC) and medial nucleus of the central amygdala (CeM). To be considered positive for Fos-like immunoreactivity, the nucleus had to be of appropriate size (area ranging from 100 to 500 μm2) and shape (at least 50 % of circularity), and be distinct from the background. Fos positive cells were automatically counted and averaged across 2 distinct rostro-caudal sections for each brain structure analyzed (Metamorph software version 6.1). The density of Fos positive cells in the CeL/CeC and CeM was calculated by dividing the number of Fos positive cells by the total area of each region.
2.8 Data Collection and Analysis
Behavior was recorded with digital video cameras (Micro Video Products, Bobcaygeon, Ontario, Canada) and freezing was measured using commercially available software (Freezescan, Clever Systems, Reston, VA, USA). Trials were averaged in blocks of two and compared with repeated-measures analysis of variance (ANOVA), followed by Tukey's post hoc comparisons (STATISTICA; Statsoft, Tulsa, OK). In some comparisons, Student's t-tests (two-tailed) were used. In addition to freezing, suppression of bar-pressing was used as a measure of conditioned fear (Quirk et al., 2000; Sierra-Mercado et al., 2006) and analyzed using Student's t-test. A suppression ratio comparing pre-tone press rates with tone press rates was calculated as follows: (pretone – tone)/(pretone + tone). A value of 0 represents no suppression (low fear), whereas a value of +1 represents complete suppression (high fear). For the open field, the total number of line crosses and percent time spent in the center were assessed.
3. Results
3.1 dMT is not necessary for acquisition of conditioned fear
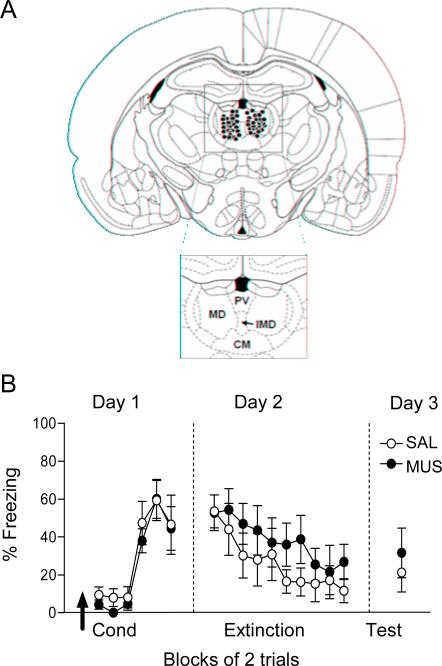
Figure 1A shows the location of injector tips for muscimol infusion within the mediodorsal nucleus for all experiments. Fifteen minutes prior to conditioning, rats were infused with 0.2 μL of physiological saline (SAL) or muscimol (0.11nmol), which was the same dose we used previously to inactivate PL and IL (Sierra-Mercado et al., 2011). We estimated a spread of approximately 1 mm diameter, based on prior studies tracking labeled muscimol (Corcoran et al., 2005; Pothuizen et al., 2005). This degree of spread would affect MD, PV, part of CM, and perhaps the more rostrally located PT. As shown in Figure 1B, inactivation of dMT prior to conditioning did not affect acquisition of fear, as repeated measures ANOVA revealed no effect of drug (F(1, 50)= 0.29, p=0.59) or interaction between drug and trial block (F(5,50)= 0.20, p= 0.95). The following day (Day 2), freezing during the first trial block was similar between SAL and MUS groups (52.71 %; 53.48 %, respectively; t10= 0.05, p= 0.95), indicating that both groups acquired similar levels of fear during conditioning. In addition, a repeated-measures ANOVA revealed a significant effect of trial block indicating that animals extinguished (F(9, 90)= 4.65, p<0.001), but no effect of drug (F(1,10)= 0.70, p= 0.42). Together these data suggest that dMT is not necessary for fear acquisition.
Fig. 1.
Inactivation of dMT does not impair acquisition of fear conditioning. (A) Schematic showing the location of injector tips in dorsal midline thalamus (dMT) for muscimol-infused rats in all experiments. Abbreviations: MD: mediodorsal nucleus, PV: paraventricular nucleus, CM: centromedial nucleus, IMD: intermediodorsal nucleus. (B) Infusion of saline (SAL) or muscimol (MUS) into dMT 15 minutes prior to conditioning (arrow) did not alter freezing during conditioning, extinction (Day 2), or retrieval of extinction (Day 3). Data are shown as mean ± SEM, in blocks of two trials (SAL n=6, MUS n=6).
3.2 dMT is not necessary for fear extinction
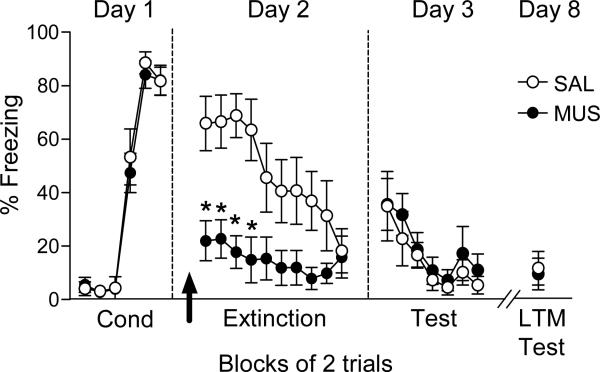
We next investigated the role of dMT in extinction of conditioned fear, by inactivating prior to extinction training on Day 2. As shown in figure 2, dMT inactivation prior to extinction decreased fear expression throughout the extinction session. A repeated-measures ANOVA revealed a main effect of drug (F(1,17) = 7.62; p= 0.013) and an interaction between drug and trial block (F(9,153)= 3.11, p= 0.002). Subsequent analysis using Tukey post hoc test confirmed a significant reduction in freezing by MUS during the first four blocks of extinction training (p<0.01). In addition to reducing freezing, repeated-measures ANOVA revealed that MUS significantly reduced conditioned suppression of bar pressing (suppression ratio for 1st block, SAL: 0.97; MUS: 0.43; F(1,17)= 10.72; p<0.01). Subsequent analysis using Tukey post hoc test confirmed a significant reduction in suppression ratio during the first 5 blocks (p<0.001).
Fig. 2.
dMT inactivation did not impair extinction. Inactivation of dMT prior to extinction training (arrow) significantly decreased freezing in trial blocks 1-4. The next day, however, MUS-infused rats showed normal retrieval of extinction learned the previous day. MUS-infused rats continued to show normal extinction retrieval after an additional 5 days. Data are shown as mean ± SEM in blocks of two trials (SAL n=9; MUS n=10). *p < 0.01 Repeated-measures ANOVA followed by Tukey's post hoc test.
Despite reduced expression of freezing, the groups significantly decreased their freezing throughout the extinction session. Repeated-measures ANOVA revealed a significant effect of trial block (F(9,153)= 5.46, p= 0.001). On day 3, both groups showed similar retrieval of extinction. Freezing during the first block of tones was similarly low in both groups (SAL: 34.94 %; MUS: 35.56 %, t18= - 0.03; p= 0.97). A repeated-measures ANOVA for the extinction test revealed a significant effect of trial block (F(6, 102)= 9.95, p< 0.001), but no effect of drug (F(1,17)= 0.03, p= 0.84) nor interaction between drug and trial block (F(6,102)= 0.80, p= 0.56). Retrieval of extinction was also unimpaired 5 days later (see figure 2). Together, these data suggest that dMT is necessary for retrieval of a previously acquired fear memory, but not for extinction learning.
3.3 dMT has a time-dependent role in retrieval of fear memories
The pattern of results above suggests that dMT becomes involved in fear expression sometime after the initial acquisition phase. To explore the time course of dMT involvement, we conditioned four groups of rats drug-free. We then inactivated dMT at one of four timepoints post-conditioning: 2 h, 4 h, 8 h or 24 h. Fifteen minutes after each infusion, rats were given two tones to test for retrieval of fear memory (each group was only infused once).
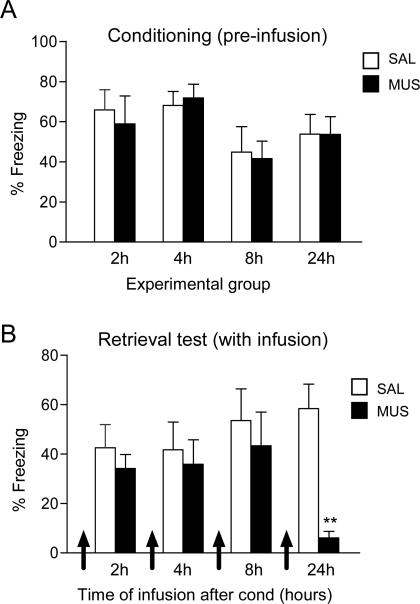
Figure 3 shows the conditioning levels (average of last three trials of conditioning) for all groups prior to infusions. As expected, two way ANOVA for conditioning showed no significant effect for drug (F(1,51)= 0.05, p= 0.82), or timepoint (F(3,51)= 0.09, p= 0.07). Subsequent dMT inactivation did not impair retrieval of fear in the 2, 4, or 8 h groups (all t's < 1.07, all p's > 0.30), but did impair retrieval in the 24 h group (SAL: 58.44 %; MUS: 6.05 %; t17 = 5.15; p= 0.001), replicating our findings from figure 2. In addition to reducing freezing, 24h MUS rats showed a significant reduction in suppression ratio during fear retrieval (SAL: 0.80; MUS: 0.26; t17= 3.54, p= 0.002). Therefore, the involvement of dMT in fear retrieval appears to be time-dependent, with dMT becoming involved 24 h following conditioning. The loss of memory in the 24 h group did not appear to be permanent, as a drug free test on day 3 showed equivalent levels of freezing in both groups (SAL: 27.62%; MUS: 30.54%; t17= -0.19; p= 0.85) as well as similar suppression ratios (SAL:0.63; MUS: 0.93; t17= -1.35, p= 0.19). Thus, activity in dMT appears to be necessary for retrieval of fear memory, rather than consolidation or retention.
Fig. 3.
Inactivation of dMT impaired retrieval of long-term, but not short-term, fear memory. (A) Freezing levels to tones in all groups during conditioning trials 5-7, on day 1, prior to infusions. (B) Freezing levels 15 minutes after infusion of SAL or MUS, at different post-conditioning delays (2 h, 4 h, 8 h, 24 h, each group was infused only once). Only at 24 h did MUS impair conditioned freezing (** p < 0.01). Data are shown as mean ± SEM in blocks of two trials (2 h: SAL n=7, MUS n=7; 4 h: SAL n=7, MUS n=7; 8h: SAL n=7, MUS n=7; 24 h: SAL n=9; MUS=9).
In addition to reduced tone fear, MUS rats infused 24 h after conditioning also showed reduced contextual fear, as indicated by the rates of bar pressing for food. In SAL rats, conditioning significantly reduced the rate of spontaneous pressing prior to the first tone (pre-cond: 23.0 presses/min; pre-ext: 11.11 press/min; t(1,9)= 2.306;p=0.004). In contrast, MUS rats showed no such reduction in press rates (pre-cond: 17.66; preext: 17.77; t(1,9)= 2.306; p=0.973) consistent with a deficit in retrieval of contextual fear.
3.4 dMT inactivation modulates Fos expression in Ce, but not in BA or mPFC
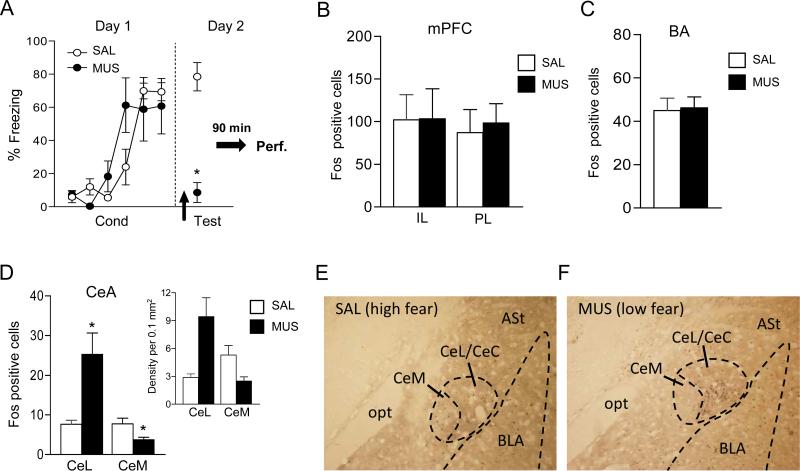
To investigate the neural circuit through which dMT might influence fear, we assessed Fos expression in rats infused with SAL or MUS 24 h after conditioning. Again, replicating our findings above (figures 2 and 3), inactivation of dMT 24 h after conditioning impaired the retrieval of fear memory, as evidenced by significantly reduced freezing during a two-tone test (MUS: 78.5 %; SAL: 17.4%; t7= 4.17, p= 0.005) (see figure 4A). Ninety minutes after the test, rats were sacrificed and processed for Fos immunoreactivity. dMT inactivation had no effect on Fos expression in PL, IL or BA (all t's < 0.31, all p's > 0.76) (see figure 4B-C). In contrast, inactivation of dMT significantly increased Fos expression in CeL (SAL: 7.67; MUS: 25.25, t7= -2.73; p=0.04), and significantly decreased Fos expression in CeM (SAL: 7.75; MUS: 3.75, t7=2.82; p= 0.03) (see figure 4D). The density of Fos positive cells in the saline group showed a trend toward higher counts in CeM compared to CeL (CeM: 5.3; CeL: 2.87, t6= -2.23; p= 0.08), consistent with fear retrieval (inset figure 4D). In contrast, under muscimol, CeL showed a significantly higher density of label than CeM (CeL: 9.4; CeM: 2.4, t8= 3.34; p= 0.01). Given that CeL contains GABAergic neurons that inhibit CeM output neurons (Ciocchi et al.; Martina et al., 1999), our findings suggest that dMT inactivation reduced fear by increasing feed-forward inhibition of CeM.
Fig. 4.
Inactivation of dMT altered Fos expression in the amygdala central nucleus. (A) Replicating our findings above (see figs 2 and 3B), dMT infusion of MUS 24 h after conditioning (arrow) impaired fear retrieval. 90 mins later, brains were processed for Fos immunohistochemistry. (B-C) dMT inactivation did not alter Fos counts in IL, PL, or BA. (D) In contrast, dMT inactivation increased Fos counts in CeL and decreased Fos counts in CeM. The density of Fos positive cells in CeM and CeL is shown in the inset. *p < 0.05 t-test between MUS and SAL groups (SAL n=3; MUS n=4). (E-F) Photomicrographs showing Fos labeled neurons in the amygdala of SAL and MUS-infused rats (10X magnification). Note the shift of label from CeM to CeL with MUS infusion.
3.5 Inactivation of dMT does not affect locomotion, anxiety, or motivation
Given the marked effects of dMT inactivation on freezing, we sought to determine if dMT inactivation produced other behavioral effects that might potentially confound freezing measurements. Inactivation of dMT did not affect motivation to press a lever for food, as indicated by similar rates of spontaneous pressing in SAL and MUS-infused rats prior to conditioning (MUS: 14.3 presses per min, SAL: 12.05 presses per min; t13=- 0.54, p= 0.59). To assess open-field behavior, a subset of rats was re-infused one week following the fear experiments. Inactivation of dMT did not increase locomotion in the open field, as indicated by the number of line crosses (MUS: 253.4, SAL: 235.0; t13= - 1.12; p = 0.28). Neither did dMT inactivation alter anxiety levels, since the amount of time spent in the center of the open field was similarly low for both MUS and SAL groups (14.0 %, 13.4 %, respectively; t13= - 0.22, p= 0.82).
4. Discussion
Previous studies using permanent lesions to assess the role of dorsal midline thalamic areas in conditioned fear have produced conflicting results. Here, we used the GABAA agonist muscimol to induce temporary inactivation of dMT during different phases of conditioning and extinction. We found that: (i) inactivation of dMT prior to conditioning or extinction training did not impair these processes; (ii) dMT was necessary for retrieval of long-term, but not short-term fear memory, and (iii) dMT inactivation did not alter Fos expression in mPFC or BA, but increased and decreased, respectively, Fos expression in CeL and CeM nuclei. These findings suggest that retrieval of a well consolidated fear memory involves dMT modulation of Ce activity.
4.1 The role of dMT in acquisition and extinction
Our negative results with respect to acquisition of fear agree with a previous finding that electrolytic lesions of MD did not affect acquisition of auditory fear conditioning (Garcia et al., 2006). Thus, dMT does not appear to be an essential site of plasticity for fear learning, at least for auditory conditioning. We also observed that inactivation of dMT did not alter extinction learning. These results were unexpected, given the extensive reciprocal connections between MD and infralimbic-mPFC (Hoover and Vertes, 2007), a key region in recall of fear extinction (Laurent and Westbrook, 2009; Quirk et al., 2006), and the fact that extinction potentiates MD-mPFC evoked potentials (Herry and Garcia, 2002; Herry et al., 1999). The critical factor here may be time, as a previous study showed that MD-mPFC evoked potentials increased 7 days after extinction training,(Hugues and Garcia, 2007). We observed stable extinction memory 7 days after extinction with pre-extinction infusions, however, the effect of MD inactivation 7 days after extinction training on recall of extinction is not known.
4.2 The time-dependent role of dMT in fear retrieval
Despite these negative findings, dMT was necessary for retrieval of fear memory learned the previous day. To our knowledge, this is the first evidence that dMT activity is essential for retrieval of a tone-shock association. In addition to impaired retrieval during the tone, we also observed impaired contextual fear, reflected in bar press rates, which agrees with prior studies examining lesions of MD made prior to (Antoniadis and McDonald, 2006; Li et al., 2004), or after (Li et al., 2004) contextual fear conditioning. A surprising finding was the time dependence of our effects: inactivation of dMT 2, 4 or 8 h after conditioning did not impair fear retrieval. This suggests that thalamic circuits may be recruited sometime between 8 and 24 h after training for retrieval of fear conditioning. Late phase involvement of dMT in fear retrieval has not yet been reported, but similar observations have been made for other structures. For example, 12 hours after inhibitory avoidance training, there is a sudden increase in Fos expression in the hippocampus (Katche et al., 2010).
It was surprising that dMT inactivation did not reduce Fos immunoreactivity in PL, given dMT's strong reciprocal connections with PL (Groenewegen, 1988; Moga et al., 1995; Van der Werf et al., 2002; Vertes and Hoover, 2008), and the role of PL in expression of conditioned fear (Burgos-Robles et al., 2009; Choi et al.; Laurent and Westbrook, 2009; Sierra-Mercado et al., 2011). At face value, this suggests that dMT modulates fear via targets other than the mPFC. PL involvement, however, cannot be completely ruled out based on these findings, because Fos may not be sufficiently sensitive to detect changes in tone responses in PL neurons, given their high baseline firing rate (Burgos-Robles et al., 2009). The critical test of PL involvement will be to record PL unit responses in rats with dMT inactivation, to determine the extent to which PL tone responses are dependent on dMT inputs. Regarding BA, our negative Fos findings agree with a prior observations that small lesions (Onishi and Xavier) or inactivation (Herry et al., 2008) of BA did not impair retrieval of auditory fear conditioning.
4.3 dMT modulation of amygdala central nucleus
In contrast to PL and BA, Ce showed pronounced Fos changes after dMT inactivation. Fos expression increased in CeL and decreased in CeM. It is well established that CeM neurons mediate amygdala control of fear via subcortical projections to midbrain and hypothalamic targets (Antoniadis and McDonald, 2006; Davis, 2000; De Oca et al., 1998; LeDoux et al., 1988), and that CeL sends inhibitory projection to CeM output neurons (Lopez de Armentia and Sah, 2004; Petrovich and Swanson, 1997). This CeL-CeM network has received increased attention recently for its role in fear acquisition and expression (Ehrlich et al., 2009; Pape and Pare, 2010; Wilensky et al., 2006). Retrieval of auditory fear memory was correlated with excitatory tone responses in CeM neurons, together with inhibitory tone responses in CeL neurons, consistent with disinhibition of CeM (Ciocchi et al., 2010; Duvarci et al., 2011). Furthermore, two subpopulations of reciprocally connected CeL neurons were identified (Haubensak et al., 2010), suggesting gating of CeL projections to CeM. Thus, dMT could drive fear responses by exerting feed-forward inhibition of the CeL neurons that project to CeM.
Given known projections of the midline thalamus (Li and Kirouac, 2008; Moga et al., 1995; Vertes and Hoover, 2008), there are several possible circuits that are consistent with the pattern of Fos immunoreactivity we observed. MD does not project to CeL, but the paraventricular nucleus (PV) does. PV could activate intra-CeL GABAergic neurons, which in turn would inhibit CeL-CeM inhibitory projections. It was previously proposed that thalamic inputs to CeL involved in retrieval of fear conditioning originated in the sensory thalamus (Ciocchi et al., 2010), but our findings suggest that PV might play this role. Consistent with this, stimulation of PV induces feed-forward inhibition of CeL neurons (Veinante and Freund-Mercier, 1998), and retrieval of conditioned fear activates Fos in PV (Beck and Fibiger, 1995). Alternatively, PV and paratenial (PT) nuclei both project directly to CeM (Vertes and Hoover, 2008), and could drive CeM directly. Lastly, PT projects to the dorsal intercalated cells of the amygdala (Royer and Pare, 2002; Vertes and Hoover, 2008), which could inhibit CeL neurons that project to CeM. Additional experiments assessing conditioned responses in different parts of dMT, and connections with Ce are needed to distinguish between these possibilities.
In further support of CeL as the likely target of dMT inactivation, Duvarci et al. (2011) observed that the number of CeL neurons with inhibitory tone responses was increased 24 h after conditioning, compared to immediately after conditioning. Together with our findings, this suggests that 8-24 hr after conditioning, there may be potentiation of auditory inputs to PV (from PFC, BA, midbrain?), which would increase the inhibitory responses in CeL, thereby disinhibiting CeM. A testable prediction from this scenario is that PV would show a time-dependent development of tone responses after conditioning. One possible advantage of recruiting midline thalamic areas such as PV and PT is that they target diverse areas (and functions) such as dorsal striatum (avoidance), bed nucleus of stria terminalis (anxiety), entorhinal cortex (spatial function), and accumbens (reward). In this way, retrieval of fear could be coordinated with other functions to generate the most adaptive behavioral response to a threatening stimulus.
Inactivation of dMT prior to training did not impair conditioning.
Inactivation of dMT 24hr after training impaired fear retrieval.
Inactivation of dMT increased cFos in amygdala central nucleus (lateral).
Acknowledgements
We thank Demetrio Sierra-Mercado for help with experiments and comments on the manuscript, and Carlos Rodriguez, Oscar Ortiz, and Zarkalys Quintero for technical help. Supported by NIH grants: MH058883 and MH081975 to GJQ, MH086400 to Suzanne Haber (Conte Center), and a MARC undergraduate fellowship (GM007821) to NPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, McDonald RJ. Fornix, medial prefrontal cortex, nucleus accumbens, and mediodorsal thalamic nucleus: roles in a fear-based context discrimination task. Neurobiol Learn Mem. 2006;85:71–85. doi: 10.1016/j.nlm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25:8978–8987. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The Amygdala. 2000. The role of the amygdala in conditioned and unconditioned fear and anxiety. [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Paré D. Central amygdala activity during fear conditioning. J Neurosci. 31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron A, Thierry AM, Le Douarin C, Glowinski J. Inhibitory influence of the mesocortical dopaminergic system on spontaneous activity or excitatory response induced from the thalamic mediodorsal nucleus in the rat medial prefrontal cortex. Brain Res. 1984;302:257–265. doi: 10.1016/0006-8993(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigg J, Tan AM, Finch DM. Glutamatergic hippocampal formation projections to prefrontal cortex in the rat are regulated by GABAergic inhibition and show convergence with glutamatergic projections from the limbic thalamus. Hippocampus. 1994;4:189–198. doi: 10.1002/hipo.450040209. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Vouimba RM, Garcia R. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol. 1999;82:2827–2832. doi: 10.1152/jn.1999.82.5.2827. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Bekinschtein P, Slipczuk L, Goldin A, Izquierdo IA, Cammarota M, Medina JH. Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage. Proc Natl Acad Sci U S A. 107:349–354. doi: 10.1073/pnas.0912931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Li XB, Inoue T, Nakagawa S, Koyama T. Effect of mediodorsal thalamic nucleus lesion on contextual fear conditioning in rats. Brain Res. 2004;1008:261–272. doi: 10.1016/j.brainres.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–1294. doi: 10.1152/jn.00211.2004. [DOI] [PubMed] [Google Scholar]
- Martina M, Royer S, Paré D. Physiological properties of central medial and central lateral amygdala neurons. J Neurophysiol. 1999;82:1843–1854. doi: 10.1152/jn.1999.82.4.1843. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Onishi BK, Xavier GF. Contextual, but not auditory, fear conditioning is disrupted by neurotoxic selective lesion of the basal nucleus of amygdala in rats. Neurobiol Learn Mem. 93:165–174. doi: 10.1016/j.nlm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic; Sydney: 1997. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Pirot S, Jay TM, Glowinski J, Thierry AM. Anatomical and electrophysiological evidence for an excitatory amino acid pathway from the thalamic mediodorsal nucleus to the prefrontal cortex in the rat. Eur J Neurosci. 1994;6:1225–1234. doi: 10.1111/j.1460-9568.1994.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Jongen-Relo AL, Feldon J. The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacology. 2005;30:683–696. doi: 10.1038/sj.npp.1300643. [DOI] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong HW, Fanselow MS. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci U S A. 107:14881–14886. doi: 10.1073/pnas.1005754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr., Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998;794:188–198. doi: 10.1016/s0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508:212–237. doi: 10.1002/cne.21679. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives F, Mogenson GJ. Electrophysiological evidence that the mediodorsal nucleus of the thalamus is a relay between the ventral pallidum and the medial prefrontal cortex in the rat. Brain Res. 1985;344:329–337. doi: 10.1016/0006-8993(85)90811-x. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]