Abstract
Bone tissue engineering is a highly interdisciplinary field that seeks to tackle the most challenging bone-related clinical issues. The major components of bone tissue engineering are the scaffold, cells, and growth factors. This review will focus on the scaffold and recent advancements in developing scaffolds that can mimic the natural extracellular matrix of bone. Specifically, these novel scaffolds mirror the nanofibrous collagen network that comprises the majority of the non-mineral portion of bone matrix. Using two main fabrication techniques, electrospinning and thermally-induced phase separation, and incorporating bone-like minerals, such as hydroxyapatite, composite nanofibrous scaffolds can improve cell adhesion, stem cell differentiation, and tissue formation. This review will cover the two main processing techniques and how they are being applied to fabricate scaffolds for bone tissue engineering. It will then cover how these scaffolds can enhance the osteogenic capabilities of a variety of cell types and survey the ability of the constructs to support the growth of clinically relevant bone tissue.
Keywords: Bone, bone tissue engineering, scaffold, biomimetic material, nanofiber
Introduction
Bone injuries and defects present a significant clinical problem. Most are caused by trauma and are relatively simple to treat; however, complex breaks and pathological fractures arising from malformation, osteoporosis, and tumors present a difficult challenge to treat effectively. In serious fractures and defects or in elderly patients, complications such as mal-union or non-union are more common and prevent the bone from healing naturally. The current gold standard for those cases is an invasive surgery to align and stabilize the bone, usually with metallic pins, screws, plates, or rods, but these surgical procedures extend the healing time and can require multiple surgeries. However, in some specific cases such as hip fractures in elderly patients, a full hip replacement with a permanent metallic implant is standard. While the use of metal for its mechanical strength and integrity make it appealing, metallic implants have numerous drawbacks that can induce stress shielding, stiffness, infections, and chronic pain, among other side effects. Bone grafting is preferable in the most complex cases with the most significant risk. Autografts are ideal to reduce any risk of immune rejection but require multiple surgeries and have associated donor site morbidity.
Since all the current techniques have significant drawbacks, tissue engineering holds promise in providing an improved clinical therapy. By combining a scaffold, cells, and growth factors, tissue engineering seeks to create an alternative solution to repair bone injuries. There are several advantages that tissue engineering has. The use of an engineered implant would reduce the need for multiple surgeries associated with the removal of metallic stabilizers and graft harvesting. Fewer surgeries results in a quicker recovery time, lower costs, and reduced risks. The tissue engineering implant can also be integrated into the existing tissue, resulting in a seamless transition between the two. This prevents stress shielding and resorption of the healthy surrounding bone. These advantages can be mostly attributed to the development of biomimetic scaffolds, and that is what this review will focus on.
The goal of the scaffold is to provide a 3D environment for cells and tissue to grow on. For a biomimetic scaffold, the model is the natural extracellular matrix (ECM). The major protein of the ECM is collagen, which arranges into nanofibers[1] ranging from 50 to 500nm in diameter[2] and controls cell behavior with its architecture[3-5]. In bone, the basic building block of the ECM is the mineralized collagen I fibrils, with collagen comprising about 90% of the protein[6]. At nucleation points on the collagen fibrils there are highly ordered carbonated apatite crystals (Ca5(PO4, CO3)3(OH))[6] only a few nanometers thick[7]. The composite structure of the mineralized collagen fibrils is what gives bone it’s lightweight strength[8]. The mineralized collagen fibrils then align and arrange in ways to form higher order structures and eventually a full bone.
Naturally then, scaffolds were developed to mimic the nanofibrous collagen ECM[9, 10]. In addition to the nanofibrous architecture, these new scaffolds also had to have a key set of characteristics that all tissue engineering scaffolds need. They need to be highly porous to allow for cell ingrowth and efficient mass transport of nutrients, oxygen, growth factors, and waste products. For larger constructs, the pores must also facilitate vascularization to avoid necrosis at the core. The scaffolds must be structurally sound to withstand the mechanical stresses during tissue neogenesis. This requirement made metal alloys[11] and osteoconductive ceramics[12] appealing, but those are either not biodegradable or minimally biodegradable. Scaffolds should be biodegradable to allow for full tissue regeneration and biocompatible to avoid an immune response.
The first requirement, high porosity, is mainly a function of the processing technique. Currently, there are two main fabrication methods used in bone tissue engineering to create nanofibrous scaffolds: electrospinning and phase separation. This review will cover how scaffolds are made using each technique and recent advancements in fabricating next generation bone scaffolds. It should be noted that other methods to make nanofibrous scaffolds also exist, such as molecular self-assembly[13-16], bacteria-derived hydrogels[17], and electrohydrodynamic printing[18], but none are as widely applied in bone tissue engineering. The second two requirements, mechanical integrity and biodegradability, in addition to being a function of processing, are also a function of material choice. There are myriad natural and synthetic polymers that can satisfy those characteristics, and some, like poly(L-lactic acid) (PLLA), have already been FDA approved for other applications[19], but this review will not dwell on the polymer landscape of the bone tissue engineering field. It will, however, cover how composite nanofibrous scaffolds are fabricated and techniques used to do so. Finally, it will delve into the osteogenic advantages of nanofibers with respect to cell attachment, stem cell differentiation, and mineralized tissue formation.
Scaffold fabrication
Electrospinning
Electrospinning was first developed in the early 1900s for applications in textiles and filters. It wasn’t until Doshi et al. expanded the polymers capable of being electrospun[20] that the field of tissue engineering began to see the potential applications of electrospinning.
The principle of electrospinning is that an electric field is used to overcome the surface tension of a polymer solution to shoot a jet of liquid out of a needle toward a conducting collector[21]. The volatile solvent evaporates in the air leaving behind, under the right conditions, a polymer fiber with a diameter that can range from tens of nanometers to microns[20]. Many parameters affect this process including polymer properties, solvent properties[22], solution flow rate, voltage, distance from needle to collector, and polymer concentration, among others.
The wide range of polymers capable of being electrospun is appealing to bone tissue engineering and gives researchers flexibility in designing nanofibrous scaffolds. Generally, there are two groups of polymers that are used: synthetic and natural. Synthetic polymers, such as poly(L-lactic acid) (PLLA)[23], poly(glycolic acid) (PGA)[24], and polycaprolactone (PCL)[25, 26], among others, provide great flexibility in synthesis, processing, and modification. However, these polymers lack bioactivity and special care needs to be taken to ensure that newly synthesized polymers are biocompatible. Many natural polymers, on the other hand, have inherent bioactivity with peptide sequences that affect cell adhesion, proliferation, and differentiation. Collagen[27], gelatin[28], silk[29], and chitosan[30], among others, are commonly used natural polymers for scaffold fabrication, but care must be taken to prevent denaturation when proteins are used[31].
Since both synthetic and natural polymers have advantages and disadvantages, research has progressed to fabricate hybrid scaffolds in an effort to maximize the benefits of both. Yang et al. combined PCL with various amounts of chitosan to create bioactive nanofibers[32]. Pure electrospun chitosan was too weak to be mechanically tested and pure PCL had reduced cell adhesion, but a 9.1% chitosan in PCL nanofibers had the maximum Young’s modulus while significantly increasing cell adhesion compared to pure PCL. This novel hybrid scaffold takes advantage of the physical properties of the synthetic polymer and the bioactivity of the natural polymer while minimizing the disadvantages of both.
In addition to creating a hybrid scaffold, to further the bioactivity of electrospun nanofibers growth factors can be incorporated into the polymer to create a controlled delivery system. Zeng et al. added bovine serum albumin (BSA) to a poly(vinyl alcohol) (PVA) solution and electrospun fibers with diameters ranging from 250 to 300nm[33]. Control of the release rate was achieved by coating fibers with different thicknesses of poly(p-xylene). Following a burst release within 2 hours, the thicker coating slowed the long term release of BSA, allowing for greater control over the protein’s release rate. By incorporating a bone morphogenic protein (BMP), this delivery strategy can be used to design an osteoinductive scaffold for bone tissue engineering. However, while the authors showed that luciferase retained its bioactivity through the electrospinning and release process, they only showed activity for up to 1 day. For this to be applied to tissue engineering, a protein from the BMP family should be incorporated and assayed for its bioactivity for a much longer period of time.
With electrospinning’s clear benefits, there are also some obstacles that need to be overcome. It remains difficult to create clinically relevant 3D constructs beyond a relatively 2D mat. For bone tissue engineering, a large 3D scaffold may be required. While new processing techniques have shown promise to increase the size and porosity of electrospun scaffolds[34], more work needs to be done to further the architectural control. Having pores large enough for not only cell penetration, but vascular ingrowth is imperative for a vascularized tissue such as bone.
Thermally-induced phase separation
Thermally-induced phase separation (TIPS) takes advantage of the thermodynamic instability of polymer solutions at certain temperatures[35, 36]. It was applied to the fabrication of tissue engineering scaffolds in the 1990s with the work of several labs[37-39], including our lab which used a novel TIPS technique to produce a 3D nanofibrous scaffold[38].
TIPS is usually a five step process involving polymer dissolution, phase separation and gelation, solvent extraction, freezing, and freeze drying. When a polymer, such as PLLA, is dissolved in a solvent, it becomes thermodynamically unstable at low temperatures and will spontaneously separate into two phases. Under the right conditions, the polymer-rich phase forms the nanofibrous matrix and the polymer-lean phase is extracted, leaving behind nanofibers ranging from 50-500nm[38].
Beyond creating ECM-like nanofibers, TIPS can be combined with other techniques to provide additional architectural control on larger size scales. For instance, the polymer solution can be cast around a paraffin[40, 41], sugar[9, 42], or salt[9] porogen network to form macropores with different sizes and shapes after the removal of the porogen. Larger pores are necessary to allow cell penetration through entire scaffold, promote efficient transport of nutrients and waste, allow ingrowth of blood vessels, and guide tissue formation[43]. The ability to have control over the pore size is critical for bone tissue engineering as numerous studies have shown that pore size has an effect on cell behavior and bone formation[44, 45]. With that effect, controlling pore size becomes another way to control tissue regeneration.
On another level, the shape of the scaffold as a whole can be controlled by combining CT scanning and reverse solid freeform fabrication (SFF)[46]. This technique can image a segment of bone and create an anatomically shaped mold to cast the polymer solution in. Bone defects are rarely geometrical so the ability to create a custom mold and scaffold for each individual patient allows for a smooth transition into the clinic.
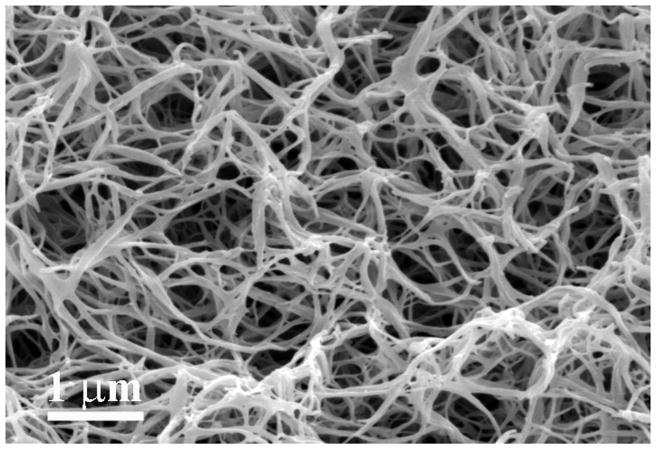
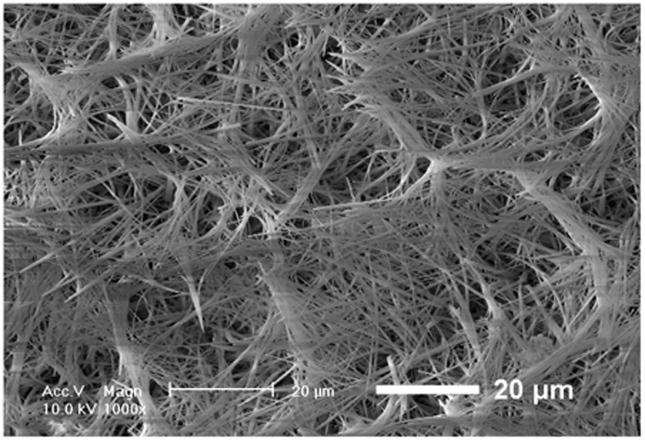
In an even simpler approach, Liu et al. used a star-shaped PLLA (SS-PLLA) polymer to fabricate nanofibrous hollow microspheres (Figure 1)[47]. Dissolved SS-PLLA was emulsified into liquid microspheres in glycerol and after phase separation, solvent extraction, and freeze drying, nanofibrous hollow microspheres were formed without the need for a template. Control over the size of the microspheres, from a few microns to a few hundred microns in diameter depending on the application, can be achieved by varying the polymer concentration and emulsification stirring speed. Furthermore, the absence of a surfactant in the process eschews any problems that arise with surfactant removal. The resulting microspheres act as injectable cell carriers that can fill complex defects without requiring a multiple step molding process. Additionally, the injectable nature of this treatment means the surgery is much less invasive, making the procedure easier on both patients and surgeons.
Figure 1.

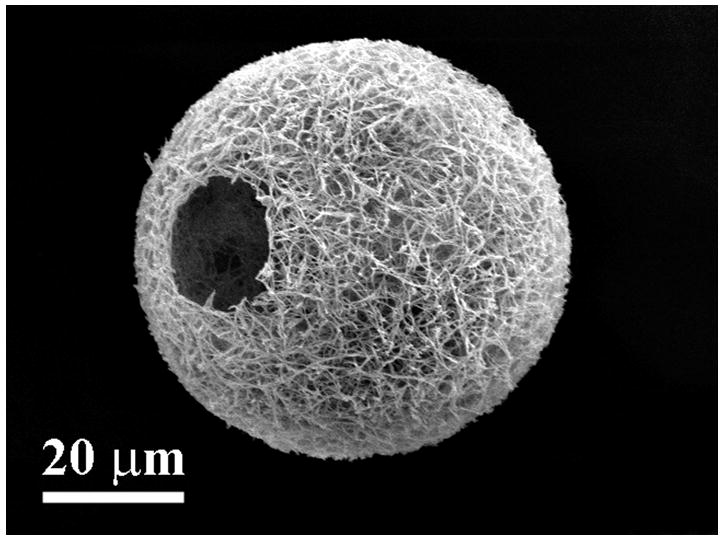
SEM images of nanofibrous hollow microspheres at (a) low, (b) medium, and (c) high magnification. From Liu et al.[47] Copyright © 2011 Nature Publishing Group. Reprinted with permission.
TIPS is a very promising technique to fabricate bone tissue engineering scaffolds stemming from its great processing flexibility. It can produce designed 3D scaffolds with control over the overall shape and pore structure or injectable cell carriers, both of which have a role to play in certain bone regeneration applications.
Composites
Bone is a mineralized structural tissue. Biomimetic composite scaffolds with a mineral component have been widely explored for bone regeneration[43]. The mineral not only adds to the structural integrity of the scaffold, but it can also be actively osteoconductive. Hydroxyapatite (HA) (Ca10(PO4)6(OH)2) is commonly used because it closely resembles the natural minerals found in bone, but other calcium phosphate (CaP) variants[44] or bioglass[48] are also used for their biocompatibility.
Minerals can be incorporated into a scaffold in many ways. In both electrospinning and TIPS, mineral particles can be introduced directly into the polymer solution before processing to create a composite fiber with both mineral and polymer surfaces. Wei et al. created a suspension of nano-hydroxyapatite in PLLA (30:70) and fabricated nano composite scaffolds using TIPS technique and dioxane/water as a solvent[49]. Using a specific dioxane/water ratio of 87:13, composite nanofibers were created. The high surface area of the nanofibrous walls allows more of the hydroxyapatite to be exposed, which is desirable for bone tissue engineering. Peng et al. fabricated needle shaped HA particles and created a 20/80 wt% HA/PLLA solution[50]. The solution was electrospun to fabricate both aligned and random PLLA nanofibers with aligned HA particles distributed throughout the fiber. However, the HA particles had a Ca/P ratio of 1.89, higher than the 1.67 of stoichiometric apatite. This discrepancy was most likely attributed to the wet processing required to synthesize the elongated HA particles. Jegal et al. also incorporated HA into electrospun nanofibers, but used a gelatin-apatite precipitate homogenized in an organic solvent with poly(lactide-co-caprolactone) (PLCL)[51]. During the precipitation reaction, the Ca/P ratio was kept to 1.67 to ensure stoichiometric apatite formation. At varying concentrations of gelatin-apatite, from 14.3 to 20 wt%, the fibers ranged from heterogeneous with slightly thicker regions, to fibers with discrete beads. In both cases, there were apatite crystals distributed through the gelatin-PLCL matrix resulting in composite nanofibers. However, only the lowest concentration of gelatin-apatite resulted in an increase in mean strength. While both of these techniques are effective in creating a composite scaffold with both organic and inorganic components, direct incorporation of the mineral into the polymer phase is not the most efficient use of the mineral. Calcium phosphates are only effective if exposed, and this technique embeds much of the mineral surface within the fiber.
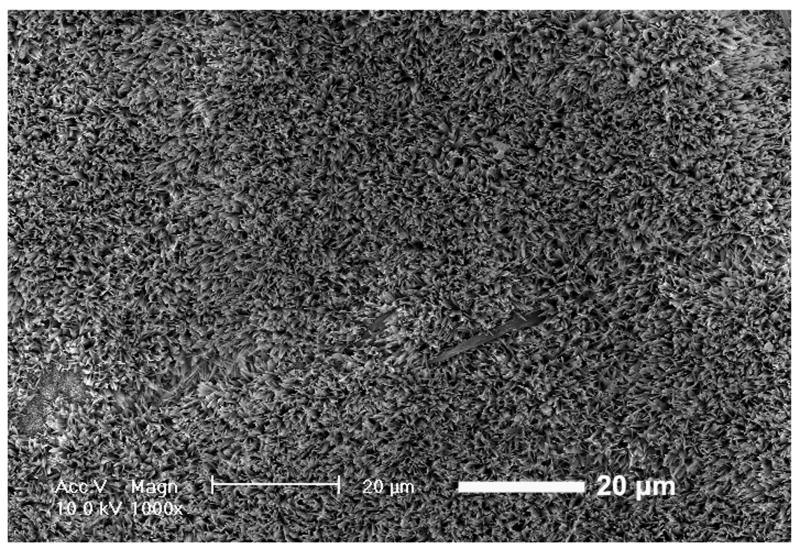
To make the mineral surface more available, calcium phosphates can be deposited directly on the polymer surface. Originally, incubation in a simulated body fluid (SBF) with ion concentrations and pH similar to that of human blood plasma was used to produce apatite deposition[42]. This technique allows the nanofibrous pore walls to be mineralized without clogging the larger pores and the interpore openings. Similarly, electrospun fibrous scaffolds from various synthetic and natural polymers also were mineralized using the SBF technique[52-54]. While the process was successful in coating calcium phosphate mineral on either electrospun or phase-separated nanofibers to improve their osteoconductivity and mechanical properties, it was a slow process, lasting days to weeks[42, 55, 56]. Recently, He et al. developed an electrodeposition process that reduces the mineralization time to under an hour[57]. Using an electrolyte solution and varying parameters like temperature and voltage, control over the surface topography (Figure 2) and Ca/P ratio was achieved. To prove the flexibility of the method, electrodeposition was successfully performed on both electrospun PLLA fibers and phase separated PLLA fibers. Thus, electrodeposition was proven to be a rapid and effective means to mineralize a bone tissue engineering scaffold.
Figure 2.
SEM images of calcium phosphate deposition on electrospun PLLA nanofibrous scaffolds. Electrodeposition was performed for 60 min at 60 °C and (a) 2, (b) 3, and (c) 5 V. From He et al.[57] Copyright © 2010 John Wiley & Sons, Inc. Reprinted with permission.
Tissue engineering applications
Cell source and stem cell differentiation
Along with the scaffold, the choice of cells is important for taking the next step to engineer bone. Primary cells are appealing since they are more differentiated and can be harvested from specific tissues. To illustrate their use, Woo et al. first showed that MC3T3-E1 osteoblasts attached better on nanofibers compared to solid walls by a factor of 1.7[58]. To explain this, they found that the nanofibrous scaffold selectively enhanced the adsorption of proteins such as fibronectin, vitronectin, and laminin. These proteins could allow the cells to anchor more tightly to the matrix, resulting in a higher number of cells attached. They then went a step further to demonstrate the ability of nanofibers to enhance the osteogenic potential of mouse calvarial osteoblasts[59]. Cells were seeded on nanofibrous scaffolds and solid-walled scaffolds and assayed for their mineral deposition and osteogenic gene expression. After 7 days, cells seeded on nanofibers expressed higher levels of osteocalcin and bone sialoprotein compared to those seeded on solid-walled scaffolds. Furthermore, after 2 weeks there was a significant increase in calcium deposition for the cells on nanofibers compared to little for the cells on solid walls as shown by von Kossa staining, revealing a large difference in scaffold mineralization (Figure 3). This osteoblastic induction was also found to be associated with the RhoA-Rock signaling pathway where inhibition of the RhoA effector ROCK promoted the expression of bone sialoprotein[60]. Thus, it is evident that nanofibers promote osteogenesis and biomineralization with primary osteoblasts. However, there are several drawbacks associated with using primary osteoblasts. First, while harvesting them is mostly straightforward, there is limited availability and inherent donor site morbidity. Second, primary cells have a limited proliferative capacity and have age dependent behavior[61]. Finally, when they are taken out of their 3D in vivo environment, there is a risk of dedifferentiation during in vitro culture.
Figure 3.
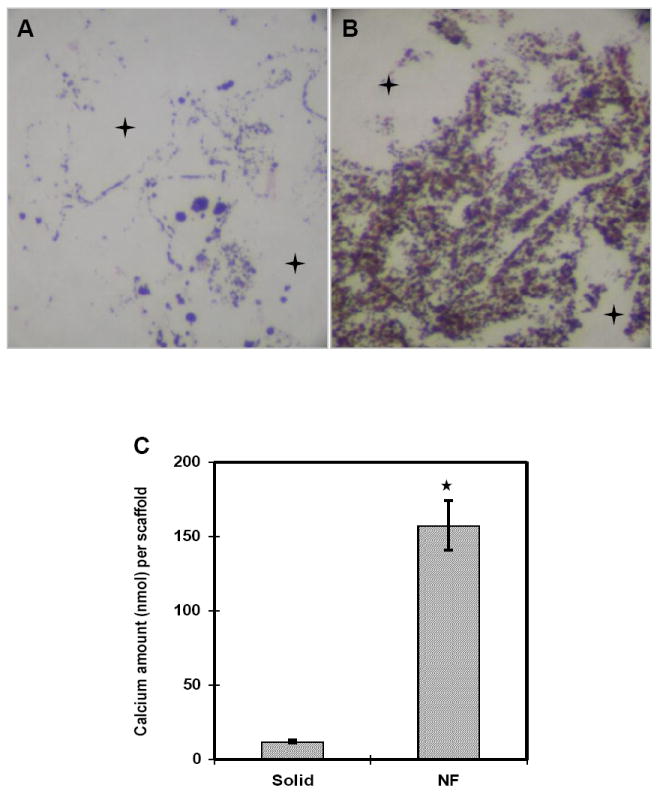
Murine calvarial osteoblasts were cultured for 14 days on (a) solid scaffolds and (b) nanofibrous scaffolds and von Kossa stained (original magnification x100). + denotes macropores. Calcium content was quantified for cells cultured on scaffolds for 7 days (*p<0.05). From Woo et al.[59] Copyright © 2007 Elsevier Ltd. Reprinted with permission.
On the other hand, stem cells are attractive for their large proliferative capacity and ability to differentiate into multiple cell types. Their benefits tend to outweigh the issues associated with their difficulty to harvest and low initial numbers.
Bone marrow stromal cells, also known as mesenchymal stem cells (MSCs), are commonly used for bone tissue engineering. Compared to other stem cells they can be harvested relatively easily and can undergo 40 population doublings in culture[62], although their proliferation rate is slow[63]. In addition, numerous studies have illustrated the ability of MSCs to readily differentiate into osteoblasts under osteogenic conditions on nanofibers[64, 65]. For those reasons, MSCs might currently be the most appealing cell source for bone tissue engineering. However, work is also being done to assess the viability of more pluripotent lines for bone regeneration.
Embryonic stem cells (ESCs) are the quintessential stem cell with the ability to differentiate into any cell type[66, 67]. Importantly, they can maintain their pluripotency while proliferating indefinitely[68]. Also, like primary osteoblasts, ESCs have been shown to attach better to nanofibrous substrates[69, 70]. Smith et al. cultured ESCs in osteogenic media on nanofibrous and solid matrices and assayed for expression of bone differentiation markers[70, 71]. After 4 weeks, cells on the nanofibrous matrix exhibited higher levels of collagen type I, Runx2, osteocalcin, and bone sialoprotein. Additionally, there was significant calcium staining on the nanofibrous matrix compared to the solid films. This shows that nanofibers enhance the osteogenic differentiation of ESCs.
However, the use of human ESCs has ethical and political baggage that hinders their potential. In order to circumvent that, induced pluripotent stem cells (iPSCs) were developed [72, 73] to provide a pluripotent stem cell line that avoided the controversy surrounding human ESCs. While there have been studies to show that iPSCs are capable of osteogenic differentiation using various techniques[74-76], more work needs to be done to apply them to bone tissue engineering and to see if nanofibers have a similar osteogenic effect.
Another alternative to ESCs are amniotic fluid-derived stem cells (AFSCs)[77]. Slightly more mature than ESCs, AFSCs can undergo upwards of 250 population doublings while still retaining the ability to differentiate into multiple lineages, including osteogenic. Sun et al. then illustrated enhanced osteogenesis of AFSCs on nanofibrous scaffolds compared to solid-walled scaffolds. After 1 week of culture in media supplemented with bone morphogenic protein-7 (BMP-7), there was significantly higher alkaline phosphatase activity, an early bone marker, on the nanofibrous scaffolds, along with increased expression levels of osteogenic genes Runx2, osterix, osteopontin, bone sialoprotein, and osteocalcin. There was also increased calcium content within the nanofibrous scaffolds after both 2 and 4 weeks compared to the solid scaffolds. This difference was confirmed with von Kossa staining showing higher mineralization levels in the nanofibrous scaffolds.
While MSCs and other pre-osteoblast lines remain the most common stem cell sources, the relatively new iPSC and AFSC lines have shown great promise in being applied to bone tissue engineering. More work needs to be done to elucidate any effect nanofibers might have on iPSCs, but AFSCs experience enhanced osteogenic differentiation when seeded on nanofibers. The ultimate goal, however, is not just to differentiate stem cells into osteocytes.
Bone tissue formation
While controlling stem cell differentiation is necessary, it is just one step on the path to bone regeneration with the aim to form new functional tissue.
Seyedjafari et al. seeded hydroxyapatite coated and uncoated electrospun PLLA fibers with human cord blood derived stem cells and implanted the scaffolds subcutaneously into mice[78]. After 10 weeks, scaffolds without hydroxyapatite showed no calcium deposition and were surrounded by a granulomatous inflammatory response while scaffolds with hydroxyapatite showed significant mineralization with little inflammatory response. Additionally, higher order bone structures such as trabeculi and bone marrow were found within the newly formed ectopic bone. Cai et al. also used an electrospun PLLA scaffold, but combined it with a collagenous guided bone regeneration membrane[79]. Using rabbit tibia defect model, implants were composed of a porous collagen membrane, a nanofibrous PLLA membrane, or a bilayer combining the two. After 3 weeks, the defects treated with the bilayer group were 91% filled with new bone tissue compared to 64% for the nanofibrous membrane alone and just 32% for the collagen membrane alone. After 6 weeks, the new bone formed on the nanofibers was found to contain a high percentage of cortical bone, 86% and 77% for the bilayer and nanofibrous membrane alone respectively, compared to just 46% for the collagen membrane.
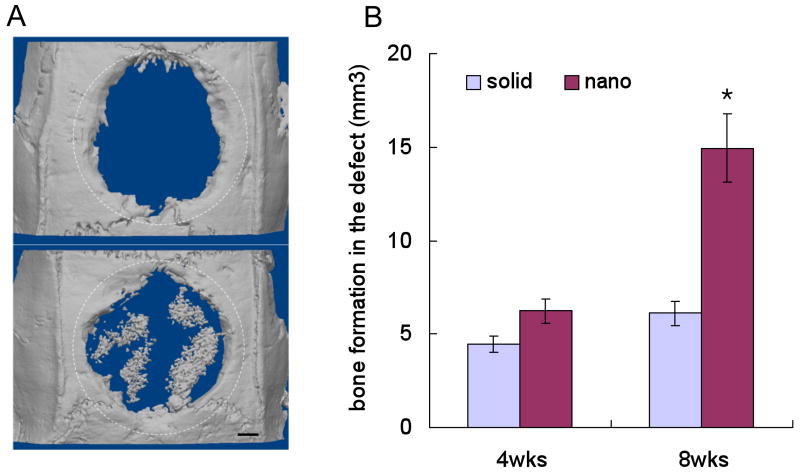
Woo et al. went a step further to directly compare the in vivo bone formation potential of nanofibrous scaffolds versus solid-walled scaffolds[80]. A critical-size calvarial defect was created in rats and either a nanofibrous or solid-walled scaffold was implanted. Micro-CT analysis revealed 2.1-fold more bone formation after 8 weeks (Figure 4). Von Kossa and H&E staining confirmed that new bone had formed throughout the nanofibrous implants, while only the periphery of the solid-walled scaffolds sustained bone ingrowth. This study clearly shows the advantage of nanofibrous scaffolds over solid-walled scaffolds in an in vivo setting.
Figure 4.
(a) Micro-CT images of the defect implanted with either a solid-walled scaffold (upper) or nanofibrous scaffold (lower) after 8 weeks. The dotted circles outline the original defect. (b) Quantification of the bone volume after subtracting the negative control where no scaffold was implanted (*p<0.05). From Woo et al.[80] Copyright © 2009 Mary Ann Liebert, Inc. Reprinted with permission.
With the clinic in mind, Wang et al. sought to create scaffolds that could be made into patient-specific digits[81]. Using a CT scan of the patient’s defect and a 3D printer, an anatomic mold was created and used to fabricate a 3D scaffold using TIPS and a paraffin porogen. MC3T3-E1 osteoblasts were seeded in the scaffold and showed high levels of mineralization and tissue formation after 6 weeks.
Conclusion
From the inception of tissue engineering as a field, it has garnered interest from biologists, materials scientists, engineers, and physicians. The interdisciplinary nature has led to rapid expansion and advancement as numerous hurdles are overcome. Arguably one of the biggest hurdles is the fabrication of a biomimetic scaffold. Since the ECM of bone is composed of primarily collagen I nanofibers, techniques were developed to fabricate fibers of similar sized. At first, an old principle of electrospinning was applied very successfully to create nanofibrous meshes. Then thermally-induced phase separation was used and combined with porogen leaching and 3D printing to create more 3D nanofibrous scaffolds. Now, even more techniques have arisen, including molecular self-assembly and bacterial cellulose, to fabricate nanofibrous scaffolds.
The nanofibrous scaffold was only part of the way to fabricating a biomimetic construct. Bone has a significant mineral component that gives it its physiological properties, including its mechanical strength. Thus, bone-like calcium phosphates, such as hydroxyapatite and tricalcium phosphate, were integrated with the polymer, either through direct incorporation or deposition, to create a composite construct. These composites took advantage of processing capabilities of polymers through electrospinning or TIPS and the osteoconductivity and mechanical strength of the mineral.
As the bone tissue engineering field continues to strive toward the ability to fully and functionally heal bone defects, nanofibrous scaffolds will continue to be the foundation. Their biomimetic nature has been shown to improve cell adhesion, osteogenic differentiation, and tissue formation. Moreover, composite scaffolds have shown promise by premineralizing the matrix, also resulting in enhanced osteogenesis. In essence, the scaffold has become, and will continue to need to be, an active player in the tissue regeneration process instead of simply a cell carrier or tissue template.
More needs to be done to translate these next generation scaffolds to the clinic. There have been many studies that illustrate the potential and promise of their work in vitro, but comparatively little has been done in vivo to follow through.
Acknowledgments
The authors would like to gratefully acknowledge support from the NIH/NIDCR (NIDCR DE14755, DE017689 and DE15384: PXM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott A. Cell culture: Biology’s new dimension. Nature. 2003;424(6951):870–872. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 4.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 5.Kadler K. Matrix loading: assembly of extracellular matrix collagen fibrils during embryogenesis. Birth Defects Res, Part C. 2004;72(1):1–11. doi: 10.1002/bdrc.20002. [DOI] [PubMed] [Google Scholar]
- 6.Weiner S, Wagner HD. The material bone: Structure mechanical function relations. Annu Rev Mater Sci. 1998;28:271–298. [Google Scholar]
- 7.Fratzl P, Groschner M, Vogl G, Plenk H, Eschberger J, Fratzlzelman N, et al. Mineral crystals in calcified tissue - A comparative study by SAXS. J Bone Miner Res. 1992;7(3):329–334. doi: 10.1002/jbmr.5650070313. [DOI] [PubMed] [Google Scholar]
- 8.Buehler MJ. Molecular nanomechanics of nascent bone: fibrillar toughening by mineralization. Nanotechnology. 2007;18(29):295102. [Google Scholar]
- 9.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res. 2000;52:430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 11.Niinomi M. Metallic biomaterials. J Artif Organs. 2008;11(3):105–110. doi: 10.1007/s10047-008-0422-7. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuki C, Kamitakahara M, Miyazaki T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J R Soc, Interface. 2009;6:S349–S360. doi: 10.1098/rsif.2008.0419.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 14.Semino CE. Self-assembling peptides: from bio-inspired materials to bone regeneration. J Dent Res. 2008;87:606–616. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]
- 15.Sargeant TD, Rao MS, Koh C-Y, Stupp SI. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials. 2008;29:1085–1098. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recouvreux DOS, Rambo CR, Berti FV, Carminatti CA, Antonio RV, Porto LM. Novel three-dimensional cocoon-like hydrogels for soft tissue regeneration. Mater Sci Eng. 2011;31(2):151–157. [Google Scholar]
- 18.Ahmad Z, Rasekh M, Edirisinghe M. Electrohydrodynamic direct writing of biomedical polymers and composites. Macromol Mater and Eng. 2010;295(4):315–319. [Google Scholar]
- 19.Yu NYC, Schindeler A, Little DG, Ruys AJ. Biodegradable poly(alpha-hydroxy acid) polymer scaffolds for bone tissue engineering. J Biomed Mater Res, Part B. 2010;93B(1):285–295. doi: 10.1002/jbm.b.31588. [DOI] [PubMed] [Google Scholar]
- 20.Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. J Electrost. 1995;35:151–160. [Google Scholar]
- 21.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. [Google Scholar]
- 22.Luo CJ, Nangrejo M, Edirisinghe M. A novel method of selecting solvents for polymer electrospinning. Polymer. 2010;51(7):1654–1662. [Google Scholar]
- 23.Badami AS, Kreke MR, Thompson MS, Riffle JS, Goldstein AS. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 24.Boland ED, Telemeco TA, Simpson DG, Wnek GE, Bowlin GL. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly(glycolic acid) for tissue engineering. J Biomed Mater Res, Part B. 2004;71B(1):144–152. doi: 10.1002/jbm.b.30105. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Ouyang H, Lim CT, Ramakrishna S, Huang ZM. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J Biomed Mater Res, Part B. 2004;72B(1):156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Oh SH, Liu J, Soker S, Atala A, Yoo JJ. The use of thermal treatments to enhance the mechanical properties of electrospun poly (epsilon-caprolactone) scaffolds. Biomaterials. 2008;29(10):1422–1430. doi: 10.1016/j.biomaterials.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Yeo M, Ahn S, Kang D-O, Jang CH, Lee H, et al. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J Biomed Mater Res, Part B. 2011;97(2):263–270. doi: 10.1002/jbm.b.31809. [DOI] [PubMed] [Google Scholar]
- 28.Sisson K, Zhang C, Farach-Carson MC, Chase DB, Rabolt JF. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J Biomed Mater Res, Part A. 2010;94(4):1312–1320. doi: 10.1002/jbm.a.32756. [DOI] [PubMed] [Google Scholar]
- 29.Jin H-J, Chen J, Karageorgiou V, Altman GH, Kaplan DL. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 2004;25(6):1039–1047. doi: 10.1016/s0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- 30.Geng X, Kwon O-H, Jang J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 2005;26(27):5427–5432. doi: 10.1016/j.biomaterials.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 31.Zeugolis DI, Khew ST, Yew ESY, Ekaputra AK, Tong YW, Yung L-YL, et al. Electro-spinning of pure collagen nano-fibres - just an expensive way to make gelatin? Biomaterials. 2008;29:2293–2305. doi: 10.1016/j.biomaterials.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Chen X, Wang H. Acceleration of osteogenic differentiation of preosteoblastic cells by chitosan containing nanofibrous scaffolds. Biomacromolecules. 2009;10:2772–2778. doi: 10.1021/bm900623j. [DOI] [PubMed] [Google Scholar]
- 33.Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 2005;6(3):1484–1488. doi: 10.1021/bm0492576. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama Y, Hattori S, Yoshikawa C, Yasuda Y, Koyama H, Takato T, et al. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater Lett. 2009;63:754–756. [Google Scholar]
- 35.Ma PX. Biomimetic materials for tissue engineering. Advanced Drug Delivery Reviews. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holzwarth JM, Ma PX. 3D nanofibrous scaffolds for tissue engineering. J Mater Chem. 2011 doi: 10.1039/c1031jm10522a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, Ma PX. Poly(alpha-hydroxyl acids)hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 38.Ma PX, Zhang R. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Nam YS, Park TG. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res. 1999;47:8–17. doi: 10.1002/(sici)1097-4636(199910)47:1<8::aid-jbm2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Chen VJ, Ma PX. Nano-fibrous poly(L-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25:2065–2073. doi: 10.1016/j.biomaterials.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 41.Ma PX, Choi J-W. Biodegradable polymer scaffolds with well-defined interconnected pherical pore network. Tissue Eng. 2001;7:23–33. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 42.Wei G, Ma PX. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res, Part A. 2006;78A:306–315. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 43.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Delivery Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sicchieri LG, Crippa GE, Oliveira PTd, Beloti MM, Rosa AL. Pore size regulates cell and tissue interactions with PLGA–CaP scaffolds used for bone engineering. J Tissue Eng Regener Med. 2011 doi: 10.1002/term.1422. [DOI] [PubMed] [Google Scholar]
- 45.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5475–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Chen VJ, Smith LA, Ma PX. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973–3979. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10(5):389–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yunos DM, Ahmad Z, Boccaccini AR. Fabrication and characterization of electrospun poly-DL-lactide (PDLLA fibrous coatings on 45S5 Bioglass (R) substrates for bone tissue engineering applications. J Chem Technol Biotechnol. 2009;85(6):768–774. [Google Scholar]
- 49.Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Peng F, Yu X, Wei M. In vitro cell performance on hydroxyapatite particles/poly(L-lactic acid nanofibrous scaffolds with an excellent particle along nanofiber orientation. Acta Biomater. 2011;7(6):2585–2592. doi: 10.1016/j.actbio.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Jegal SH, Park JH, Kim JH, Kim TH, Shin US, Kim TI, et al. Functional composite nanofibers of poly(lactide–co-caprolactone) containing gelatin–apatite bone mimetic precipitate for bone regeneration. Acta Biomater. 2011;7(4):1609–1617. doi: 10.1016/j.actbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Ito Y, Hasuda H, Kamitakahara M, Ohtsuki C, Tanihara M, Kang IK, et al. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J Biosci Bioeng. 2005;100(1):43–49. doi: 10.1263/jbb.100.43. [DOI] [PubMed] [Google Scholar]
- 53.Zuo Y, Yang F, Wolke JG, Li Y, Jansen JA. Incorporation of biodegradable electrospun fibers into calcium phosphate cement for bone regeneration. Acta Biomater. 2010;6(4):1238–1247. doi: 10.1016/j.actbio.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez K, Renneckar S, Gatenholm P. Biomimetic calcium phosphate crystal mineralization on electrospun cellulose-based scaffolds. ACS Appl Mater Interfaces. 2011;3(3):681–689. doi: 10.1021/am100972r. [DOI] [PubMed] [Google Scholar]
- 55.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J Biomed Mater Res. 1990;24(6):721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 56.Zhang R, Ma PX. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999;45(4):285–293. doi: 10.1002/(sici)1097-4636(19990615)45:4<285::aid-jbm2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 57.He C, Xiao G, Jin X, Sun C, Ma PX. Electrodeposition of nanofibrous polymer scaffolds: Rapid mineralization, tunable calcium phosphate composition and topography. Adv Funct Mater. 2010;20(20):3568–3576. doi: 10.1002/adfm.201000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res, Part A. 2003;67A(2):531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 59.Woo KM, Jun J-H, Chen VJ, Seo J, Baek J-H, Ryoo H-M, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28(2):335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Hu J, Liu X, Ma PX. Induction of osteoblast differentiation phenotype on poly(L-lactic acid nanofibrous matrix. Biomaterials. 2008;29(28):3815–3821. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montjovent MO, Burri N, Mark S, Federici E, Scaletta C, Zambelli PY, et al. Fetal bone cells for tissue engineering. Bone. 2004;35(6):1323–1333. doi: 10.1016/j.bone.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64(2):278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 63.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5(1):91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28(2):316–325. doi: 10.1016/j.biomaterials.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30(28):5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans MJ, Kaufman MH. Establishment in cluture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 67.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Siergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 68.Zeng X, Rao MS. Human embryonic stem cells: Long term stability, absence of senescence and a potential cell source for neural replacement. Neuroscience. 2007;145(4):1348–1358. doi: 10.1016/j.neuroscience.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 69.Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24(2):426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 70.Smith LA, Liu X, Hu J, Ma PX. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30(13):2516–2522. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith LA, Liu X, Hu J, Wang P, Ma PX. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng, Part A. 2009;15(7):1855–1864. doi: 10.1089/ten.tea.2008.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 74.Tashiro K, Inamura M, Kawabata K, Sakurai F, Tamanishi K, Hayakawa T, et al. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells. 2009;27(8):1802–1811. doi: 10.1002/stem.108. [DOI] [PubMed] [Google Scholar]
- 75.Kao CL, Tai LK, Chiou SH, Chen YJ, Lee KH, Chou SJ, et al. Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev. 2010;19(2):247–258. doi: 10.1089/scd.2009.0186. [DOI] [PubMed] [Google Scholar]
- 76.Bilousova G, Jun DH, King KB, Langhe SD, Chick WS, Torchia EC, et al. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011;29(2):206–216. doi: 10.1002/stem.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coppi PD, Georg Bartsch J, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 78.Seyedjafari E, Soleimani M, Ghaemi N, Shabani I. Nanohydroxyapatite-coated electrospun poly(L-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11(11):3118–3125. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- 79.Cai YZ, Wang LL, Cai HX, Qi YY, Zou XH, Ouyang HW. Electrospun nanofibrous matrix improves the regeneration of dense cortical bone. J Biomed Mater Res, Part A. 2010;95A(1):49–57. doi: 10.1002/jbm.a.32816. [DOI] [PubMed] [Google Scholar]
- 80.Woo KM, Chen VJ, Jung HM, Kim TI, Shin HI, Baek JH, et al. Comparative evaluation of nanofibrous scaffolding for bone regeneration in critical-size calvarial defects. Tissue Eng, Part A. 2009;15(8):2155–2162. doi: 10.1089/ten.tea.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang P, Hu J, Ma PX. The engineering of patient-specific, anatomically shaped, digits. Biomaterials. 2009;30(14):2735–2740. doi: 10.1016/j.biomaterials.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]